Abstract
Introduction and hypothesis
In interstitial cystitis/bladder pain syndrome (IC/BPS), pelvic floor dysfunction may contribute significantly to pelvic pain. To determine if pelvic floor hypertonicity manifests alterations on magnetic resonance imaging (MRI) in patients with IC/BPS, we retrospectively compared pelvic measurements between patients and controls.
Methods
Fifteen women with IC/BPS and 15 age-matched controls underwent pelvic MRI. Two blinded radiologists measured the pelvic musculature, including the H- and M lines, vaginal length, urethral length and cross-sectional area, levator width and length, and posterior puborectalis angle. MRI measures and clinical factors, such as age, parity, and duration of symptoms, were compared using a paired, two-tailed t test.
Results
There were no significant differences in age, parity, or symptom duration between groups. Patients with IC/BPS exhibited shorter levator muscles (right: 5.0 ± 0.7 vs. 5.6 ± 0.8, left: 5.0 ± 0.8 vs. 5.7 ± 0.8 cm, P < 0.002) and a wider posterior puborectalis angle (35.0 ± 8.6 vs. 26.7 ± 7.9°, P < 0.01) compared with controls. The H line was shorter in patients with IC/BPS (7.8 ± 0.8 vs. 8.6 ± 0.9 cm, P < 0.02), while M line did not differ. Total urethral length was similar, but vaginal cuff and bladder neck distances to the H line were longer in patients with IC/BPS (5.7 ± 0.6 vs. 5.1 ± 0.9 cm, P < 0.02; 1.9 ± 0.4 vs. 1.4 ± 0.2 cm, P < 0.001, respectively).
Conclusions
Patients with IC/BPS have pelvic floor hypertonicity on MRI, which manifests as shortened levator, increased posterior puborectalis angles, and decreased puborectal distances. We identified evidence of pelvic floor hypertonicity in patients with IC/BPS, which may contribute to or amplify pelvic pain. Future studies are necessary to determine the MRI utility in understanding pelvic floor hypertonicity in patients with IC/BPS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is characterized by refractory discomfort referable to the lower urinary tract and associated with urinary urgency, frequency, and pain. Previously, IC/BPS was thought to be a primary inflammatory or infectious condition, but evidence now suggests it is a manifestation of afferent neurologic dysfunction [1] in which abnormal processing of sensory information related to the genitourinary tract results in bladder and pelvic pain and voiding dysfunction. This pathophysiology is shared by other functional somatic syndromes, such as irritable bowel syndrome (IBS), Chronic Fatigue Syndrome (CFS), and fibromyalgia, which are characterized more by painful or uncomfortable symptoms, suffering, and disability than by demonstrable abnormalities of organ structure or function [2].
While this abnormal processing is fundamental in the pathophysiology of IC/BPS, pelvic floor dysfunction likely contributes to the cycle of pain experienced by affected women. Myofascial pain and hypertonic pelvic floor dysfunction coexist in 85 % of patients with IC/BPS and other chronic pain syndromes [3]. Patients with IC/BPS frequently have coexisting levator pain (87–94 %), vulvar pain (50–51 %), and sexual dysfunction/dyspareunia (71–72 %) [4, 5]. Urodynamics of patients with severe urgency and IC [6] demonstrated that abnormal behavior of the external urinary sphincter paralleled pain occurrences. On physical exam, palpation of pelvic trigger points and taut muscle bands in patients with IC/BPS elicits pain in the bladder, vulva, vagina, or perineum [7]. Such myofascial trigger points were observed in 78 % of patients with IC/BPS; their presence and number correlated with symptom duration and severity [8].
The importance of myofascial dysfunction and pain in patients with IC/BPS is demonstrated by patient responses to therapies aimed at pelvic floor relaxation. Injection and stretch techniques aimed at muscular trigger points referred to the pelvis resulted in quality of life (QoL) improvements [9]; 59 % of patients reported improvements with myofascial physical therapy in contrast to 26 % who underwent generalized therapeutic massage [10]. Pelvic floor manual therapy generated improvements in 70 % of patients with IC/BPS [11]. Perianal/perivaginal electromyography correlated decreases in pelvic floor tension with better symptom scores. These data strongly support a role for pelvic floor dysfunction in a subset of patients with IC/BPS and recommend the use of interventions targeting the pelvic floor.
Trigger points in the pelvic floor musculature generate pain referred to the perineum and vagina, the sensation of fullness in the rectum, and urgency to void. The main component of the pelvic floor is the levator ani muscle complex, which contains slow-twitch fibers that provide continuous tone to the pelvic floor. Our hypothesis is that abnormal over-tensioning of these muscles (as primary dysfunction or secondary to pain itself) perpetuates severe pelvic pain and disability seen in patients with IC/BPS.
Materials and methods
After approval by the Institutional Review Board (IRB#11-003181), 15 women between the ages 18 and 55 years with a diagnosis of IC/BPS, defined as significant pelvic pain in conjunction with urinary urgency and frequency without an anatomic cause, and 15 control individuals without pain, prolapse, or lower urinary tract symptoms were selected from a retrospective review of University of California, Los Angeles (UCLA) Urology and Gynecology clinical records between January 2005 and December 2010. Patients were diagnosed according to the IC/BPS definition recommended by the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU): “An unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than 6 weeks’ duration, in the absence of infection or other identifiable causes.” All of these patients also underwent cystoscopy, which was normal in all cases. All patients with IC/BPS were assessed with the O’Leary-Sant (OS) validated questionnaire, with an average OS Symptom Index (OSSI) of 13.2 ± 1.5 and OS Problem Index (OSPI) of 10.5 ± 1.3. MRI was performed in these patients to rule out other pelvic pathology as a cause of their symptoms. Patients with prior pelvic radiation, pelvic malignancy, pelvic prolapse, prior pelvic surgery, culture-proven urinary tract infection, or pelvic vascular congestion were excluded. The most common indication for MRI in controls was menorrhagia; all but one of these patients had uterine fibroids. We selected controls with similar demographics to the IC/BPS group. Postmenopausal women were excluded to avoid a potential confounder of hormonal status.
Patients underwent standardized pelvic MRI [12] in the supine position, either half-Fourier-acquisition single-shot turbo spin echo (HASTE) sequence in a 1.5-Tesla magnet with phased-array coils (Siemens) or single-shot fast spin echo (SSFSE) T2-weighted sequence in a 1.5-Tesla magnet (General Electric). These MRI sequences are functionally equivalent and used a slice thickness of 3 mm, table speed of 5 mm/s, and 2-mm reconstruction interval. This imaging protocol, previously described in great detail [13], provides a reproducible method for assessing the pelvic floor and is significantly less expensive than a standard MRI of the pelvis, costing only a few hundred US dollars per study in contrast to the several thousand US dollars required for a standard MRI.
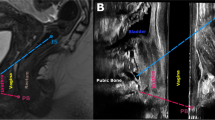
Axial and sagittal images were reviewed by two blinded radiologists. We used the standardized Health Maintenance Organization (HMO) classification [13] to determine H and M lines, which assess relative positions of the pelvic floor musculature and genitourinary structures (Fig. 1a). Additional pelvic structures were assessed, as previously described [14–17]. Total vaginal and urethral lengths and perpendicular distance of the urethral meatus to the puborectal line (UPRL) were measured in midline sagittal images. Puborectalis length, thickness, urethral cross-sectional area, and posterior puborectalis angle were measured in axial images at the levels of the pubic symphysis and bladder neck (Fig. 1b). As body mass index (BMI) was not available for all patients, we measured subcutaneous adipose tissue (SCAT), the greatest thickness of the anterior body-wall adipose layer in axial sections at the level of the umbilicus, a measure that correlates with BMI [18].
Pelvic floor measurements on axial and sagittal magnetic resonance imaging. a On midline sagittal images, the H line extends from the inferior ramus of the pubis to the posterior margin of the rectum. The pubococcygeal line extends from the inferior pubic ramus to the coccyx. The M line measures the descent of the levator plate from the pubococcygeal line. Urethral and vaginal lengths follow the anatomic structures and are not indicated in the figure. The urethral distance to the puborectal line measures the distance from the bladder neck to the H line. b On axial images in the pelvis at the midurethra, we measured the lengths and widths of the bilateral puborectalis muscles. Urethral diameter was a function of the product of the urethral width and depth. The posterior puborectalis angle was measured at the posterior insertions of the muscles, as shown
For each parameter, measurements taken by each radiologist were averaged for statistical analysis. Measurements for which there was >10 % difference between reviewing radiologists were re-evaluated by a third, blinded, radiologist. If the discordant value was >10 % different from the re-evaluated measurement, this value was discarded. If not, all three values were averaged for analysis. Statistically significant differences between the IC/BPS and control groups were examined using a two-tailed, paired Student’s t test. To evaluate interrater reliability of MRI measurements, we determined Lin’s concordance class correlation (CCC) and the corresponding bootstrap 95 % confidence intervals (CI) to measure agreement between these two continuous variables. For reference, CCC values 0–0.4 are considered poor agreement, 0.4–0.7 moderate agreement, 0.7–0.9 high agreement, and >0.9 very high agreement.
Using receiver operating characteristic (ROC) curve analysis, we evaluated the accuracy of puborectalis length as a test to diagnose patients with IC/BPS, as previously described [19, 20]. Data were examined using STATA, version 12 (StataCorp LP). P values <0.05 were considered statistically significant.
Results
There were no significant differences between the IC/BPS and control populations in age, parity, or age at menarche (Table 1). We also assessed symptom severity using OS index and self-reported symptom duration in the IC/BPS population. As body habitus influences pelvic architecture, we also assessed SCAT [18]. There was no significant difference in this surrogate for BMI between women with and without IC/BPS.
IIC/BCS
We calculated the total number of comorbidities self-reported for each patient at the time of MRI assessment. Patients with IC/BPS had a multitude of comorbidities, including depression (n = 6), IBS (n = 4), anxiety (n = 3), hypothyroidism (n = 3), hyperlipidemia (n = 3), fibromyalgia (n = 2), migraines (n = 2), chronic back pain (n = 2), asthma (n = 2), hypertension (n = 2), hemolytic anemia (n = 1), gastritis (n = 1), CFS (n = 1), gastroesophageal reflux disease (n = 1), Von Willebrand’s disease (n = 1), Hashimoto’s thyroiditis (n = 1), osteoarthritis (n = 1), attention-deficit/hyperactivity disorder (n = 1), type 1 diabetes (n = 1), and endometriosis (n = 1). Patients with IC/BPS had more comorbidities than controls, a difference accounted for by psychiatric and neurologic disorders. No significant differences were seen in the prevalence of diabetes, hypertension, hyperlipidemia, asthma, anemia, hypothyroidism, or heart disease; 60 % of women with IC/BPS, however, had a comorbid psychiatric condition, most commonly depression (33 %) or anxiety (13 %), requiring medication. Nearly half of patients with IC/BPS reported a chronic neurologic/pain condition, e.g., migraine, IBS, fibromyalgia, or CFS. Control patients denied these comorbidities, suggesting IC/BPS coexists with other functional somatic syndromes [21]. A significant proportion of the patients with IC/BPS also exhibited drug allergies (40 vs. 13.3 % in controls), as seen previously [4].
Independent measurements of pelvic anatomy by two blinded radiologists demonstrated good interrater reliability; for puborectalis length, the CCC was 0.959 (CI 0.952–0.967). Similar results (>0.95) were found for all measurements. The M line, a measure of pelvic prolapse, did not differ between study populations (Table 2). The H line, the distance from pubis to posterior anal canal, differed significantly (7.8 ± 0.8 cm IC/BPS vs. 8.6 ± 0.9 cm controls, P < 0.02), reflecting shortening of the anteroposterior (AP) pelvic musculature in IC/BPS. Pelvic tightening was also reflected in bilateral puborectalis shortening (right: 5.0 ± 0.7 cm IC/BPS vs. 5.6 ± 0.8 cm controls, left: 5.0 ± 0.8 cm IC/BPS vs. 5.7 ± 0.8 cm controls, P < 0.002). This tightening of the pelvic floor in patients with IC/BPS shifted the posterior levators anteriorly, resulting in a wider posterior puborectalis angle (Fig. 2) (35.0 ± 8.6° IC/BPS vs. 26.7 ± 7.9° controls, P < 0.01). There were no significant differences in puborectalis width or urethral diameter, suggesting levator shortening is not due to distortion of the pelvic floor.
Puborectalis shortening in patients with interstitial cystitis/bladder pain syndrome (IC/BPS). Axial slices of the pelvis at the level of the pubic symphysis demonstrate the relationship of the puborectalis to the pelvic organs. a In comparison with a control patient, shortening of the bilateral puborectalis muscles in b the patient with IC/BPS shifts the posterior insertion of the muscle anteriorly, widening the posterior puborectalis angle (arrow)
Urethral length measures the distance from the urethral meatus to the bladder neck, while UPRL measures the perpendicular distance of the meatus to the H line, which is not necessarily along the urethral axis. While overall urethral length did not differ between populations, UPRL was significantly longer in the IC/BPS cohort (1.9 ± 0.4 cm vs. 1.4 ± 0.2 cm controls, P < 0.001), again reflecting tightening of the AP anatomy of the pelvis; the bladder neck moves anteriorly in the pelvis, resulting in an increase in UPRL without increasing urethral length.
Multiparity is associated with greater levator laxity, longer levator length, and narrower posterior puborectalis angles [16]. Despite a trend in increased parity over controls, patients with IC/BPS exhibited shorter levators on imaging. To rule out a bias from parity, we performed a subset analysis comparing nulliparous women from each group. The differences in levator angle (34.0 ± 6.8 cm IC/BPS vs. 26.4 ± 7.7 cm controls, P = 0.05), left levator length (4.9 ± 0.7 cm IC/BPS vs. 5.7 ± 0.7 cm controls, P = 0.04), and right levator length (4.9 ± 0.6 cm IC/BPS vs. 5.8 ± 0.8 cm controls, P = 0.04) persisted. In a multivariate analysis of clinical factors, only the diagnosis of IC/BPS was an independent predictor of shorter levator length.
As the greatest percent changes were observed in levator lengths, we evaluated the diagnostic performance of MRI puborectalis length to discriminate patients with IC/BPS from control patients using ROC curve analysis. While the trend toward shorter levator lengths in IC/BPS can be seen graphically (Fig. 3), we could not identify a levator length cutoff value with sufficient specificity and sensitivity to be useful in differentiating patients with IC/BPS. An MRI levator length cutoff of 5.6 cm would provide a sensitivity of 0.81 and a specificity of 0.57, while a cutoff of 5.4 would provide a sensitivity of 0.68 and a specificity of 0.63, making levator length a poor diagnostic predictor in this small sample.
Distribution of levator length in patients with interstitial cystitis/bladder pain syndrome (IC/BPS) in contrast to control women. Bilateral puborectalis lengths determined on axial magnetic resonance imaging (MRI) slices were pooled for each population, then expressed graphically as a function of the number of patients
Discussion
While studies suggest a relationship between pelvic floor myofascial dysfunction and IC/BPS, no objective evidence of increased pelvic floor hypertonicity exists. In our study, women with IC/BPS exhibited shortening of the puborectalis, resulting in a wider posterior puborectalis angle. This tightening of the AP pelvic musculature correlates with a smaller levator hiatus (H line) and an anterior shift in the pelvic floor, evidenced by an increased UPRL. This study is the first to demonstrate an association between IC/BPS and objective, reproducible differences in pelvic floor tonicity on imaging. Many women with IC/BPS have symptoms attributable to abnormalities of the pelvic floor musculature; the increased pelvic floor tonicity seen in these patients, independent of age, parity, and body habitus, supports the involvement of pelvic floor over-tensioning in IC/BPS as a contributor to or consequence of the underlying pathophysiology.
There are several limitations to this pilot analysis, the foremost of which is the small cohort size. We observed no correlation between pelvic floor measurements and either symptom duration or severity as assessed by OSS (data not shown), which is not surprising, as the small size of our study population is not sufficient to detect any such correlation. In addition, the control group received pelvic MRI for a nonpain indication, most commonly menorrhagia. Given our small population size, factors associated with menorrhagia could confound our analysis. As morphometric measurements of pelvic structures vary considerably in normal individuals [22], natural variation could generate false-positive correlations between levator shortening and IC/BPS. All of our subset and multivariate analyses, however, reiterated this correlation, which would be unlikely if resulting from random population variations alone.
As the study was performed retrospectively, there is a risk that we did not appropriately match control individuals to patients with IC/BPS. While there were no significant differences in population demographics, our analysis may lack the power to detect unrelated confounders. Still, analysis of several possible confounders lends support to our underlying conclusion: Both increased parity and BMI are associated with longer levator lengths, smaller posterior puborectalis angles, greater H lines, and apical descent [23]; observation of the opposite results in patients with IC/BPS, despite greater parity and body adiposity, implicates pelvic floor over-tensioning in IC/BPS pathophysiology.
Prior studies have linked the duration and severity of IC/BPS symptoms with worsening myofascial trigger-point pain on palpation [8]. Ours was a pilot study to evaluate the feasibility of MRI to distinguish pelvic floor changes in individuals with IC/BPS and was not sufficiently powered to correlate the severity or duration of IC/BPS symptoms with MRI measures. The high concordance of measurements by independent radiologists suggests these findings are objective and reproducible, in contrast to the controversial assessment of trigger points on examination. Future analyses correlating MRI findings with both disease duration and validated measures of symptom severity may elucidate the contribution of pelvic floor dysfunction to the pathophysiology of IC/BPS and provide an objective phenotypic characteristic by which to identify a unique subset of patients with IC/BPS.
Phenotypic variants of IC/BPS exhibit different predominant symptoms, such as dyspareunia, pelvic pain, bladder pain, and urinary dysfunction, despite a common pathophysiology [24]. Such phenotyping predicts symptom severity and QoL but has not been useful to predict therapeutic outcomes. Only a subset of patients responds to treatments like pelvic floor physiotherapy [10], neurostimulation, or antidepressants; disparate responses likely result from varying contributions of muscular hypertonicity to afferent pain perpetuation. The high concordance between radiologists suggests MRI measurements are objective, allowing reproducible isolation of patients with a significant pelvic floor component to their disease. As manual assessment of the pelvic floor has been criticized as being highly subjective, the most important finding of this study is the identification of objective differences in the appearance of the pelvic floor, which is reproducible by independent radiologists. Future studies will be necessary to determine if these differences can be correlated with disease phenotypes or responses to therapy, such as in patients who might benefit from therapies aimed at pelvic floor relaxation. For patients with IC/BPS, such predictions would be invaluable and have a positive economic impact. Such determination will require analysis of a large population with IC/BPS with careful correlation of MRI measurements and symptom severity, duration, and pain characteristics.
The current model of IC/BPS pathophysiology suggests that central nervous system dysfunction results in aberrant responses to stimulation of pelvic visceral and somatic neurons manifest as chronic pelvic pain out of proportion to demonstrable pathology. As a result of limbic efferent stimulation, the pelvic musculature undergoes tonic contraction [8]. Pain afferents from the pelvic floor and bladder transmit this noxious stimulus back to the sensitized limbic system, reinforcing efferent contraction of pelvic muscles and perpetuating chronic pain [25]. Given cross-talk between the pelvic floor and bladder and bowel neurons in the spinal cord [26–29], pelvic floor dysfunction may perpetuate voiding dysfunction, defecatory dysfunction, urinary urgency and frequency, and pelvic pain. The increased tension in the pelvic floor musculature seen on MRI may be the radiographic correlate of the altered activation of cortex visceromotor areas and increased connectivity between CNS areas controlling the pelvic musculature and bladder function seen in patients with IC/BPS [30]. Using MRI to examine the pelvic floor may not only further our understanding of IC/BPS pathophysiology but may provide a better methodology for classifying patient disease phenotypes. If larger studies confirm correlation of pelvic floor hypertonicity with responses to specific therapies, such classification may also help guide patient treatment and provide specific physiologic and anatomic targets for future therapies.
Patients with IC/BPS are a heterogeneous population in whom unrelieved chronic pain and urinary complaints are frequently refractory to treatment. The lack of effective interventions may be due to our lack of understanding of the disease pathophysiology and promoting factors. This study identifies pelvic floor hypertonicity on MRI in patients with IC/BPS, supporting the theory that pelvic muscle over-tensioning contributes to and amplifies patient pain. Identification of patients with severe pelvic floor dysfunction may distinguish those likely to respond to treatments aimed at pelvic floor rehabilitation. Future prospective studies will be necessary to determine MRI utility in helping further our understanding of IC/BPS phenotype and pathophysiology and clinical management of this condition.
Abbreviations
- AP:
-
Anteroposterior
- BMI:
-
Body mass index
- CCC:
-
Concordance class correlation
- IC/BPS:
-
Interstitial cystitis/bladder pain syndrome
- MRI:
-
Magnetic resonance imaging
- ROC:
-
Receiver operating characteristic
- SCAT:
-
Subcutaneous adipose tissue
- UPRL:
-
Urethral meatus to the puborectal line
References
Clemens JQ (2010) Afferent neurourology: a novel paradigm. Neurourol Urodyn 29(Suppl 1):S29–S31. doi:10.1002/nau.20792
Barsky AJ, Borus JF (1999) Functional somatic syndromes. Ann Intern Med 130(11):910–921
Butrick CW (2003) Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis, and treatment. Clin Obstet Gynecol 46(4):811–823
Peters KM, Carrico DJ, Diokno AC (2008) Characterization of a clinical cohort of 87 women with interstitial cystitis/painful bladder syndrome. Urology 71(4):634–640. doi:10.1016/j.urology.2007.11.013
Peters K, Girdler B, Carrico D, Ibrahim I, Diokno A (2008) Painful bladder syndrome/interstitial cystitis and vulvodynia: a clinical correlation. Int Urogynecol J Pelvic Floor Dysfunct 19(5):665–669. doi:10.1007/s00192-007-0501-y
Schmidt RA, Vapnek JM (1991) Pelvic floor behavior and interstitial cystitis. Semin Urol 9(2):154–159
Simons DG, Travell JG, Simons LS (1999) Travell & Simons’ myofascial pain and dysfunction: the trigger point manual, 2nd edn. Williams & Wilkins, Baltimore
Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S (2011) Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. Int Urogynecol J 22(4):413–418. doi:10.1007/s00192-010-1301-3
Doggweiler-Wiygul R, Wiygul JP (2002) Interstitial cystitis, pelvic pain, and the relationship to myofascial pain and dysfunction: a report on four patients. World J Urol 20(5):310–314. doi:10.1007/s00345-002-0298-8
FitzGerald MP, Payne CK, Lukacz ES, Yang CC, Peters KM, Chai TC, Nickel JC, Hanno PM, Kreder KJ, Burks DA, Mayer R, Kotarinos R, Fortman C, Allen TM, Fraser L, Mason-Cover M, Furey C, Odabachian L, Sanfield A, Chu J, Huestis K, Tata GE, Dugan N, Sheth H, Bewyer K, Anaeme A, Newton K, Featherstone W, Halle-Podell R, Cen L, Landis JR, Propert KJ, Foster HE Jr, Kusek JW, Nyberg LM (2012) Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol 187(6):2113–2118. doi:10.1016/j.juro.2012.01.123
Weiss JM (2001) Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J Urol 166(6):2226–2231
Gousse AE, Barbaric ZL, Safir MH, Madjar S, Marumoto AK, Raz S (2000) Dynamic half Fourier acquisition, single shot turbo spin-echo magnetic resonance imaging for evaluating the female pelvis. J Urol 164(5):1606–1613
Comiter CV, Vasavada SP, Barbaric ZL, Gousse AE, Raz S (1999) Grading pelvic prolapse and pelvic floor relaxation using dynamic magnetic resonance imaging. Urology 54(3):454–457
Pannu HK (2003) Dynamic MR imaging of female organ prolapse. Radiol Clin N Am 41(2):409–423
Singh K, Reid WM, Berger LA (2002) Magnetic resonance imaging of normal levator ani anatomy and function. Obstet Gynecol 99(3):433–438
Hsu Y, Summers A, Hussain HK, Guire KE, Delancey JO (2006) Levator plate angle in women with pelvic organ prolapse compared to women with normal support using dynamic MR imaging. Am J Obstet Gynecol 194(5):1427–1433. doi:10.1016/j.ajog.2006.01.055
Ansquer Y, Fernandez P, Chapron C, Frey C, Bennis M, Roy C, Salomon L, Mandelbrot L, Carbonne B (2006) Static and dynamic MRI features of the levator ani and correlation with severity of genital prolapse. Acta Obstet Gynecol Scand 85(12):1468–1475. doi:10.1080/00016340600984837
Ludescher B, Rommel M, Willmer T, Fritsche A, Schick F, Machann J (2011) Subcutaneous adipose tissue thickness in adults - correlation with BMI and recommendations for pen needle lengths for subcutaneous self-injection. Clin Endocrinol (Oxf) 75(6):786–790. doi:10.1111/j.1365-2265.2011.04132.x
Metz CE (1978) Basic principles of ROC analysis. Semin Nucl Med 8(4):283–298
Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39(4):561–577
Wessely S, Nimnuan C, Sharpe M (1999) Functional somatic syndromes: one or many? Lancet 354(9182):936–939. doi:10.1016/S0140-6736(98)08320-2
Tunn R, Delancey JO, Howard D, Ashton-Miller JA, Quint LE (2003) Anatomic variations in the levator ani muscle, endopelvic fascia, and urethra in nulliparas evaluated by magnetic resonance imaging. Am J Obstet Gynecol 188(1):116–121
Hsu Y, Chen L, Huebner M, Ashton-Miller JA, DeLancey JO (2006) Quantification of levator ani cross-sectional area differences between women with and those without prolapse. Obstet Gynecol 108(4):879–883. doi:10.1097/01.AOG.0000233153.75175.34
Nickel JC, Shoskes D, Irvine-Bird K (2009) Clinical phenotyping of women with interstitial cystitis/painful bladder syndrome: a key to classification and potentially improved management. J Urol 182(1):155–160. doi:10.1016/j.juro.2009.02.122
Fenton BW (2007) Limbic associated pelvic pain: a hypothesis to explain the diagnostic relationships and features of patients with chronic pelvic pain. Med Hypotheses 69(2):282–286. doi:10.1016/j.mehy.2006.12.025
Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI (2006) Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 18(10):936–948. doi:10.1111/j.1365-2982.2006.00807.x
Kaddumi EG, Hubscher CH (2007) Changes in rat brainstem responsiveness to somatovisceral inputs following acute bladder irritation. Exp Neurol 203(2):349–357. doi:10.1016/j.expneurol.2006.08.011
Rudick CN, Chen MC, Mongiu AK, Klumpp DJ (2007) Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol 293(3):R1191–R1198. doi:10.1152/ajpregu.00411.2007
Pezzone MA, Liang R, Fraser MO (2005) A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 128(7):1953–1964
Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, Farmer MA, Apkarian AV, Mackey S, Martucci KT, Clauw DJ, Harris RE, Deutsch G, Ness TJ, Yang CC, Maravilla K, Mullins C, Mayer EA (2014) Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol 192(3):947–955. doi:10.1016/j.juro.2014.03.093
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ackerman, A.L., Lee, U.J., Jellison, F.C. et al. MRI suggests increased tonicity of the levator ani in women with interstitial cystitis/bladder pain syndrome. Int Urogynecol J 27, 77–83 (2016). https://doi.org/10.1007/s00192-015-2794-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-015-2794-6