Abstract
Purpose of Review
We assessed the differences in the 2020 European Society of Cardiology (ESC) versus 2015 ESC and 2014 American College of Cardiology (ACC) guidelines on the management of non-ST-segment elevation acute coronary syndromes (NSTE-ACS).
Recent Findings
The recent publication of the 2020 ESC has provided a comprehensive series of recommendations on diagnosis and management of patients presenting with NSTE-ACS. However, there are discrepancies between the 2020 ESC versus 2015 ESC and 2014 ACC guidelines, creating uncertainty among clinicians in routine practices. Our investigation provides insights into several domains, including diagnosis, risk stratification, pharmacological treatments, invasive treatment, and special populations.
Summary
Overall, it seems that the 2020 version of the ESC guideline for the management of NSTE-ACS provides the most evidence-based recommendations for clinicians; although due to the lack of validated investigation across some of the proposed recommendations, further longitudinal multicenter studies are warranted to address the current questions.
Graphical abstract
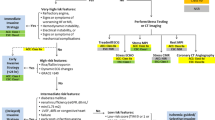
Diagnostic algorithm in NSTE-ACS. Abbreviations: ACC = American College of Cardiology; CABG = coronary artery bypass grafting; CCTA = coronary computed tomography angiography; CMR = cardiac magnetic resonance; CS = cardiogenic shock; ECG = electrocardiography; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; GRACE = Global Registry of Acute Coronary Events; HF = heart failure; LVEF = left ventricular ejection fraction; MPI = myocardial perfusion imaging; MR = mitral regurgitation; NSTE-ACS = non-ST-segment elevation acute coronary syndromes; PCI = percutaneous coronary intervention; TIMI = thrombolysis in myocardial infarction

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an essential barrier to sustainable development in the new millennium, coronary artery disease (CAD) has imposed a great global burden on public health [1]. Despite the abundant developments in the treatment of CAD, it remains among the leading causes of mortality and disease burden, resulting in approximately 9.1 million deaths and 180.0 million disability-adjusted life years globally in 2019 [1,2,3]. Owing to the dynamic nature of CAD, it contributes to a spectrum, which can be categorized as either chronic coronary syndromes (CCS) or acute coronary syndromes (ACS) encompassing non-ST-segment elevation ACS (NSTE-ACS) and ST-segment elevation myocardial infarction (STEMI) [4, 5••].
The recent publication of the 2020 European Society of Cardiology (ESC) has provided comprehensive series of recommendations on diagnosis, risk assessment, and management of patients presenting with NSTE-ACS [5••]. Meanwhile, there exist some discrepancies between the 2020 ESC versus 2015 ESC and 2014 American College of Cardiology (ACC) guidelines [6••, 7••]. From an overall point of view, the recommendations by the ESC and ACC guidelines are nearly consistent, with some differences regarding the classes of recommendations and management approaches. In this viewpoint, the 2014 ACC guideline has more recommendations; however, European guidelines provide a significantly higher level of evidence (Supplementary Table 1). Besides, compared to the previous versions, the 2020 ESC guideline dedicated new concepts, including myocardial infarction with non-obstructive coronary arteries (MINOCA), spontaneous coronary artery dissection (SCAD), and quality indicators (QIs). In this communication, we have dissected the entangled packages of management approaches and compared the proposed recommendations by the aforementioned guidelines.
Diagnosis
Chest pain is the initial presentation of patients with ACS, characterized by a sensation of pain with radiation to both arms, the jaw, and neck, lasting more than 20 min (ESC) at rest or at least 10 min with minimal exertion or rest (ACC). From a diagnostic perspective, troponin elevation serves as an important diagnostic and prognostic tool [5••, 7••]. The 2014 ACC guideline recommends the measurement of cardiac troponin at presentation and within 3–6 h (I-A). While the 2015 ESC guideline takes a more restrictive approach by applying the 0 h/1 h protocol as an alternative to the 0 h/3 h pathway (I-B). In the 2020 edition of the ESC guideline, both the 0 h/1 h and the 0 h/2 h approaches are suggested for patients presenting with low detectable concentrations of cardiac troponin by a class I recommendation (Table 1). Overall, the 0 h/1 h algorithm is introduced as the best approach regarding safety and efficacy by the ESC 2020. However, this guideline takes a more flexible strategy to maximize the feasibility of the process by recommending either one of the 0 h/1 h and the 0 h/2 h approaches. In addition, for those with intermediate troponin concentrations, further observation strategy is recommended by the ESC 2020, which might create a potential uncertainty with regard to the best approach. Across other biomarkers than troponin, the 2015 ESC recommends the creatinine kinase-MB (CK-MB) and copeptin measurement for diagnostic assessments. On the other hand, the 2020 ESC forbids the routine measurement of these biomarkers (III-B), which is nearly consistent with that of the 2014 ACC guideline (III-A).
Further discrepancies are present regarding non-invasive imaging. Across low-risk patients without any abnormal findings in the initial examination, while both ESC guidelines prefer stress imaging over exercise electrocardiogram (ECG) concerning its higher diagnostic and prognostic accuracy (IIa-A/B), the 2014 ACC provides a less specific approach, which includes both sets of the aforementioned strategies (I-A/B). Besides, both the 2014 ACC and 2015 ESC guidelines assign a class II recommendation to coronary computed tomography angiography (CCTA), although the 2020 version of the ESC guideline applies the same strategy but with a higher degree of recommendation (I), suggesting CCTA as an alternative to invasive angiography for low to intermediate-risk patients for CAD (Fig. 1). The upregulated approach by the ESC guideline is driven from two studies, demonstrating that the implementation of upfront CCTA had an NPV of 90.9% and reduced the requirement of invasive coronary angiography in patients with NSTE-ACS [8, 9].
Summary of the diagnostic algorithm of patients with NSTE-ACS. (1) The initial assessment in all three sets of guidelines is derived from clinical evaluation, the 12-lead ECG, and the cardiac troponin concentration. (2) Rapid “rule-in” and “rule-out” algorithms are defined in 2015 ESC and 2020 ESC guidelines. In the 2020 ESC, the ESC 0 h/1 h algorithm is recommended, while the 2015 ESC highly relies on the rapid rule-out protocol at 0 h and 3 h. (3) Intermediate-risk criteria are only elucidated in the 2014 ACC and 2015 ESC guidelines. (4) The mentioned low-risk criteria are based on the 2014 ACC guideline. Both sets of ESC guidelines indicate low-risk patients with the exclusion of other categories. (5) This category is based on the 2015 ESC and 2020 ESC guidelines. The observed group is assigned to patients who do not qualify for “rule-in” and “rule-out” criteria. According to the 2020 ESC, echocardiography and a third measurement of cardiac troponin at 3 h are highly suggested as the next steps in these patients. Abbreviations: ACC = American College of Cardiology; CABG = coronary artery bypass grafting; CCTA = coronary computed tomography angiography; CMR = cardiac magnetic resonance; CS = cardiogenic shock; ECG = electrocardiography; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; GRACE = Global Registry of Acute Coronary Events; HF = heart failure; LVEF = left ventricular ejection fraction; MPI = myocardial perfusion imaging; MR = mitral regurgitation; NSTE-ACS = non-ST-segment elevation acute coronary syndromes; PCI = percutaneous coronary intervention; TIMI = thrombolysis in myocardial infarction
Risk Stratification
Risk assessment plays a pivotal role in providing essential clinical insight for guiding ACS management [5••]. The 2014 ACC guideline states that B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) “may be considered” to evaluate the risk–benefit ratio in patients with suspected ASC. Meanwhile, the 2020 ESC includes an additional recommendation over the previous version of the ESC guideline and enhances the classes of recommendations compared with the 2014 ACC by stating that BNP or NT-pro-BNP “should be considered” to achieve increased prognostic value (Table 1). In the field of prognostic information, the 2020 ESC has downgraded the value of the GRACE risk score (2020: IIa-B, 2015: I-B), which is due to the failure of the AGRIS cluster-randomized trial in illustrating the add-on value with the routine measurement of GRACE risk scores [10]. Drawing from the proposed definition by the Academic Research Consortium for High Bleeding Risk (ARC-HBR), the 2020 ESC introduces and applies the novel definition entitled “High Bleeding Risk (HBR)” to determine the bleeding risk in patients with NSTE-ACS [11]. Moreover, to ascertain the out-of-hospital bleeding risk during dual antiplatelet therapy (DAPT), the 2020 ESC recommends the application of new scores, including the DAPT and the PRECISE-DAPT, representing the integration of ischemic and bleeding risks [12, 13•]. Taken together, the proposed concepts by the 2020 ESC are evolving fields. Hence, further studies should be conducted to delineate the prognostic roles of these risk scores.
Pharmacological Treatments
Antithrombotic treatment is the cornerstone in the management of patients with NSTE-ACS [5••]. The apposed hazards of ischemia and bleeding impose the greatest discrepancy between the ESC and ACC guidelines (Table 2). First, the recommended loading and maintenance dose of non-enteric-coated aspirin in the 2014 ACC guideline is 162–325 mg/day and 81–162 mg/day, respectively, with lower doses favored in all patients (I-A). In contrast, both sets of ESC guidelines encourage the loading and maintenance dose of 150–300 mg and 75–100 mg/day, with the administration of both lower and higher doses in patients undergoing an invasive strategy (I-A). Second, in the domain of applying the P2Y12 receptor inhibitors, the 2014 ACC recommends the administration of ticagrelor or clopidogrel, irrespective of treatment approach (I-B). Nevertheless, both ESC guidelines restrict the administration of clopidogrel to the settings in which ticagrelor or prasugrel are contraindicated, cannot be tolerated, or not available (I-B/C).
Notably, interpretation of new clinical trial findings has enriched the 2020 ESC guideline for providing some additional insights in terms of pre-treatment of patients with NSTE-ACS. With regard to the results of the ISAR-REACT 5 trial, the guideline recommends the administration of prasugrel over ticagrelor in patients proceeding to percutaneous coronary intervention (PCI) (IIa-B) [14•]. In line with the findings of the ACCOAST study, data of the SCAAR registry failed to demonstrate any association between prasugrel pre-treatment and ischemic benefit, but inversely, with notably higher bleeding risk in patients with NSTE-ACS [15, 16•]. Hence, the 2020 ESC restricts the use of routine pre-treatment with P2Y12 in those with unknown coronary anatomy as well as in patients scheduled for invasive strategy (III-A). Of note, the mentioned pre-treatment strategy could be applied for patients who are not scheduled for an invasive approach and do not have HBR (IIb-C). In this perspective, the 2020 ESC has provided a greater nuance with regard to the pre-treatment management in patients with NSTE-ACS; however, it suffers from a notable shortcoming as there still exists a great discrepancy regarding the definition of pre-treatment among clinicians. Besides, for the first time ever, the change in a recommendation regarding “pre-treatment” is based on a non-published observational study from a registry (SCAAR) that was unable to identify any differences between the two studied strategies. The guidelines also refer to an open-label underpowered study with biologically implausible findings to change the recommendations on the selection of antithrombotic agents.
Across post-interventional and maintenance treatment, a risk-based approach is recommended by all three sets of guidelines; however, compared to the 2015 version of the ESC guideline, the latest version enhances the classes of recommendations in the field of long-term secondary prevention (IIa-A versus IIb-A). This upregulation is driven from the DAPT, PEGASUS, and COMPASS trials, representing either rivaroxaban, ticagrelor, or prasugrel could be applied in patients at high risk of ischemic events and without increased HBR [17, 18, 19•]. In addition, with respect to the evidence of the AFIRE study, the ESC 2020 goes a step further and gives a class I recommendation to discontinuation of antiplatelet therapy after 1 year in patients on oral anticoagulation [20]. The third difference in the light of antithrombotic treatment, existing among ACC and ESC guidelines, is the criteria for the administration of glycoprotein (GP) II-b/III-a antagonists. While the 2014 ACC recommends the administration of GP II-b/III-a antagonists in patients not adequately treated with P2Y12 antagonists at the time of PCI (I-A), in patients on DAPT treatment, undergoing cardiac catheterization (IIa–B), or in those treated with unfractionated heparin (UFH) and P2Y12 antagonists (IIa–B), the ESC guidelines advocate the utilization of these drugs for thrombotic complications as well as bail-out situations (IIA-C).
Confronting the accumulated thrombin generation necessitates the suitable implementation of anticoagulation treatment [5••]. The ACC and ESC guidelines recommend the administration of parenteral anticoagulation during PCI (I-C) and at the time of admission (I-B), respectively. The essential discrepancy between guidelines is the selection approaches among diverse anticoagulant treatments. The 2014 ACC limits the administration of bivalirudin to the patients undergoing PCI (I-B) and suggests fondaparinux (I-B), UFH (I-C), or enoxaparin (I-A), irrespective of treatment approach. Enoxaparin at the time of PCI is recommended for patients who have received fewer than two subcutaneous doses, for patients who received the last dose of subcutaneous enoxaparin dose within 8–12 h (I-B), or for those pre-treated with upstream subcutaneous enoxaparin (IIb-B). According to the 2015 ESC guideline, fondaparinux is considered to have the most favorable efficacy, irrespective of treatment approach (I-B). Meanwhile, UFH and enoxaparin are recommended as reasonable alternatives (I-B). On the other hand, the 2020 ESC states that UFH is the first choice for PCI (I-A) and advocates the fondaparinux administration only in medical limitations for transferring the patient to PCI (I-B). Consistent with the recommendation of the ACC guideline, enoxaparin is suggested for PCI in patients pre-treated with subcutaneous enoxaparin by both ESC guidelines (IIa-B). Besides, based on ESC guidelines, an additional dose of UFH should be administrated before PCI performance in patients who were commenced on fondaparinux (I-B). Across bivalirudin administration, the 2020 ESC lowers the classes of recommendations (IIb-A). This approach is thought to be the result of the findings of the MATRIX and VALIDATE-SWEDEHEART studies, indicating that bivalirudin was linked with a remarkable increase in the risk of stent thrombosis and a notable decrease in bleeding risk [21, 22]. It is noteworthy to mention that all three sets of guidelines recommend discontinuation of anticoagulant treatment immediately after PCI, except in particular clinical conditions.
In special clinical conditions such as the presence of atrial fibrillation (AF) or left ventricle (LV) aneurysm with thrombus formation, triple antithrombotic therapy (TAT) (commonly the combination of anticoagulation therapy with DAPT) should be initiated after PCI performance. The 2014 ACC states a lack of evidence on the risks of ticagrelor and prasugrel in TAT regarding the higher bleeding complications than clopidogrel. Both ESC guidelines restrict the use of ticagrelor and prasugrel in TAT (I-A). Across the duration of TAT, the 2015 ESC recommends TAT for 1 month in patients at high bleeding risk and 6 months for patients at a low to intermediate risk of bleeding (IIA-C). Besides, both treatments with non-vitamin K antagonist oral anticoagulants (NOAC) or vitamin K antagonists (VKA) were confirmed equally in combination with antiplatelet agents (I-C). The 2020 ESC guideline determines a shorter duration of TAT, with 1 month of medication in patients at high risk of stent thrombosis (IIA-C) and 1 week of treatment for others (I-A). Also, administration of NOAC is preferred to VKA. Strikingly, dual therapy with OAC and ticagrelor or prasugrel is recommended as an alternative to TAT (IIB-C), although no evidence is cited in support of this concept, indicating a necessity to conducting further investigations.
Invasive Treatment
Invasive coronary angiography plays a critical role in elucidating the cause of chest pain in patients with NSTE-ACS [5••]. The 2014 ACC suggests the performance of the ischemia-guided strategy, delayed invasive strategy, or stress testing for initially stabilized individuals by a class II recommendation, providing additional leeway for clinicians to guide NSTE-ACS management. Despite the discrepancy that exists between the two versions of ESC guidelines with respect to risk criteria, both sets underscore the selective invasive strategy for initially stabilized patients by a class I recommendation, representing a more restricted approach compared with the 2014 ACC guideline. In light of the risk criteria, both the 2014 ACC and 2015 ESC guidelines recommend an immediate early strategy (< 2 h) for very high-risk patients, an early invasive strategy (< 24 h) for high-risk individuals, and a delayed strategy (< 72 h) for intermediate-risk patients. Meanwhile, with eliminating the intermediate-risk group and integrating this group with the low-risk group, the 2020 ESC guideline appears to advocate an all or nothing strategy (Supplementary Table 2 and Fig. 1). As another key change, high-risk patients could be defined as anyone with an established non-ST-segment elevation myocardial infarction (NSTEMI). However, the class 1A recommendation that all patients with NSTEMI should undergo invasive coronary angiography < 24 h is not well supported by evidence, and it might have huge practical clinical implications.
Notably, the 2020 ESC guideline expands the time window to perform coronary angiography in patients resuscitated after an out-of-hospital cardiac arrest with a hemodynamically stable setting and without ST-segment elevation (IIa-C). This recommendation is originated from the insights of the COACT trial, which enrolled 552 patients and revealed no disparity in 90-day survival between delayed and immediate coronary angiography strategies [23]. In contrast, the 2014 ACC guideline takes a more restrictive approach, underscoring a necessity to perform early coronary angiography in these subsets of patients.
In terms of desired revascularization strategy, the 2014 ACC guideline states that multivessel PCI “may be reasonable” as part of the revascularization strategy. Nevertheless, the 2020 ESC guideline enhances the classes of recommendations by stating that complete revascularization “should be considered” in those with multivessel coronary artery disease and without cardiogenic shock (CS). This insight is driven from a British study, demonstrating remarkably lower cumulative mortality rates with a multivessel approach compared with culprit-lesion-only PCI [24]. Citing evidence from the SMILE trial, the 2020 version of the ESC guideline also suggests the implementation of complete revascularization during index PCI in patients with multivessel disease (IIb-B) [25]. Furthermore, the 2015 ESC guideline questioned the value of fractional flow reserve (FFR)-guided PCI; however, based on the results of the FAMOUS-NSTEMI trial, the latest version of ESC guideline recommends the FFR-guided revascularization of a non-culprit lesion during index PCI (IIb-B) [26].
Special Populations
For the sake of NSTE-ACS management in special populations, all three sets of guidelines highlight the need to tailor management strategies to each individual’s situation. First, the 2015 ESC advocates the consideration of invasive strategy or revascularization in the elderly after appraising the potential risk and benefits (IIa-A). Meanwhile, the 2014 ACC recommends the use of guideline-directed medical therapy as well as early invasive strategy and revascularization with a higher class of recommendation in older patients (> 75 years of age) (I-A). Besides, the 2014 ACC prefers coronary artery bypass grafting (CABG) over PCI in older patients, especially in those with complex 3-vessel CAD (e.g., SYNTAX score > 22), or diabetes mellitus (DM), with or without the involvement of the proximal left anterior descending artery (IIa-B). In this regard, the latest version of the ESC guideline recommends the same diagnostic and invasive strategy in elderly patients as for younger ones (I-B). Second, across patients with DM, all sets of guidelines declare the same invasive and antithrombotic strategy in patients with DM as for patients without DM (ESC: I-C, ACC: I-A), with recommending a moderate blood glucose target of less than 180 mg/dL to reduce hyperglycemia along with preventing hypoglycemia. In line with the 2014 ACC statements, the 2015 ESC recommends the CABG over PCI in patients with DM, who have stabilized multivessel CAD with acceptable surgical risk (I-A). Besides, the invasive strategy is recommended over the non-invasive approach (I-A) in the 2015 ESC, although the ESC 2020 takes a more conservative strategy, suggesting a multifactorial approach in the management of patients with NSTE-ACS and DM (IIa-B).
Third, ascertaining glomerular filtration rate in patients with chronic kidney disease (CKD), the ESC and ACC guidelines recommend monitoring kidney function by measuring estimated glomerular filtration rate and creatinine clearance levels, respectively. Across invasive management, while the 2015 ESC recommends coronary angiography or revascularization after the assessment of the risk–benefit ratio (I-B), the 2014 ACC provides a higher specified recommendation, suggesting invasive management in patients with mild (stage 2) and moderate (stage 3) CKD (IIa-B). Both ESC guidelines recommend applying the same diagnostic and therapeutic strategy in patients with CKD as for patients with normal kidney function with a class I recommendation. In addition, CABG is preferred over PCI in patients with multivessel CAD, who have an acceptable surgical risk profile and a life expectance of higher than 1 year (IIa-B).
Fourth, an essential casualty of NSTE-ACS is CS in addition to heart failure. With respect to the management of CS in patients with NSTE-ACS, all three sets of guidelines recommend the emergent use of revascularization strategy. Noticeably, analysis of recent clinical trials empowered the 2020 ESC to specify the invasive management in patients with CS and NSTE-ACS. In this viewpoint, the 2020 ESC recommends applying emergency PCI of culprit lesions in patients with CS due to NSTE-ACS and with amenable coronary anatomy (I-B). Besides, routine immediate revascularization of non-culprit lesions is inhibited in the aforementioned patients. This recommendation is driven by the CULPRIT-SHOCK trial, indicating that the risk of all-cause mortality in the culprit-lesion-only PCI strategy was remarkably lower than immediate multivessel PCI at 30-day follow-up. Last but not the least, across patients with anemia and no evidence of active bleeding, the 2020 ESC suggests the blood transfusion strategy in patients with hemoglobin level < 8 g/dL, and the 2015 ESC applies a different cut-off value with the hemoglobin level of less than 7 g/dL (IIb-C). Consistent with the statement of the 2020 ESC, the 2014 ACC dispraises blood transfusion in hemodynamically stable patients with hemoglobin level > 8 g/dL (III-B).
New Concepts
The terms SCAD and MINOCA have been newly introduced by the 2020 ESC guideline. SCAD is determined as separation of the coronary arterial tunics, which is not due to traumatic, iatrogenic, or atherosclerotic conditions. From a diagnostic point of view, implementation of intracoronary imaging is recommended in addition to CCTA. The guideline recommends the conservative approach except for very high-risk profile patients, although the suitable strategy remains a matter of discussion since the treatment strategy has not been adequately validated by clinical trials. Besides, the guideline illustrates a diagnostic strategy for the term so-called MINOCA. This approach states that advanced imaging strategies such as cardiac MRI and coronary vascular imaging play an essential role in distinguishing this group from other conditions such as myocarditis and Takotsubo cardiomyopathy. The 2020 ESC guideline also provides a robust metric, which could be applied by health care providers for appraising the quality of care. Besides, a summary of gaps in the evidence for NSTE-ACS syndrome is included in the latest version of the ESC guideline, which could be applied by ongoing investigations to achieve aspirational targets in the management of patients with NSTE-ACS.
Conclusion
The 2020 version of the ESC guideline for the management of acute coronary syndromes in patients presenting without ST-segment elevation provides the most evidence-based recommendations for clinicians on how to accurately diagnose and manage these groups of patients. The latest version of ESC guideline has merged the previous investigations to make a better congruency in the management approach of NSTE-ACS patients, resulting in the creation of statistically and clinically oriented algorithms; however, due to the lack of validated investigation, it might be difficult to achieve at the best decision across some of the proposed recommendations. Besides, the existing disparities between the ESC and ACC guidelines could create uncertainty among physicians regarding the diagnosis and management of NSTE-ACS. Hence, further longitudinal multicenter studies, randomized controlled trials, and meta-analysis are warranted to respond to the current questions.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1736–88. doi:https://doi.org/10.1016/s0140-6736(18)32203-7.
GBD Compare. Institute for Health Metrics and Evaluation. https://vizhub.healthdata.org/gbd-compare/. Accessed February 6 2021.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77. https://doi.org/10.1093/eurheartj/ehz425.
•• Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European heart journal. 2020. https://doi.org/10.1093/eurheartj/ehaa575. The 2020 ESC guideline for the management of NSTE-ACS provides the most evidence-based recommendations for clinicians on how to accurately diagnose and manage these groups of patients.
•• Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). European heart journal. 2016;37(3):267-315. https://doi.org/10.1093/eurheartj/ehv320. Comparing the results of this study (2015 ESC) with the 2020 ESC guideline for the management of patients with NSTE-ACS could provide critical insights about the latest updates in the management of NSTE-ACS.
•• Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr., Ganiats TG, Holmes DR, Jr. et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;64(24):e139-e228. https://doi.org/10.1016/j.jacc.2014.09.017. Comparing the findings of this study (2014 ACC) with the 2020 ESC guideline for the management of patients with NSTE-ACS could pave the way for better understanding the latest insights for these group of patients.
Smulders MW, Kietselaer B, Wildberger JE, Dagnelie PC, Brunner-La Rocca HP, Mingels AMA, et al. Initial imaging-guided strategy versus routine care in patients with non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2019;74(20):2466–77. https://doi.org/10.1016/j.jacc.2019.09.027.
Linde JJ, Kelbæk H, Hansen TF, Sigvardsen PE, Torp-Pedersen C, Bech J, et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2020;75(5):453–63. https://doi.org/10.1016/j.jacc.2019.12.012.
Chew DP, Astley CM, Luker H, Alprandi-Costa B, Hillis G, Chow CK, et al. A cluster randomized trial of objective risk assessment versus standard care for acute coronary syndromes: rationale and design of the Australian GRACE Risk score Intervention Study (AGRIS). Am Heart J. 2015;170(5):995-1004.e1. https://doi.org/10.1016/j.ahj.2015.07.032.
Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40(31):2632–53. https://doi.org/10.1093/eurheartj/ehz372.
Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315(16):1735–49. https://doi.org/10.1001/jama.2016.3775.
• Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet (London, England). 2017;389(10073):1025-34. doi:https://doi.org/10.1016/s0140-6736(17)30397-5. This study revealed that the PRECISE-DAPT score could provide a robust tool for the prediction of out-of-hospital bleeding during DAPT in patients with NSTE-ACS.
•Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. The New England journal of medicine. 2019;381(16):1524–34. https://doi.org/10.1056/NEJMoa1908973. Findings of this study indicated that the administration of prasugrel is preferred over ticagrelor in the pre-treatment of patients with NSTE-ACS.
Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay JF, et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med. 2013;369(11):999–1010. https://doi.org/10.1056/NEJMoa1308075.
• Dworeck C, Redfors B, Angerås O, Haraldsson I, Odenstedt J, Ioanes D et al. Association of pretreatment with P2Y12 receptor antagonists preceding percutaneous coronary intervention in non-ST-segment elevation acute coronary syndromes with outcomes. JAMA network open. 2020;3(10):e2018735. https://doi.org/10.1001/jamanetworkopen.2020.18735. The ACCOAST study demonstrated no association between prasugrel pre-treatment and ischemic benefit, but inversely, with notably higher bleeding risk in patients with NSTE-ACS.
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–66. https://doi.org/10.1056/NEJMoa1409312.
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–800. https://doi.org/10.1056/NEJMoa1500857.
• Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. The New England journal of medicine. 2017;377(14):1319-30. https://doi.org/10.1056/NEJMoa1709118. This study represented either rivaroxaban, ticagrelor, or prasugrel could be applied for post-interventional and maintenance treatment in NSTE-ACS patients at high risk of ischemic events and without increased HBR.
Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381(12):1103–13. https://doi.org/10.1056/NEJMoa1904143.
Han Y, Guo J, Zheng Y, Zang H, Su X, Wang Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA. 2015;313(13):1336–46. https://doi.org/10.1001/jama.2015.2323.
Nührenberg TG, Hochholzer W, Mashayekhi K, Ferenc M, Neumann FJ. Efficacy and safety of bivalirudin for percutaneous coronary intervention in acute coronary syndromes: a meta-analysis of randomized-controlled trials. Clin Res Cardiol. 2018;107(9):807–15. https://doi.org/10.1007/s00392-018-1251-1.
Bonello L, Laine M, Puymirat E, Lemesle G, Thuny F, Paganelli F, et al. Timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes and clinical outcomes: an updated meta-analysis. JACC Cardiovasc Interv. 2016;9(22):2267–76. https://doi.org/10.1016/j.jcin.2016.09.017.
Rathod KS, Koganti S, Jain AK, Astroulakis Z, Lim P, Rakhit R, et al. Complete versus culprit-only lesion intervention in patients with acute coronary syndromes. J Am Coll Cardiol. 2018;72(17):1989–99. https://doi.org/10.1016/j.jacc.2018.07.089.
Sardella G, Lucisano L, Garbo R, Pennacchi M, Cavallo E, Stio RE, et al. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients: the SMILE trial. J Am Coll Cardiol. 2016;67(3):264–72. https://doi.org/10.1016/j.jacc.2015.10.082.
Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J. 2015;36(2):100–11. https://doi.org/10.1093/eurheartj/ehu338.
Author information
Authors and Affiliations
Contributions
MM: original draft preparation, conceptualization, methodology, investigation. HA: original draft preparation, conceptualization, methodology, investigation. SR: original draft preparation, conceptualization, methodology, investigation. HF: original draft preparation, conceptualization, methodology, investigation. BH: review and editing, investigation, data curation. SZ: review and editing, investigation, data curation. SB: review and editing, investigation, data curation. RF: review and editing, investigation, data curation. SA: review and editing, investigation, data curation. AA: review and editing, investigation, data curation. SKS*: review and editing, conceptualization, methodology, supervision. SJ*: review and editing, conceptualization, methodology, supervision. All authors read and approved the final manuscript. *Corresponding authors.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretation
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keykhaei, M., Ashraf, H., Rashedi, S. et al. Differences in the 2020 ESC Versus 2015 ESC and 2014 ACC/AHA Guidelines on the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation. Curr Atheroscler Rep 23, 77 (2021). https://doi.org/10.1007/s11883-021-00976-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-021-00976-7