Abstract
Purpose of Review
Despite significant progress in plasma lipid lowering strategies, recent clinical trials highlight the existence of residual cardiovascular risk. Angiopoietin-like protein 3 (ANGPTL3) and apolipoprotein C-III (Apo C-III) have been identified as novel lipid-lowering targets.
Recent Findings
Apo C-III and ANGPTL3 have emerged as novel regulators of triglyceride (TG) and low-density lipoprotein-cholesterol (LDL-C) levels. ANGPTL3 is an inhibitor of lipoprotein lipase (LPL), reducing lipolysis of Apo B-containing lipoproteins. Loss-of-function ANGPLT3 mutations are associated with reduced plasma cholesterol and TG, while novel ANGPLT3 inhibition strategies, including monoclonal antibodies (evinacumab), ANGPLT3 antisense oligonucleotides (IONIS-ANGPTL3-LRx), and small interfering RNA (siRNA) silencing techniques (ARO-ANG3), result in increased lipolysis and significant reductions of LDL-C and TG levels in phase I and II clinical trials. Similarly, Apo C-III inhibits LPL while promoting the hepatic secretion of TG-rich lipoproteins and preventing their clearance. Loss-of-function APOC3 mutations have been associated with reduced TG levels. Targeting of Apo C-III with volanesorsen, an APOC3 siRNA, results in significant reduction in plasma TG levels but possibly also increased risk for thrombocytopenia, as recently demonstrated in phase I, II, and III clinical trials. ARO-APOC3 is a novel siRNA-based agent targeting Apo C-III which is currently under investigation with regard to its lipid-lowering efficiency.
Summary
ANGPTL3 and Apo C-III targeting agents have demonstrated striking lipid-lowering effects in recent clinical trials; however, more thorough safety and efficacy data are required. Here, we evaluate the role of ANGPLT3 and Apo C-III in lipid metabolism, present the latest clinical advances targeting those molecules, and outline the remaining scientific challenges on residual lipid-associated cardiovascular risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysregulation of lipid metabolism, characterized by increased plasma levels of low density lipoprotein cholesterol (LDL-C) and triglycerides (TG), has long been identified as a core pathogenic aetiology of atherosclerosis [1, 2]. However, despite the use of statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, recent clinical studies revealed the presence of residual cardiovascular risk associated with increased morbidity and mortality [3, 4]. Interestingly, accumulating evidence suggests that this residual risk exists even at very low levels of circulating LDL-C [5] and can be better quantified by non-high-density lipoprotein cholesterol (non-HDL-C) than by LDL-C [6]. Optimal lipid reduction thus remains an elusive therapeutic target in cardiovascular disease (CVD) prevention, warranting further investigation [7].
Angiopoietin-like protein 3 (ANGPLT3), a member of the angiopoietin-like proteins that exert angiogenic properties, participates in the regulation of lipoprotein metabolism [8]. Under normal conditions, it is primarily important for the maintenance of physiological levels of plasma TG, though it also regulates plasma LDL cholesterol levels. Due to its latter property, it has attracted a lot of attention as a potential target for the efficient management of dyslipidaemia [9]. The main mechanism underlying the effect of ANGPTL3 on lipid levels involves the inhibition of lipoprotein lipase (LPL), hepatic lipase (HL), and endothelial lipase, thereby inhibiting the lipolysis of plasma lipoprotein TG and phospholipids [9, 10]. Importantly, pharmacological strategies for efficient inhibition of ANGPTL3 function and expression have been developed with promising preliminary results [11, 12].
Apolipoprotein (Apo) C-III is found in TG-rich lipoproteins and high-density lipoprotein (HDL) [13, 14] and genetic studies have linked reduced Apo C-III levels with low TG levels [11, 15]. Apo C-III increases the hepatic synthesis of TG-rich lipoproteins while reducing their clearance by inhibiting LPL and hepatic lipoprotein receptor uptake [11, 15, 16]. As such, Apo C-III is a promising target in hypertriglyceridaemia; thus, gene silencing techniques such as antisense oligonucleotides (ASO) and small interfering RNA (siRNA) have been applied in human phase III trials, demonstrating significant reductions in plasma TG levels [11, 15]. Ongoing research is focusing on optimizing the in vivo treatment characteristics in order for Apo C-III inhibition to find its path towards clinical practice [11, 17].
The aim of this review is the comprehensive evaluation of the current role of ANGPTL3 and Apo C-III in lipid reduction strategies. At first, we provide a summary of lipid pathophysiology in atherosclerosis, the underlying role of ANGPTL3 and Apo C-III as well as the mechanistic evidence for their pharmacological targeting. We next summarize the clinical studies investigating the efficacy of ANGPTL3 and Apo C-III as targets for lipid reduction, providing insights into their potential integration in clinical practice.
Lipids and Atherosclerosis: an Ongoing Challenge
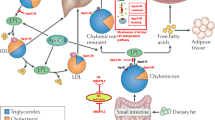
Lipids in the circulation form macromolecular assemblies with plasma apolipoproteins such as Apo B-48, B-100, E, C-II, C-III, A-I, and Lp(a) which are collectively termed as lipoproteins [12, 18,19,20]. TG are mainly circulated with the TG-rich lipoproteins that are very low-density lipoprotein (VLDL) particles, chylomicrons, and their remnants. VLDL particles are produced by the liver and contain Apo B-100 as their main apolipoprotein. Chylomicrons are assembled postprandially by the intestine and contain Apo B-48 [12, 19]. TG-rich lipoproteins are circulated to the adipose tissue, cardiac and skeletal muscle and the liver, where TG are sequentially hydrolyzed by the endothelium-bound LPL to form free fatty acids (FFA) that are taken up by the tissues [12, 21]. Sequential LPL-mediated hydrolysis leads to the formation of lipoprotein particles of decreasing size, decreasing TG content and increasing cholesterol content [12, 21]. These include chylomicron remnants, intermediate density lipoproteins (IDL) and low density lipoproteins (LDL) that are atherogenic lipid particles (Fig. 1) [12, 21]. Dysregulated lipid levels, particularly elevated LDL-C and TG levels as well reduced HDL levels, have consistently been linked with the pathogenesis of atherosclerosis [2, 22, 23].
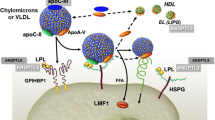
Overview of lipid turnover and effects of angiopoietin-like protein 3 (ANGPTL3) and apolipoprotein C-III (Apo C-III) inhibition. Chylomicrons (CM) are formed by triglycerides (TG) and Apo B-48 in the intestine following dietary lipid absorption. Endothelial lipoprotein lipase (LPL) converts CM to chylomicron remnants (CMR), which are internalized by the liver to contribute to the hepatic lipid pool. Hepatic cholesterol and TG, partly synthesized de novo from citrate and acetyl co-enzyme A, interact with Apo B-100 to form very low-density lipoprotein (VLDL) particles, which are secreted in the circulation. Triglycerides are hydrolyzed by LPL converting VLDL particles to intermediate density lipoproteins (IDL) and subsequently to low density lipoproteins (LDL), the main carrier of cholesterol in the bloodstream. Triglyceride lysis by LPL forms free fatty acids (FFA) which are taken up by peripheral tissues and the liver. ANGPTL3 inhibits LPL activity; therefore, its inhibition by the monoclonal antibody evinacumab or by the gene-silencing IONIS-ANGPTL3-LRx facilitates TG-rich particle lipolysis by LPL, reducing most Apo B-containing lipoprotein particles. Apo C-III enhances hepatic VLDL synthesis and also inhibits LPL; therefore, its inhibition by gene-silencing agents such as volanesorsen reduces TG-rich lipoprotein levels. The aforementioned agents have demonstrated their lipid-lowering abilities in vivo and are being further investigated in large randomized clinical trials
ANGPTL3 and Atherosclerosis
Role and Disease Mechanisms
ANGPTL3 belongs to the protein family of ANGPTLs, angiopoietin-like proteins involved in various processes such as lipid metabolism and angiogenesis. ANGPTL3 is exclusively expressed in the liver (hence being considered a hepatokine [9]), where undergoes a variety of post-translational modification such as protein glycosylation and cleavage, which prompt its activation [24]. Its C-terminal fibrinogen-like domain interacts with integrin ανβ3 receptor regulating angiogenesis [24], while its N-terminal end inhibits LPL, HL and EL activities [8, 10].
The ability of ANGPTL3 to inhibit LPL and HL activities which are primarily responsible for the hydrolysis of lipoprotein TG has attracted significant interest. ANGLPTL8 promotes ANGPTL3 cleavage, while it has been demonstrated that interaction of the N-terminal ANGPTL3 fragment with ANGPTL8 significantly increases the ability of the former to allosterically inhibit lipoprotein lipases at physiological concentrations [25]. Inhibition of ANGPTL3 would be expected to reduce lipid levels via enhanced LPL activity facilitating lipoprotein lysis and clearance. In line with this hypothesis, gain-of-function and loss-of-function transgenic mouse models have confirmed that ANGPTL3 regulates lipid levels in vivo, with loss of ANGPTL3 resulting in global hypolipidaemia while overexpression of ANGPTL3 in increased blood lipids concentration [26•]. This has also been described in human genome-wide association and Mendelian randomization studies causally linking homozygous loss of ANGPTL3 with reduced levels of LDL-C, VLDL-CHOLESTEROL (VLDL-C), TG-rich lipoproteins and HDL CHOLESTEROL (HDL-C) (but not Lp(a)), such as in the case of homozygous hypobetalipoproteinaemia-2 [11, 27••, 28]. Additionally, in vitro, ANGPLT3 silencing reduces the biosynthesis, lipidation, and secretion of VLDL by human hepatocytes in response to various stimuli [29, 30], which may be a complementary mechanism, beyond LPL regulation, via which ANGPTL3 inhibition reduces serum VLDL levels. The exact mechanism of LDL-C reduction following ANGPTL3 inhibition is not well-known. It has been proposed that it involves increased uptake [31] as well as decreased hepatic secretion of Apo B-containing lipoproteins (in a way similar to other lipid-lowering drugs blocking ApoB-containing lipoprotein synthesis, namely niacin [32], mipomersen [33], and lomitapide [34]). However, other experimental studies showed that ANGPTL3 inhibition did not affect the number of ApoB-containing lipoproteins secreted by the liver [35]. The effect of ANGPTL3 inhibition on LDL-C levels was attenuated in LDL RECEPTOR (LDLR)-deficient mice, suggesting dependence on LDLR-mediated clearance of LDL particles [31]. Furthermore, other investigators showed that ANGPTL3 inhibition reduced the lipidation of VLDL and LDL particles in mice [36], and these altered particles are possibly cleared more rapidly from the circulation through noncanonical pathways [35].
Besides research directly linking ANGPTL3 with lipid levels, several studies have also associated ANGPTL3 function with disease phenotypes such as obesity, diabetes and atherosclerosis at an observational level [37,38,39]. Hepatic ANGPTL3 was increased in insulin-deficient and insulin-resistant experimental models of diabetic mice, while genetic variants of ANGPTL3 were associated with future risk of incident diabetes in humans [39]. Loss-of-function ANGPTL3 mutations have been associated with reduced risk for diabetes and obesity [30], as well as with reduced plaque burden and overall atherosclerosis risk in humans [40]. Despite the fact that HDL-C has been previously linked with decreased cardiovascular risk [41], ANGPLT3 loss is associated with decreased cardiovascular risk despite reducing HDL-C, apparently due to the overarching effect on LDL-C and TG reduction [27••].
Targeting of ANGPTL3
ANGPTL3 is a promising therapeutic target considering its ability to modulate lipid levels including LDL-C, VLDL-C and TG. To date, two strategies have been developed to target ANGPTL3, namely monoclonal antibody targeting and transcriptional modulation by ASO [11].
-
i)
Monoclonal antibody targeting
Evinacumab is a fully human monoclonal antibody specifically binding to ANGPTL3. It has displayed its efficacy in inhibiting ANGPTL3 and lipoprotein lipases in several animal in vivo studies and human clinical studies. In a representative study using an APOE*3Leiden/CETP mouse model, evinacumab reduced plasma cholesterol and TG levels, as well as atherosclerotic lesion size, compared to controls [27••]. Human studies have shown a dose-dependent reduction of TG levels up to 76% and LDL-C up to 23% in response to evinacumab treatment, administered as weekly doses ranging from 75 to 450 mg subcutaneously and 5 to 20 mg/kg intravenously [27, 42••]. Other studies have further confirmed the efficacy of evinacumab in patients with homozygous familiar hypercholesterolaemia in open-label phase 2 studies [43, 44••], which was found to be independent to LDL receptor (LDLR) activity [43], resulting in marked LDL-C reductions, even in the context of homozygous familial hypercholesterolemia (Table 1).
Importantly, evinacumab appears to have additive benefit in reducing TG and LDL-C levels on top of statin and PCSK9 inhibitor treatment as evidenced in both recent animal studies in APOE*3Leiden/CETP mice [47, 48] and in humans with homozygous hypercholesterolaemia [49]. This triple combination therapy was accompanied by striking reduction in atherosclerosis burden in mice [47]. Preliminary data indicate that evinacumab is well-tolerated, however, large outcome trials assessing the safety of evinacumab and its relationship with cardiovascular events are lacking.
-
ii)
Antisense oligonucleotide targeting
The use of ASO comprises another approach that has been used to inhibit ANGPTL3 [11, 46••] by inhibiting its translation [50]. N-acetylgalactosamine (GalNAc)–conjugated mouse Angptl3 ASOs (IONIS-ANGPTL3-LRx) have been developed and tested in a variety of mouse models, resulting in reduced levels of all ApoB-containing lipoproteins and attenuated systemic insulin resistance [46••].
Importantly, the safety and efficacy of IONIS-ANGPTL3-LRx have been investigated in a phase 1 trial of 44 human participants, using single (subcutaneous 20, 40, or 80 mg injections) versus multiple (subcutaneous 10, 20, 40, or 60 mg weekly injections for 6 weeks) administration. In the single-dose groups, a non-significant, dose-dependent trend for lower VLDL-C, TG and LDL-C levels was reported [46••]. Clearer, statistically significant lipid reductions were achieved in the multiple-administrations groups, in a dose-dependent manner, inducing a maximum of 63% TG reduction and 36.6% reduction in non-HDL-C levels [46••]. No serious side effects or significant alterations of blood cell counts, renal or liver function were observed, while headache and dizziness were reported by few participants [46••]. These observations suggest that IONIS-ANGPTL3-LRx may be a viable option for reducing ApoB-containing lipoproteins in humans [46••] (Table 1). However, larger clinical trials linking ANGPTL3 ASOs with cardiovascular outcomes are required.
-
iii)
siRNA silencing
Recently, another gene silencing agent has been developed (ARO-ANG3), which is able to target ANGPTL3 expression via siRNA [51]. ARO-ANG3 is currently being tested in phase I/II trials, where single drug doses caused TG reduction of up to 66%, accompanied by dose-dependent reductions in LDL-C, albeit not significant probably due to small effect size [51]. It remains to be seen whether this siRNA technique could be a similarly efficient means of ANGPTL3 targeting in humans.
Apo C-III and Atherosclerosis
Role and Disease Mechanisms
Apo C-III, encoded by the gene APOC3 located on chromosome 11q23, is an apolipoprotein which primarily comprises part of VLDL and chylomicrons [11], while it can also be found in LDL and HDL particles [13, 14, 16, 52, 53]. It is mainly expressed in the intestine, where it helps form chylomicrons postprandially, and in the liver, where it modulates central lipid metabolism and turnover [16].
The in vivo relationship of Apo C-III with hyperlipidaemia, particularly TG levels, has been confirmed in genome wide association studies (GWAS) [48, 54,55,56]. In addition, Mendelian randomization studies have demonstrated reduced TG levels and coronary calcification in humans with null mutations in APOC3, characterized by markedly decreased plasma Apo C-III levels [57, 58]. Loss-of-function mutations of APOC3 have since been linked with both low TG levels and decreased cardiovascular risk in large studies [59••, 60••]. Furthermore, association studies have independently associated circulating Apo C-III with the presence of atherosclerosis as well as adverse cardiovascular outcomes [61,62,63].
At a cellular level, Apo C-III inhibits lipoprotein lipases and prevents the interaction of ApoB and ApoE apolipoproteins with their hepatic receptors, thereby increasing the bioavailability of circulating TG-rich lipoproteins via reduced lipolysis and reduced hepatic uptake [64]. This is further augmented by the ability of Apo C-III to stimulate hepatic synthesis and secretion of VLDL [65], while it has also been revealed that Apo C-III also decreases the hepatic clearance of TG-containing lipoproteins via regulating the LDLR/low density lipoprotein receptor-related protein 1 (LRP1) pathway [66]. Furthermore, a higher Apo C-III content has been noticed in HDL particles from patients with obesity and atherosclerosis [64]. In addition to TG-rich lipoproteins, Apo CIII may also be capable of promoting the de novo biogenesis of HDL (Apo C-III-HDL) in the absence of Apo A-I, which is the essential component of classical HDL [13]. Interestingly, the extent of Apo CIII accumulation on TG-rich lipoproteins, and thus the extent of inhibition of their LPL-mediated lipolysis, appears dependent on the activity of the lipid transporter ABCA1 which is key to free cholesterol uptake by very small, nascent HDL, and which also regulates the relative partitioning of Apo C-III on HDL vs other lipoproteins [13]. In experimental mice, Apo C-III-HDL was found to be a poorer acceptor of free [14C]-cholesterol in RAW 264.7 cells than was Apo A-I-HDL, indicating reduced cholesterol efflux, which, in turn, appears to be a key property for the effective unloading of free cholesterol from peripheral tissues and its shuttling back to the liver for catabolism [14]. Moreover, Apo C-III potentiated the effect of lipopolysaccharides on tumor necrosis factor (TNF)-α release in RAW 264.7 macrophage cells suggesting a proinflammatory role in circulation [14]. Apo C-III-HDL had a much higher antioxidant capacity than classical HDL, suggesting a positive role in reducing oxidative stress [14]. In case-control studies Apo C-III-HDL appeared to be associated with increased cardiovascular risk as opposed to Apo A-I-HDL, implying that Apo C-III may be related to HDL dysfunction in humans [64].
Despite having attracted scientific attention mainly due to its direct relationship with TG levels, Apo C-III appears to have pleiotropic biological effects beyond lipid metabolism [64, 67]. Apo C-III promotes proinflammatory nuclear factor kappa beta (NFkB) signaling, which in turns activates monocytes and increases their integrin-mediated interaction with ECs [68], while it also upregulates vascular cell adhesion molecule-1 (VCAM1) expression in ECs [69], thus facilitating local macrophage recruitment and inflammation [64]. Apo C-III also induces reactive oxygen species-mediated proliferation of VSMCs, which is also involved in early atherogenesis [64]. In addition, Apo C-III has been associated with increased thrombin production which could promote a hyper-coagulant state [70]. It should be noted however that Apo C-III-HDL stimulates brown adipose tissue (BAT) metabolic activation in mice [14], which explains the sensitivity of apoc3-deficient (apoc3-/-) mice towards diet-induced obesity that has been previously reported in literature [71].
Targeting of Apo C-III
Modulation of Apo C-III has been attempted by lifestyle and dietary means, while several approved medications have shown moderate Apo C-III-lowering effects [15]. On the other hand, more recent techniques specifically targeting Apo C-III involve gene silencing via siRNA, which is currently being tested in large clinical trials [15].
-
i)
Lifestyle and dietary interventions
Experimental studies have revealed opposing direct effects of glucose and insulin on hepatocellular APOC3 expression [15]. Glucose stimulates hepatic APOC3 expression via transcriptional regulation involving hepatocyte nuclear factor 4α [72]. On the other hand, insulin induces Foxo1-mediated transcriptional pathways downregulating APOC3 expression in hepatocytes [73]. Therefore, glucose intolerance in the context of systemic insulin resistance, metabolic syndrome or diabetes could dually increase APOC3 expression in the liver by the direct effect of hyperglycaemia and the loss of the inhibitory insulin effect (due to hepatic insulin resistance) [15]. Consistently, several human observational studies have indicated that high-carbohydrate diet is associated with increased serum Apo C-III levels [74, 75], while low-carbohydrate diets may reduce Apo C-III [76].
Beyond carbohydrate consumption, Apo C-III levels have also been linked with the type of dietary lipids. Consumption of saturated fats has been associated with elevated Apo C-III levels [77], while unsaturated fats may reduce Apo C-III levels, with 3-poly-unsaturated fats (3-PUFA) demonstrating a robust ability in that regard [78].
Recently, aerobic exercise was demonstrated to decrease TG levels via reducing Apo C-III in patients with coronary artery disease. The underlying mechanism is not fully understood, but it may involve favorable changes in skeletal muscle and hepatic insulin sensitivity as well other metabolic adjustments owing to aerobic exercise [79].
-
ii)
Clinically used drugs with pleiotropic effects on Apo C-III
Statins are anti-hyperlipidaemic drugs mainly inhibiting de novo cholesterol biosynthesis, however human studies have revealed a dose-dependent role of statins such as rosuvastatin in reducing Apo C-III [80, 81], while this has been confirmed in meta-analyses of large randomized clinical trials with statins [82].
Fibrates are drugs mainly used to reduce TG levels. Fibrates exert their main actions via peroxisome proliferator-activated receptor alpha (PPARα), and part of their actions may be due to Apo C-III reduction as evidenced by mechanistic studies on the effects of PPARα [83] and demonstrated in human studies [84,85,86]. Interestingly, Apo C-III data from large clinical trials with fibrates are quite limited, raising the question as to the clinically relevant contribution of Apo C-III reduction to the overall TG-decreasing effects of fibrates [15].
In a randomized study of hypercholesterolaemic, obese individuals, circulating Apo C-III levels were reduced in the subgroups treated with ezetimibe, a drug targeting enteric cholesterol absorption, and orlistat, an intestinal lipase inhibitor used to treat obesity [87]. Therefore, Apo C-III may mediate the minor to modest TG-lowering effects of ezetimibe and orlistat respectively, which however require more definite investigation.
-
iii)
APOC3 silencing
Gene silencing by ASOs is a molecular technique which has also been used to target APOC3, volanesorsen (IONIS-APO-CIIIRx) being an ASO that inhibits Apo C-III expression [15]. Volanesorsen is a second-generation 2′-O-methoxyethyl (2′-MOE) chimeric ASO targeting the APOC3 mRNA, preventing its translation [88, 89•].
Volanesorsen has been validated in several animal models with regard to its TG-lowering abilities [89•]. Importantly, subcutaneous injection doses ranging from 50 mg to 400 mg of volanesorsen markedly and dose-dependently reduced TG levels in apparently healthy volunteers [89•]. Human studies in patients with hypertriglyceridaemia have demonstrated significant reductions of TG levels with volanesorsen (weekly 300 mg injections) [88], while, in a 15-week trial in patients with diabetes, volanesorsen, besides reduction of TG levels by ~69%, also ameliorated glucose intolerance [90•] (Table 2). In phase II clinical trials, volanesorsen, administered weekly at a dose range from 100 mg to 300 mg, was able to reduce TG levels dose-dependently by up to ~90% in patients with hypertriglyceridaemia, both as a monotherapy and as an add-on therapy on top of standard fibrate therapy [91••, 92]. Volanesorsen was associated with decreased familial chylomicronemia syndrome (FCS) symptom burden in 22 FCS patients according to ReFOCUS, a retrospective web-based study [95].
Two phase III randomized clinical trials have further explored the safety and efficacy of volanesorsen in reducing TG levels (Table 2). The APPROACH trial (NCT02211209) evaluated the effect of volanesorsen (300 mg administered subcutaneously on a weekly basis) vs placebo on TG levels in 66 FCS patients over a period of 52 weeks [93•, 96•]. Volanesorsen reduced TG levels by 77% as opposed to the 18% increase observed in the placebo group [96•]. On the other hand, the COMPASS trial (NCT02300233) evaluated the effect of volanesorsen (300 mg delivered as a weekly subcutaneous injection) on TG levels in 113 patients with fasting triglycerides greater than 500 mg/dL [94••]. Volanesorsen significantly reduced TG levels by 71.8% vs 0.8% in the placebo group [94••]. Clinical trials directly comparing volanesorsen with fibrates or n-3 fatty acids as a means of reducing TG levels are still lacking; however, the striking TG reductions achieved with volanesorsen appear to be far greater than those achieved with the other agents [92•]. Interestingly, volanesorsen may also reduce hypertriglyceridaemia-associated acute pancreatitis risk [97]. Finally, clinical trials exploring the potential effect of volanesorsen on cardiovascular events are needed.
A major side effect of volanesorsen revealed by the APPROACH study comprised thrombocytopenia, which was in rare cases severe, but not associated with bleeding events and was corrected after stopping the drug [15, 96•]. Although the COMPASS trial did not show an increase in thrombocytopenia and in bleeding events [11, 94], the FDA did not approve volanesorsen for use in FCS patients [11]. However, there is a lot of positivity with regard to the clinical utility of APOC3 silencing, while the side effect on platelet count is proposed to be a consequence of off-target properties of the specific agent rather than being a general result of APOC3 silencing [15]. Indeed, the 2′-MOE modification found in volanesorsen has previously been associated with dose-dependent thrombocytopenia in both humans and animal models [98]. APOC3 silencing with a GalNAc-conjugated ASO (IONIS-APO-CIIILRx) instead of 2′-MOE has recently been used in healthy volunteers, resulting in significant TG reduction without inducing thrombocytopenia [17]. These considerations might pave the way for clinical approval of APOC3-targeting ASOs to reduce TG both efficiently and without severe side effects, even in FCS.
Conclusion
Despite significant progress in the field of hyperlipidaemia management, recent clinical trials indicate an unmet need for more aggressive and efficient lipid-lowering therapy in high-risk patient subgroups. Apo C-III has long been known to be a key regulator of TG and HDL metabolism. Recently, ANGPTL3 has been revealed as a novel regulator of lipid metabolism in genetic studies. They have both been validated in a multitude of animal and human studies, including in relationship to obesity and diabetes (Fig. 1). In vivo targeting of ANGPTL3 and Apo C-III has recently been possible with novel monoclonal antibodies and gene silencing techniques, yielding marked reductions of LDL-C, VLDL-C and TG in phase I, II and III trials.
It should be kept in mind that to this date we do not have long-term safety and efficacy data for inhibition of these targets like we do for other drugs such as statins. ANGPTL3 and Apo CIII may likely also have beneficial long-term properties and their extreme inhibition might be harmful, as suggested by the ancient Greek saying “μέτρον άριστον,” or “everything in moderation.” Regarding atherosclerotic CVD risk reduction, the ability of ANGPTL3 inhibition to reduce LDL-C levels (the main lipid CVD risk factor), along with TG concentration, seems more promising. Both ANGPTL3 and Apo C-III inhibition may be beneficial in patients with mixed dyslipidaemia and increased residual CVD risk. The relatively stronger TG-lowering ability of Apo-CIII inhibition may benefit patients with severe hypertriglyceridaemia and increased risk of pancreatitis, while combination treatment may confer additive effects. Additionally, a combination of these drug classes could result in significant improvement of overall lipid profile and confer CVD protection. Long-term clinical trials will be required to resolve all these emerging issues and proof-of-concept hypotheses.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gofman JW, Lindgren F, Elliott H, Mantz W, Hewitt J, Strisower B, et al. The role of lipids and lipoproteins in atherosclerosis. Science (80- ). 1950;111:166–86. https://doi.org/10.1126/science.111.2877.166.
Pirillo A, Bonacina F, Norata GD, Catapano AL. The interplay of lipids, lipoproteins, and immunity in atherosclerosis. Curr Atheroscler Rep. 2018;20:12.
Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Residual hypertriglyceridemia and estimated atherosclerotic cardiovascular disease risk by statin use in U.S. adults with diabetes: National Health and Nutrition Examination Survey 2007–2014. Diabetes Care. 2019;42:2307–14.
Lawler PR, Akinkuolie AO, Harada P, Glynn RJ, Chasman DI, Ridker PM, et al. Residual risk of atherosclerotic cardiovascular events in relation to reductions in very-low-density lipoproteins. J Am Heart Assoc. 2017;6(12):e007402
Lawler PR, Akinkuolie AO, Chu AY, Shah SH, Kraus WE, Craig D, et al. Atherogenic lipoprotein determinants of cardiovascular disease and residual risk among individuals with low low-density lipoprotein cholesterol. J Am Heart Assoc. 2017;6:e005549.
Rana JS, Boekholdt SM, Kastelein JJP, Shah PK. The role of non-HDL cholesterol in risk stratification for coronary artery disease. Curr Atheroscler Rep. 2012;14:130–4.
Filippatos TD, Liontos A, Christopoulou EC, Elisaf MS. Novel hypolipidaemic drugs: mechanisms of action and main metabolic effects. Curr Vasc Pharmacol. 2019;17:332–40.
Hassan M. ANGPLT3: a novel modulator of lipid metabolism. Glob Cardiol Sci Pract. 2017;2017(1): e201706.
Kersten S. Angiopoietin-like 3 in lipoprotein metabolism. Nat Rev Endocrinol. 2017;13:731–9.
Shimamura M, Matsuda M, Yasumo H, Okazaki M, Fujimoto K, Kono K, et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol. 2007;27:366–72.
Hegele RA, Tsimikas S. Lipid-lowering agents. Circ Res. 2019;124:386–404.
Kypreos KE, Bitzur R, Karavia EA, Xepapadaki E, Panayiotakopoulos G, Constantinou C. Pharmacological management of dyslipidemia in atherosclerosis: limitations, challenges, and new therapeutic opportunities. Angiology. 2019;70:197–209.
Kypreos KE. ABCA1 Promotes the de novo biogenesis of apolipoprotein CIII-containing HDL particles in vivo and modulates the severity of apolipoprotein CIII-induced hypertriglyceridemia †. Biochemistry. 2008;47:10491–502.
Zvintzou E, Lhomme M, Chasapi S, Filou S, Theodoropoulos V, Xapapadaki E, et al. Pleiotropic effects of apolipoprotein C3 on HDL functionality and adipose tissue metabolic activity. J Lipid Res. 2017;58:1869–83.
Taskinen M-R, Packard CJ, Borén J. Emerging evidence that ApoC-III inhibitors provide novel options to reduce the residual CVD. Curr Atheroscler Rep. 2019;21:27.
Kohan AB. Apolipoprotein C-III. Curr Opin Endocrinol Diabetes Obes. 2015;22:119–25.
Alexander VJ, Digenio A, Xia S, Hurh E, Hughes S, Geary RS, et al. Inhibition of apolipoprotein C-III with galnac conjugated antisense drug potently lowers fasting serum apolipoprotein C-III and triglyceride levels in healthy volunteers with elevated triglycerides. J Am Coll Cardiol. 2018;71:A1724.
Brewer HB, Gregg RE, Hoeg JM, Fojo SS. Apolipoproteins and lipoproteins in human plasma: an overview. Clin Chem. 1988;34(8B):B4–8.
Shepherd J. Lipoprotein Metabolism. Drugs. 1994;47:1–10.
Kei AA, Filippatos TD, Tsimihodimos V, Elisaf MS. A review of the role of apolipoprotein C-II in lipoprotein metabolism and cardiovascular disease. Metabolism. 2012;61:906–21.
Chait A, Eckel RH. Lipids, Lipoproteins, and cardiovascular disease: clinical pharmacology now and in the future. J Clin Endocrinol Metab. 2016;101:804–14.
Tabas I. Lipids and atherosclerosis. Chapter in “Biochemistry of Lipids, Lipoproteins and Membranes”. 2008;579–605.
Djeric M, Stokic E, Vuckovic B, Kojic-Damjanov S, Cabarkapa V. Lipids and atherosclerosis. Jugosl Med biohemija. 2006;25:325–33.
Ono M, Shimizugawa T, Shimamura M, Yoshida K, Noji-Sakikawa C, Ando Y, et al. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3). J Biol Chem. 2003;278:41804–9.
Chi X, Britt EC, Shows HW, Hjelmaas AJ, Shetty SK, Cushing EM, et al. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab. 2017;6:1137–49.
Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30:151–7 A landmark study introducing the role of ANGPTL3 in lipid metabolism.
Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377:211–21 A crucial study causally linking ANGPTL3 with cardiovascular disease in humans.
Minicocci I, Santini S, Cantisani V, Stitziel N, Kathiresan S, Arroyo JA, et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J Lipid Res. 2013;54:3481–90.
Tikka A, Soronen J, Laurila P-P, Metso J, Ehnholm C, Jauhiainen M. Silencing of ANGPTL 3 (angiopoietin-like protein 3) in human hepatocytes results in decreased expression of gluconeogenic genes and reduced triacylglycerol-rich VLDL secretion upon insulin stimulation. Biosci Rep. 2014;34:e00160.
Christopoulou E, Elisaf M, Filippatos T. Effects of angiopoietin-like 3 on triglyceride regulation, glucose homeostasis, and diabetes. Dis Markers. 2019;2019:1–8.
Xu YX, Redon V, Yu H, Querbes W, Pirruccello J, Liebow A, et al. Role of angiopoietin-like 3 (ANGPTL3) in regulating plasma level of low-density lipoprotein cholesterol. Atherosclerosis. 2018;268:196–206.
Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:S20–6.
Crooke ST, Geary RS. Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br J Clin Pharmacol. 2013;76:269–76.
Goulooze SC, Cohen AF, Rissmann R. Lomitapide. Br J Clin Pharmacol. 2015;80:179–81.
Wang Y, Gusarova V, Banfi S, Gromada J, Cohen JC, Hobbs HH. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J Lipid Res. 2015;56:1296–307.
Tikka A, Jauhiainen M. The role of ANGPTL3 in controlling lipoprotein metabolism. Endocrine. 2016;52:187–93.
Lupo M, Ferri N. Angiopoietin-like 3 (ANGPTL3) and atherosclerosis: lipid and non-lipid related effects. J Cardiovasc Dev Dis. 2018;5:39.
Cinkajzlová A, Mráz M, Lacinová Z, Kloučková J, Kaválková P, Kratochvílová H, et al. Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: the effect of weight reduction and realimentation. Nutr Diabetes. 2018;8:21.
Inukai K, Nakashima Y, Watanabe M, Kurihara S, Awata T, Katagiri H, et al. ANGPTL3 is increased in both insulin-deficient and -resistant diabetic states. Biochem Biophys Res Commun. 2004;317:1075–9.
Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69:2054–63.
Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–25.
Ahmad Z, Banerjee P, Hamon S, Chan K-C, Bouzelmat A, Sasiela WJ, et al. Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertriglyceridemia. Circulation. 2019;140:470–86 An important study supporting the efficacy of ANGPTL3 inhibition with a monoclonal antibody in reducing triglycerides in humans.
Banerjee P, Chan K-C, Tarabocchia M, Benito-Vicente A, Alves AC, Uribe KB, et al. Functional analysis of LDLR (low-density lipoprotein receptor) variants in patient lymphocytes to assess the effect of evinacumab in homozygous familial hypercholesterolemia patients with a spectrum of LDLR activity. Arterioscler Thromb Vasc Biol. 2019;39:2248–60.
Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med. 2017;377:296–7 An important work linking ANGPTL3 inhibition with lipid reduction in homozygous familiar hypercholesterolaemia in humans.
Gaudet D, Gipe D, Hovingh K, Ahmad Z, Cuchel M, Shah P, et al. Safety and efficacy of evinacumab, a monoclonal antibody to ANGPTL3, in patients with homozygous familial hypercholesterolemia: a single-arm, open-label, proof-of-concept study. Atherosclerosis. 2017;263:e9 Further in vivo validation of ANGPTL3 inhibition efficacy in reducing lipids in human homozygous familiar hypercholesterolaemia.
Graham MJ, Lee RG, Brandt TA, Tai L-J, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. 2017;377:222–32 Pivotal work introducing the applicability of antisense oligonucleotide targeting in ANGPTL3 inhibition in humans.
Hurt-Camejo E. ANGPTL3, PCSK9, and statin therapy drive remarkable reductions in hyperlipidemia and atherosclerosis in a mouse model. J Lipid Res. 2020;61:272–4.
Pouwer MG, Pieterman EJ, Worms N, Keijzer N, Jukema JW, Gromada J, et al. Alirocumab, evinacumab, and atorvastatin triple therapy regresses plaque lesions and improves lesion composition in mice. J Lipid Res. 2020;61:365–75.
Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, JJP K, Rubba P, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383:711–720.
Bennett CF. Therapeutic antisense oligonucleotides are coming of age. Annu Rev Med. 2019;70:307–21.
Nurmohamed NS, Dallinga-Thie GM, Stroes ESG. Targeting apoC-III and ANGPTL3 in the treatment of hypertriglyceridemia. Expert Rev Cardiovasc Ther. 2020;18:355–361.
Kavo AE, Rallidis LS, Sakellaropoulos GC, Lehr S, Hartwig S, Eckel J, et al. Qualitative characteristics of HDL in young patients of an acute myocardial infarction. Atherosclerosis. 2012;220:257–64.
Zvintzou E, Skroubis G, Chroni A, Petropoulou P-I, Gkolfinopoulou C, Sakellaropoulos G, et al. Effects of bariatric surgery on HDL structure and functionality: results from a prospective trial. J Clin Lipidol. 2014;8:408–17.
Mar R, Pajukanta P, Allayee H, Groenendijk M, Dallinga-Thie G, Krauss RM, et al. Association of the apolipoprotein A1/C3/A4/A5 gene cluster with triglyceride levels and LDL particle size in familial combined hyperlipidemia. Circ Res. 2004;94:993–9.
Lai C-Q, Parnell LD, Ordovas JM. The APOA1/C3/A4/A5 gene cluster, lipid metabolism and cardiovascular disease risk. Curr Opin Lipidol. 2005;16:153–66.
Qi L, Liu S, Rifai N, Hunter D, Hu FB. Associations of the apolipoprotein A1/C3/A4/A5 gene cluster with triglyceride and HDL cholesterol levels in women with type 2 diabetes. Atherosclerosis. 2007;192:204–10.
Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science (80- ). 2008;322:1702–5.
Crawford DC, Dumitrescu L, Goodloe R, Brown-Gentry K, Boston J, McClellan B, et al. Rare variant APOC3 R19X is associated with cardio-protective profiles in a diverse population-based survey as part of the epidemiologic architecture for genes linked to environment study. Circ Cardiovasc Genet. 2014;7:848–53.
Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31 Landmark work causally linking Apo CIII with triglyceride levels and implications in human coronary artery disease.
Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41 Important work first to causally associate Apo CIII with human ischaemic vascular disease.
Ooi EMM, Barrett PHR, Chan DC, Watts GF. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin Sci. 2008;114:611–24.
Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, et al. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the cholesterol and recurrent events (CARE) trial. Circulation. 2000;102:1886–92.
Dittrich J, Beutner F, Teren A, Thiery J, Burkhardt R, Scholz M, et al. Plasma levels of apolipoproteins C-III, A-IV, and E are independently associated with stable atherosclerotic cardiovascular disease. Atherosclerosis. 2019;281:17–24.
Luo M, Peng D. The emerging role of apolipoprotein C-III: beyond effects on triglyceride metabolism. Lipids Health Dis. 2016;15:184.
Yao Z. Human apolipoprotein C-III–a new intrahepatic protein factor promoting assembly and secretion of very low density lipoproteins. Cardiovasc Hematol Disord Targets. 2012;12:133–40.
Gordts PLSM, Nock R, Son N-H, Ramms B, Lew I, Gonzales JC, et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016;126:2855–66.
Christopoulou E, Tsimihodimos V, Filippatos T, Elisaf M. Apolipoprotein CIII and diabetes. Is there a link? Diabetes Metab Res Rev. 2019;35:e3118.
Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase Cα-mediated nuclear factor-κB activation. Arterioscler Thromb Vasc Biol. 2007;27:219–25.
Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114:681–7.
Olivieri O, Martinelli N, Girelli D, Pizzolo F, Friso S, Beltrame F, et al. Apolipoprotein C-III predicts cardiovascular mortality in severe coronary artery disease and is associated with an enhanced plasma thrombin generation. J Thromb Haemost. 2010;8:463–71.
Duivenvoorden I, Teusink B, Rensen PC, Romijn JA, Havekes LM, Voshol PJ. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice. Diabetes. 2005;54:664–71.
Caron S, Verrijken A, Mertens I, Samanez CH, Mautino G, Haas JT, et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol. 2011;31:513–9.
Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. 2004;114:1493–503.
Mendoza S, Trenchevska O, King SM, Nelson RW, Nedelkov D, Krauss RM, et al. Changes in low-density lipoprotein size phenotypes associate with changes in apolipoprotein C-III glycoforms after dietary interventions. J Clin Lipidol. 2017;11:224-233.e2.
Furtado JD, Campos H, Appel LJ, Miller ER, Laranjo N, Carey VJ, et al. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr. 2008;87:1623–30.
Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Räsänen SM, et al. An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metab. 2018;27:559-571.e5.
Faghihnia N, Mangravite LM, Chiu S, Bergeron N, Krauss RM. Effects of dietary saturated fat on LDL subclasses and apolipoprotein CIII in men. Eur J Clin Nutr. 2012;66:1229–33.
Sahebkar A, Simental-Mendía LE, Mikhailidis DP, Pirro M, Banach M, Sirtori CR, et al. Effect of omega-3 supplements on plasma apolipoprotein C-III concentrations: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2018;50:565–75.
Wang Y, Shen L, Xu D. Aerobic exercise reduces triglycerides by targeting apolipoprotein C3 in patients with coronary heart disease. Clin Cardiol. 2019;42:56–61.
Ooi EMM, Watts GF, Chan DC, Chen MM, Nestel PJ, Sviridov D, et al. Dose-dependent effect of rosuvastatin on VLDL-apolipoprotein C-III kinetics in the metabolic syndrome. Diabetes Care. 2008;31:1656–61.
Kostapanos MS, Milionis HJ, Filippatos TD, Nakou ES, Bairaktari ET, Tselepis AD, et al. A 12-week, prospective, open-label analysis of the effect of rosuvastatin on triglyceride-rich lipoprotein metabolism in patients with primary dyslipidemia. Clin Ther. 2007;29:1403–14.
Sahebkar A, Simental-Mendía LE, Mikhailidis DP, Pirro M, Banach M, Sirtori CR, et al. Effect of statin therapy on plasma apolipoprotein CIII concentrations: a systematic review and meta-analysis of randomized controlled trials. J Clin Lipidol. 2018;12:801–9.
Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr Rev. 2018;39:760–802.
Staels B, Vu-Dac N, Kosykh VA, Saladin R, Fruchart JC, Dallongeville J, et al. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest. 1995;95:705–12.
Haubenwallner S, Essenburg AD, Barnett BC, Pape ME, DeMattos RB, Krause BR, et al. Hypolipidemic activity of select fibrates correlates to changes in hepatic apolipoprotein C-III expression: a potential physiologic basis for their mode of action. J Lipid Res. 1995;36:2541–51.
Filippatos TD, Tsimihodimos V, Kostapanos M, Kostara C, Bairaktari ET, Kiortsis DN, et al. Analysis of 6-month effect of orlistat administration, alone or in combination with fenofibrate, on triglyceride-rich lipoprotein metabolism in overweight and obese patients with metabolic syndrome. J Clin Lipidol. 2008;2:279–84.
Nakou ES, Filippatos TD, Agouridis AP, Kostara C, Bairaktari ET, Elisaf MS. The effects of ezetimibe and/or orlistat on triglyceride-rich lipoprotein metabolism in obese hypercholesterolemic patients. Lipids. 2010;45:445–50.
Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, et al. Very-low-density lipoprotein–associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol. 2017;69:789–800.
Graham MJ, Lee RG, Bell TA, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–90 Novel study introducing the role of Apo CIII antisense targeting in reducing triglyceride levels in humans.
Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, et al. Antisense-mediated lowering of plasma apolipoprotein C-III by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care. 2016;39:1408–15 Important work demonstrating pleitropic effects of volanesorsen on insulin sensitivty and type 2 diabetes phenotypes in humans.
Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–47 Important work linking volanesorsen with triglycerides in humans with hypertriglyceridaemia.
Yang X, Lee S-R, Choi Y-S, Alexander VJ, Digenio A, Yang Q, et al. Reduction in lipoprotein-associated apoC-III levels following volanesorsen therapy: phase 2 randomized trial results. J Lipid Res. 2016;57:706–13 Important work linking volanesorsen with lipid levels in humans.
Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381:531–42 Important work exploring the role of volanesorsen in the familiar chylomicronemia syndrome in humans.
Gouni-Berthold I, Alexander V, Digenio A, DuFour R, Steinhagen-Thiessen E, Martin S, et al. Apolipoprotein C-III inhibition with volanesorsen in patients with hypertriglyceridemia (COMPASS): a randomized, double-blind, placebo-controlled trial. J Clin Lipidol. 2017;11:794–5 Landmark study evaluating the efficacy and safety of volanesorsen in reducing triglycerides in humans.
Arca M, Hsieh A, Soran H, Rosenblit P, O’Dea L, Stevenson M. The effect of volanesorsen treatment on the burden associated with familial chylomicronemia syndrome: the results of the ReFOCUS study. Expert Rev Cardiovasc Ther. 2018;16:537–46.
Gaudet D, Digenio A, Alexander VJ, Arca M, Jones AF, Stroes E, et al. The APPROACH study: a randomized, double-blind, placebo-controlled, phase 3 study of volanesorsen administered subcutaneously to patients with familial chylomicronemia syndrome (FCS). J Clin Lipidol. 2017;11:814–5 Crucial work exploring the role of volanesorsen in the familiar chylomicronemia syndrome in human.
Shemesh E, Zafrir B. Hypertriglyceridemia-related pancreatitis in patients with type 2 diabetes: links and risks. Diabetes, Metab Syndr Obes Targets Ther. 2019;12:2041–2052.
Narayanan P, Shen L, Curtis BR, Bourdon MA, Nolan JP, Gupta S, et al. Investigation into the mechanism(s) that leads to platelet decreases in cynomolgus monkeys during administration of ISIS 104838, a 2′-MOE-modified antisense oligonucleotide. Toxicol Sci. 2018;164:613–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Theodosios D. Filippatos reports personal fees from Boehringer Ingelheim, personal fees from Mylan, personal fees from Astra Zeneca, personal fees from Lilly, personal fees from Recordati, personal fees from Bausch Health and personal fees from Servier outside the submitted work. Ioannis Akoumianakis, Evangelia Zvintzou and Kyriakos Kypreos declare no conflicts of interest relevant to this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiometabolic Disease and Treatment
Rights and permissions
About this article
Cite this article
Akoumianakis, I., Zvintzou, E., Kypreos, K. et al. ANGPTL3 and Apolipoprotein C-III as Novel Lipid-Lowering Targets. Curr Atheroscler Rep 23, 20 (2021). https://doi.org/10.1007/s11883-021-00914-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11883-021-00914-7