Abstract
The new guideline on acute coronary syndrome (ACS) of the European Society of Cardiology (ESC) replaces two separate guidelines on ST-elevation myocardial infarction (STEMI) and non-ST-elevation (NSTE) ACS. This change of paradigm reflects the experts view that the ACS is a continuum, starting with unstable angina and ending in cardiogenic shock or cardiac arrest due to severe myocardial ischemia. Secondary, partly non-atherosclerotic-caused myocardial infarctions (“type 2”) are not integrated in this concept.
With respect to acute care in the setting of emergency medicine and the chest pain unit structures, the following new aspects have to be taken into account:
1. New procedural approach as “think A.C.S.” meaning “abnormal ECG,” “clinical context,” and “stable patient”
2. New recommendation regarding a holistic approach for frail patients
3. Revised recommendations regarding imaging and timing of invasive strategy in suspected NSTE-ACS
4. Revised recommendations for antiplatelet and anticoagulant therapy in STEMI
5. Revised recommendations for cardiac arrest and out-of-hospital cardiac arrest
6. Revised recommendations for in-hospital management (starting in the CPU/ED) and ACS comorbid conditions
In summary, the changes are mostly gradual and are not based on extensive new evidence, but more on focused and healthcare process-related considerations.
Zusammenfassung
Die neue Leitlinie zum akuten Koronarsyndrom (ACS) der Europäischen Gesellschaft für Kardiologie (ESC) ersetzt 2 separate Leitlinien zum ST-Hebungs-Infarkt („ST-elevation myocardial infarction“, STEMI) und zum ACS ohne ST-Hebungen („non-ST-elevation“, NSTE-ACS). Dieser Paradigmenwechsel spiegelt die Expertenperspektive wider, dass das ACS ein Kontinuum darstellt, das mit einer instabilen Angina pectoris beginnt und mit einem kardiogenen Schock oder Herzstillstand aufgrund einer schweren Myokardischämie endet. Sekundäre, teilweise nichtatherosklerotisch bedingte Myokardinfarkte („Typ 2“) sind in dieses Konzept nicht integriert. Im Hinblick auf die Akutversorgung im Rahmen der Notfallmedizin und der Chest-Pain-Unit-Strukturen (CPU) sind folgende neue Aspekte zu berücksichtigen:
1. Neuer prozessualer Ansatz als „think A.C.S.“, d. h. „abnormales EKG“, „klinischer Kontext“ und „stabiler Patient“
2. Neue Empfehlung für einen ganzheitlichen Ansatz bei gebrechlichen Patienten
3. Überarbeitete Empfehlungen zur Bildgebung und zum Zeitpunkt der invasiven Strategie bei Verdacht auf NSTE-ACS
4. Überarbeitete Empfehlungen zur Thrombozytenaggregationshemmer- und Antikoagulanzientherapie bei STEMI
5. Revidierte Empfehlungen für Herzstillstand und außerklinischen Herzstillstand
6. Überarbeitete Empfehlungen für das Management im Krankenhaus (beginnend in der Notaufnahme/CPU) und für ACS-Komorbiditäten
Zusammenfassend lässt sich sagen, dass die Änderungen größtenteils schrittweise erfolgen und nicht auf massiven neuen Erkenntnissen beruhen, sondern eher auf gezielten und prozessbezogenen Überlegungen zum Versorgungsprozess.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The implementation of the concept of acute coronary syndrome (ACS) as a continuum into a guideline, which consequently replaces separate entries for ST-segment elevation myocardial infraction (STEMI) and non-ST-segment elevation (STE)-ACS, is the central new aspect of the current ACS guideline [1]. The concept is driven by the pathophysiology of coronary atherosclerosis, which enters “instability” at a certain point and then progresses to atherosclerotic plaque rupture, coronary occlusion, acute heart failure, and shock or death. Previous guidelines were driven more by practical aspects such as diagnostic pathways and therapeutic concepts. Corresponding evidence is still different for STEMI, NSTEMI, and type 2 myocardial infarction. Nevertheless, the continuity hypothesis has been outlined for decades and is clearly scientifically based and all steps of coronary atherosclerosis are reflected by different biomarkers [2, 3] and pathophysiological concepts. The hypothesis allows us also to locate a specific patient on their atherosclerotic journey and prescribe adequate and effective drugs and measures to prevent progression and complications. The current guideline changes some recommendations without a new large body of evidence. Therefore, there are some scientific debates related to certain aspects such as the primary diagnostic approach to chest pain, which has continued since the previous NSTE-ACS guideline [4, 5] and is of relevance for the practical work in the chest pain unit (CPU) and the emergency department (ED).
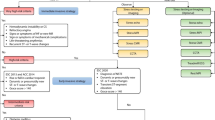
Figure 1 shows the standard care for patients with acute chest pain including new aspects of the current guideline.
Essentials of the ESC guideline an acute coronary syndrome (ACS) in the setting of the emergency department (ED)/chest pain unit (CPU). EMS emergency medical services, ICA invasive coronary angiography: 1 STEMI and very high-risk NSTE-ACS Immediate ICA; 2 high-risk ACS, assessment of frailty and comorbidities, early ICA as standard procedure; 3 low-risk patients after ECG/biomarkers, treatment and workup in regional networks
Chest pain unit and emergency department relevant news
New procedural approach of “think A.C.S.”
The guidelines start with a central illustration [1, Fig. 1], outlining the spectrum concept of ACS and introducing “think A.C.S.” It is recommended that if an ACS is suspected to think of the initialism A.C.S. for the initial assessment. This includes an electrocardiogram (ECG) to check primarily for evidence of ischemia or other abnormalities such as arrhythmias; taking a targeted clinical history to assess the clinical “context” of the presentation; and performing a focused clinical examination to judge the clinical and hemodynamic “stability.” Based on this initial assessment, the first decision is made regarding an immediate or delayed invasive management. Previous concepts for STEMI and very high risk NSTE-ACS remain unchanged. The “think A.C.S.” concept underlines the necessity of a structured and standardized line of action, which also affects the interprofessional CPU medical team.
New recommendation regarding a holistic approach for frail patients
The guidelines newly state, with a class of recommendation I and level of evidence B, that “for frail older patients with comorbidities, a holistic approach is recommended to individualize interventional and pharmacological treatments after careful evaluation of the risks and benefits.” This means that the assessment of frailty is a “must do” within the primary assessment of patients. Although the guidelines nicely lay out why frailty influences outcome and therefore needs to be considered in decision-making for treatment and care, it is also stated that “there is a lack of consensus on which frailty assessment tool is optimal in older patients with CV disease” [1]. In a review article, Chung et al. compare different scores and also report on studies using these scores in the ACS setting [6]. One of the most widely used and also practical scores in the acute situation is the Clinical Frailty Score (CFS; [7]), which has been successfully applied in ACS studies [8, 9]. Nevertheless, frailty assessment remains challenging in the emergency setting since many of the variables are influenced by the acute event itself and patients may look more frail than they really are.
The CFS is based on a large cohort of the Canadian Study of Health and Aging and has seven grades from “very fit” to “severely frail,” meaning “completely dependent on others for the activities of daily living, or terminally ill.”
Revised recommendations on imaging and timing of invasive strategy in suspected NSTE-ACS
Recommendations for imaging for patients with suspected ACS, non-elevated (or uncertain) high-sensitivity cardiac troponin (hs-cTn), no ECG changes, and no recurrence of pain have been downgraded to IIa, level of evidence A. A coronary CT angiography (CCTA) or a non-invasive stress imaging test should be considered as part of the initial workup.
Current studies have been considered [10] and overenthusiasm, as found in the previous guidelines, has been corrected. Availability, expert interpretation, and clear indications are needed to use CCTA for the benefit of patients in the future. Again, the diagnostic approach to ACS patients needs to be evidence based, standardized, and personalized but not individual according to the attending physician.
The recommendations regarding the timing of an invasive strategy were slightly changed with the same level of evidence, but a lower class of recommendation (2023: IIa, 2020: I). The new recommendation is similar to the earlier one:
“An early invasive strategy within 24 h should be considered in patients with at least one of the following high-risk criteria: Confirmed diagnosis of NSTEMI based on current recommended ESC hs-cTn algorithms; dynamic ST-segment or T wave changes; transient ST-segment elevation; GRACE risk score > 140.”
The focus on troponin only is new, although it is in line with the previous guideline, where “the diagnostic algorithm recommended in section 3” was noted and in “section 3” troponin-based algorithms were already recommended for most, but not all, situations [4]. With respect to the clinical practice in the CPU, the current recommendations indicate the use of hs-cTn and to follow the fourth universal definition of MI [11]. The currently recommend “ESC hs-Tn algorithms” mean that it is recommended to measure cardiac troponins immediately after presentation and to obtain the results within 60 min of blood sampling. It is recommended to use serial hs-cTn measurements (0 h/1 h or 0 h/2 h) to rule in and rule out NSTEMI. Additional testing after 3 h is recommended if the first two hs-cTn measurements of the 0 h/1 h algorithm are inconclusive and no alternative diagnoses explaining the condition have been made. All these recommendations are listed as IB. Point-of-care tests are not recommended as long as no high-sensitivity tests are available. Other markers, such as copeptin, are only recommended in combination with conventional troponin assays, although clear evidence exist for instant rule-out strategies of this dual-marker strategy also in combination with hs-Tn. Single hs-Tn use is generally no longer recommend. In fact, the clinical value of troponin-only concepts strongly depend on the specific population investigated [12,13,14,15,16,17,18]. In “pure” ACS cohorts, other biomarkers such as copeptin maybe of limited additional benefit. In older patients [19] with a high number having nonspecific complaints [13] and relatively high baseline troponin levels [15], additional markers may speed up the processes and avoid unnecessary admissions and invasive procedures. Given the fact of the aging population and the specific endorsement of a frailty assessment in the actual guidelines, more individualized diagnostic strategies will be needed [20,21,22]. Coming back to the primary diagnostic strategy in the CPU/ED in light of the current guidelines, after evaluating the ECG and sending a STEMI patient directly to the catheterization laboratory, hs-Tn must be measured. In most patients, a second troponin will be obtained after 1 or 2 h. A standardized assessment of all patients is needed as outlined earlier. Within a standardized approach, in clearly defined subgroups, additional biomarkers with high evidence such as copeptin can be used for fast rule-out.
Revised recommendations for antiplatelet and anticoagulant therapy in STEMI
The current guidelines clearly change the recommendation for pretreatment of STEMI patients, which was level IA and is now only IIbB: “Pre-treatment with a P2Y12 receptor inhibitor may be considered in patients undergoing a primary PCI strategy.” The change was made due to a revised appraisal of the current evidence, which does not clearly support pre-treatment in randomized trials [19], but more so in older observational data [23], which are nevertheless convincing related to the pathomechanism. In addition, in the randomized ATLANTIC study, an interaction with the co-medication of morphine may have limited the pre-treatment effect [24]. Nevertheless, in STEMI patients, a routine pre-treatment with a P2Y12 receptor inhibitor is no longer recommended. In the CPU/ED, an invasive cardiologist could set the indication for pre-treatment based on individual patient considerations.
Revised recommendations for cardiac arrest and out-of-hospital cardiac arrest
There are several new recommendations related to cardiac arrest in the new guideline. Two aspects are highly relevant for the CPU/ED:
-
1.
“Routine immediate angiography after resuscitated cardiac arrest is not recommended in hemodynamically stable patients without persistent ST-segment elevation (or equivalents)” (IIIA)
This new recommendation is based on two randomized studies including long-term follow-up data, which do not show benefit of early invasive coronary angiography (ICA) in these patients [25,26,27,28]. “Routine” in this recommendation means that on an individual basis, experts in the field may decide differently, but since in post-resuscitation situations standards are helpful, these must no longer include immediate ICA.
-
2.
Regarding temperature control, the new recommendation is that “continuous monitoring of core temperature and active prevention of fever (i.e., > 37.7 °C) is recommended after either out-of-hospital or in-hospital cardiac arrest for adults who remain unresponsive after return of spontaneous circulation.” This means that for the setting of the CPU/ED, cooling rarely needs to be started, which falls in the field of advanced post-resuscitation care on the intensive care unit (ICU). The new recommendation is based on recently published data, which show that active prevention of fever is the central goal of temperature control [29].
Revised recommendations for in-hospital management (starting in the CPU/ED) and ACS comorbid conditions
Several new recommendations of the new guideline do not apply at the very beginning of the hospital care in the CPU/ED. Nevertheless, some of the revised recommendations relate to decisions that have to be made in CPU/ED. Beyond the screening and assessment for frailty (see above), these points mainly relate to patients with cancer.
-
1.
An invasive strategy is recommended for cancer patients presenting with high-risk ACS with an expected survival of ≥ 6 months. This is important, because ACS is a time- critical disease and a general therapeutic nihilism in patients with cancer has to be actively avoided as there is evidence that they profit from standard ACS therapies [30].
-
2.
A temporary interruption of cancer therapy is recommended for patients in whom the cancer therapy is suspected to be a contributing cause of ACS. This is an important aspect and should be assessed in the CPU/ED as it could be out of sight during the subsequent hospital stay. Active cancer therapies should be assessed and documented in the CPU/ED for all ACS patients.
-
3.
A conservative non-invasive strategy should be considered for ACS patients with poor cancer prognosis (i.e., with expected survival < 6 months) and/or very high bleeding risk. This is important for the CPU/ED as here the first switch is set in the ensuing therapeutic pathway. Nevertheless, point 1 of this list has to be kept in mind and when in doubt, time-critical measures should not be withheld.
-
4.
Aspirin is not recommended for cancer patients with a platelet count of < 10,000/μL; clopidogrel is not recommended for cancer patients with a platelet count < 30,000/μL; and in ACS patients with cancer and < 50,000/μL platelet count, prasugrel or ticagrelor are not recommended.
These recommendations should be known by all members of the CPU/ED medical team.
Special entities and the ACS continuum concept
As outlined earlier, the new guideline combines previous ones on STEMI and NST-ACS looking at the ACS as a continuous spectrum. This is true for coronary artery disease with its several stages of development and the beginning of acuity at a certain point in time. Nevertheless, there are several conditions that need to be distinguished. It is important to recognize that ACS is not the same as myocardial infarction (MI). Acute MI is defined according to the universal definition of MI as cardiomyocyte necrosis in the clinical setting of acute myocardial ischemia. This includes MI due to atherothrombotic events (spontaneous, type 1 MI) and also other potential causes of myocardial ischemia and myocyte necrosis (types 2–5 MI; [11]). The current guideline is largely focused on the management of patients with spontaneous (type 1) MI or unstable angina. This is consequently a step back to the roots, leaving special situations to further scientific recommendations, as the very broad universal MI definition has caused irritation in the clinically practicing community. This also applies for the use of biomarkers strategies, i.e., cardiac-troponin only algorithms, which are recommended in the current guideline for ACS, but not generally for the whole spectrum of acute cardiac events.
Conclusion
The new acute coronary syndrome (ACS) guideline follows a changed paradigm of ACS as a continuum. This concept is based on pathophysiological considerations and enables early personalized and targeted diagnostics and therapies. With respect to the chest pain unit/emergency department, several new aspects as outlined in detail in this article should be implemented in the current standard of care.
References
Byrne RA, Rossello X, Coughlan JJ et al (2023) 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 44(38):3720–3826
Apple FS, Wu AH, Mair J et al (2005) Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem 51(5):810–824
Searle J, Danne O, Müller C, Mockel M (2011) Biomarkers in acute coronary syndrome and percutaneous coronary intervention. Minerva Cardioangiol 59(3):203–223
Collet JP, Thiele H, Barbato E et al (2021) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 42(14):1289–1367
Giannitsis E, Blankenberg S, Christenson RH et al (2021) Critical appraisal of the 2020 ESC guideline recommendations on diagnosis and risk assessment in patients with suspected non-ST-segment elevation acute coronary syndrome. Clin Res Cardiol 110(9):1353–1368
Chung K, Wilkinson C, Veerasamy M, Kunadian V (2021) Frailty scores and their utility in older patients with cardiovascular disease. Interv Cardiol 16:e5
Rockwood K, Song X, MacKnight C et al (2005) A global clinical measure of fitness and frailty in elderly people. Cmaj 173(5):489–495
Ekerstad N, Pettersson S, Alexander K et al (2018) Frailty as an instrument for evaluation of elderly patients with non-ST-segment elevation myocardial infarction: a follow-up after more than 5 years. Eur J Prev Cardiol 25(17):1813–1821
Yoshioka N, Takagi K, Morita Y et al (2019) Impact of the clinical frailty scale on mid-term mortality in patients with ST-elevated myocardial infarction. Int J Cardiol Heart Vasc 22:192–198
Lee KK, Bularga A, O’Brien R et al (2021) Troponin-guided coronary computed tomographic angiography after exclusion of myocardial infarction. J Am Coll Cardiol 78(14):1407–1417
Thygesen K, Alpert JS, Jaffe AS et al (2019) Fourth universal definition of myocardial infarction (2018). Eur Heart J 40(3):237–269
Giannitsis E, Garfias-Veitl T, Slagman A et al (2022) Biomarkers-in-cardiology 8 RE-VISITED-consistent safety of early discharge with a dual marker strategy combining a normal hs-cTnT with a normal copeptin in low-to-intermediate risk patients with suspected acute coronary syndrome—a secondary analysis of the randomized biomarkers-in-cardiology 8 trial. Cells 11(2)
Lindner T, Slagman A, Senkin A et al (2015) Medical history of elderly patients in the emergency setting: not an easy point-of-care diagnostic marker. Emerg Med Int 2015:490947
Möckel M, Searle J, Hamm C et al (2015) Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. Eur Heart J 36(6):369–376
Riedlinger D, Möckel M, Müller C et al (2018) High-sensitivity cardiac troponin T for diagnosis of NSTEMI in the elderly emergency department patient: a clinical cohort study. Biomarkers 23(6):551–557
Searle J, Slagman A, Stockburger M et al (2015) Use of copeptin in emergency patients with cardiac chief complaints. Eur Heart J Acute Cardiovasc Care 4(5):393–402
Slagman A, Searle J, Müller C, Möckel M (2015) Temporal release pattern of copeptin and troponin T in patients with suspected acute coronary syndrome and spontaneous acute myocardial infarction. Clin Chem 61(10):1273–1282
Vafaie M, Slagman A, Möckel M et al (2016) Prognostic value of undetectable hs Troponin T in suspected acute coronary syndrome. Am J Med 129(3):274–282.e2
Montalescot G, van ’t Hof AW, Lapostolle F et al (2014) Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med 371(11):1016–1027
Möckel M (2023) Perspectives on cardiovascular biomarkers: one fits all biomarkers are out, personalization is in. Biomarkers 28(4):353
Möckel M (2020) One fits all hs troponin or more personalized dual markers strategies in the primary diagnostic assessment of patients with suspected acute coronary syndrome? Biomarkers 25(8):611–612
Möckel M (2022) Actual guidelines on non-ST-elevation acute coronary syndrome: how do they help in the emergency department? Eur J Emerg Med 29(1):2–4
Dörler J, Edlinger M, Alber HF et al (2011) Clopidogrel pre-treatment is associated with reduced in-hospital mortality in primary percutaneous coronary intervention for acute ST-elevation myocardial infarction. Eur Heart J 32(23):2954–2961
Lapostolle F, Van’t Hof AW, Hamm CW et al (2019) Morphine and ticagrelor interaction in primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: ATLANTIC-morphine. Am J Cardiovasc Drugs 19(2):173–183
Desch S, Freund A, Akin I et al (2021) Angiography after out-of-hospital cardiac arrest without ST-segment elevation. N Engl J Med 385(27):2544–2553
Desch S, Freund A, Akin I et al (2023) Coronary angiography after out-of-hospital cardiac arrest without ST-segment elevation: one-year outcomes of a randomized clinical trial. JAMA Cardiol 8(9):827–834
Lemkes JS, Janssens GN, van der Hoeven NW et al (2019) Coronary angiography after cardiac arrest without ST-segment elevation. N Engl J Med 380(15):1397–1407
Lemkes JS, Janssens GN, van der Hoeven NW et al (2020) Coronary angiography after cardiac arrest without ST segment elevation: one-year outcomes of the COACT randomized clinical trial. JAMA Cardiol 5(12):1358–1365
Hassager C, Schmidt H, Møller JE et al (2023) Duration of device-based fever prevention after cardiac arrest. N Engl J Med 388(10):888–897
Bharadwaj A, Potts J, Mohamed MO et al (2020) Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J 41(23):2183–2193
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Möckel declares speakers honoraria and consulting fees from BRAHMS GmbH, Roche Diagnostics and AstraZeneca and research support from Roche Diagnostics.
For this review article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Möckel, M. The new ESC acute coronary syndrome guideline and its impact in the CPU and emergency department setting. Herz 49, 185–189 (2024). https://doi.org/10.1007/s00059-024-05241-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-024-05241-6