Abstract
Electrolysis-assisted calciothermic reduction method is proposed and successfully used to prepare ferrotitanium alloy from ilmenite by using equal-molar CaCl2-NaCl molten salt as electrolyte, molybdenum rod as cathode, and graphite as anode at 973 K with cell voltages of 3.2–4.4 V under inert atmosphere. Thermodynamics analysis of the process is presented, and the products obtained are examined with x-ray diffraction, scanning electron microscopy, and energy-dispersive spectroscopy. It is demonstrated that the calciothermic reduction of ilmenite is a stepwise process since intermediate CaTiO3 is observed in the products partially reduced. In the calciothermic reduction process, the reduction of FeTiO3 first gives rise to the formation of Fe and CaTiO3, which as intermediates will further react with calcium metal to form ferrotitanium alloys. This is in good agreement with the prediction of thermodynamics. Experimental results also show that increasing cell voltage can accelerate the formation of calcium metal through electrolysis of CaO and CaCl2 and, hence, promote the calciothermic reduction of ilmenite. As the electrolytic zone and reduction zone are combined in the same bath, the theoretical energy requirement for the production of FeTi in the calciothermic process is lower than that in the aluminothermic process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ferrotitanium, used as a well-known hydrogen-storage material and deoxidizer, is traditionally prepared by carbothermic, silicothermic, and aluminothermic reduction of oxide ores and concentrates1–4 or by melting iron and titanium scraps together at high temperatures. However, these methods have many disadvantages, such as high-energy consumption and low quality of products. For example, the carbothermic process should be operated in an electric arc furnace at high temperatures over 1473 K,5,6 which leads to not only an extensive energy consumption but also to a high carbon residue in the Fe-Ti products. This production technique is rarely used and largely replaced now by an aluminothermic technique.7 However, the aluminothermic process consumes a large quantity of aluminum primarily for maintaining a sufficient heat balance, and the energy consumption of this technique is also high.
Over the years, many efforts have been made to find new ways to prepare FeTi alloys. Among these methods, the Fray-Farthing-Chen Cambridge (FFC) process provides a promising route.8–11 Researchers have used the pretreated ferro-titanium oxide as cathode, graphite as anode, and CaCl2 as electrolyte. They have found that FeTi alloys can be successfully prepared from the ferro-titanium oxides.
Another metallurgical extraction process is the calciothermic reduction process, known as the OS process, as proposed by K. Ono and R.O. Suzuki.12–16 The mechanism of this process can be summarized with examples of the calciothermic reduction of TiO2 17–19 as follows: (I) Calcium metal is used as a reductant to reduce titanium dioxide powder directly to elemental titanium; (II) the by-product CaO is then re-electrolyzed to metallic Ca in the fused CaCl2 salts; and (III) all the reactions can be operated in one molten salt. In the OS process, Ca metal is believed to act as the reductant for the extraction of metal oxides. To verify the calciothermic reduction process, the metal oxide, such as TiO2, was positioned at several points that were not directly connected to the cathode current collector to ensure no electron transport. Finally, titanium suboxides TiO x (x < 1.5) are found.
The calciothermic reduction process has been applied successfully to extract metals from the corresponding oxides, such as Ti, Ta, and Nb.20–25 However, the reduction of ilmenite, especially the phase transformation during the calciothermic process, has received no attention. Therefore, in this article, FeTi alloys are prepared for the first time from ilmenite by electrolysis-assisted calciothermic reduction similar to the OS process.
Experimental
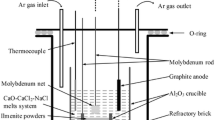
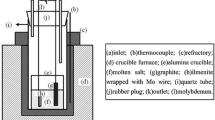
Figure 1 shows the schematic of experiment apparatus. The Al2O3 crucible (57 mm in inner diameter, 63 mm in outer diameter, and 75 mm in depth) was installed in the electric furnace. The graphite stick (99.9% in purity, 6 mm in diameter, 80 mm in length) tied by molybdenum rod was served as the anode, and the molybdenum plate (10 mm × 10 mm) tied by molybdenum rod (99.95% in purity, 3 mm in diameter, 200 mm in length) was used as the cathode, respectively. The two electrodes were immersed in fused salts about 30 mm in depth. The distance was kept at about 40 mm. The ilmenite pellet (20 mm in diameter, 5 mm in thickness, 1.5 g in weight) was then put into a Al2O3 crucible (25 mm in inner diameter, 30 mm in outer diameter, and 8 mm in depth), and it was located at the bottom of the Al2O3 vessel, as shown in Fig. 1. The distance between raw material pellet and the molybdenum plate cathode was 10 mm. In this study, continuous electrolysis was carried out at cell voltages ranging from 3.2 V to 4.4 V for 3–12 h. The ilmenite pellet was kept immersed at the bottom of the furnace during the whole process. All the experiments were commonly performed at 973 K under argon atmosphere with equal-molar molten CaCl2-NaCl (100 g) as electrolyte.
After the experiments, the molten salts were left to cool in argon. All samples were then removed from the cooled salts, rinsed in distilled water and alcohol, and dried in vacuum. The existing phases were analyzed by x-ray diffraction (XRD; D/max-2200pc model) with Cu Ka radiation at a scan rate of 10°/min in the range of 2θ = 10°–90°, characterized by x-ray energy-dispersive spectroscopy (EDS) and scanning electron microscope (SEM) by using a field emission gun scanning electron microscope (LEO-FEGSEM) operated at 20.0 keV.
Results and Discussion
Thermodynamic Analysis
The electrolysis-assisted calciothermic reduction of ilmenite is a complex procedure that includes the formation of Ca derived from CaCl2-NaCl molten salt through electrolysis of CaO and CaCl2 and the reduction of ilmenite by Ca formed. Since the solubility of CaO in fused CaCl2-NaCl salt is 5.5% by weight,26 it will dissolve in the molten salt and dissociate into calcium ions and oxygen ions before saturation. During electrolysis, calcium ions are electrochemically reduced to calcium metal at molybdenum cathode. The calcium metal formed further dissolves immediately into the CaCl2-NaCl molten salt because the solubility of Ca in the fused salt is 2.1% by weight,27 and it acts as a reductant to reduce the ilmenite pellet by diffusing from the cathode region to the interface between ilmenite and molten salt. At the same time, oxygen ions are discharged at graphite anode and removed in the form of O2 or CO and CO2. Table I lists the possible electrochemical reactions and their theoretical decomposition potentials as a function of temperature in the electrolysis process of interest. It can be seen from Table I that the upper limit of CaCl2-NaCl molten salt is 3.36 V at 973 K. If the applied cell voltage is over the theoretical composition potential of fused CaCl2-NaCl, the salt will be decomposed and produce the reductants, namely, Ca and Na metals, at the anode and Cl2 at the cathode by reactions 4 and 5, which could be regarded as the supplement of Ca metal. It is also suggested from Table I that theoretical potentials for the reactions of CaO with C to form Ca and CO/CO2 are the lowest among those of the reactions listed, indicating that metallic Ca can be preferentially produced by the electrochemical reduction of CaO when using molybdenum as cathode and the graphite as consumable anode in the fused CaCl2-NaCl salt. Furthermore, the CaO produced in the reduction process of ilmenite can be re-dissolved into the molten salt and electrochemically reduced to calcium metal again. Thus, the dissolution–electrodeposition process of CaO offers a circular route for the production of metallic calcium in the electrolysis-assisted calciothermic reduction process of ilmenite.
As the calcium metal produced in the electrolysis process diffuses to the interface of the ilmenite particles, the calciothermic reduction process occurs. In this process, a series of intermediate products may be formed, such as Fe, Ca, CaTiO3, and Fe2Ti upon the previous results.3,4 Accordingly, the possible intermediate reactions are proposed, as shown in Table II. The corresponding changes in Gibbs free energy (\( \Delta_{r} G_{m}^{\theta } \)) as a function of temperature and the respective equilibrium constant (\( K^{\theta } \)) at 973 K are shown in Fig. 2. Basically, the equilibrium constant for reactions 6–10 is calculated by Eq. 1 and represented by Eq. 2 as follows:
where a [CaO] and a [Ca] are activities of CaO and Ca dissolved in CaCl2-NaCl molten salt; b is the stoichiometric coefficient of CaO in reactions 7–10; and R is the gas constant, 8.314 J/(K mol).
By comparing changes in Gibbs free energy between reactions 6 and 7, it can be found that reaction 6 is more favorable than reaction 7 theoretically. This indicates that ilmenite should be preferentially reduced to Fe and CaTiO3 by Ca through reaction 6 rather than directly reduced to FeTi via reaction 7. The intermediate product CaTiO3 near Fe phases is further reduced and combined with Fe to form Fe2Ti or FeTi via reactions 8 or 9. In addition, CaTiO3 may be also reduced and combined with Fe2Ti to form FeTi via reaction 10. The by-product CaO produced in the previous reactions will be re-dissolved into molten salt and then reduced to calcium metal again through electrolysis. On the basis of this analysis, ilmenite can be reduced by the electrolysis-assisted calciothermic method, the reduction path of which is a stepwise procedure until the formation of the final product FeTi.
Current–Time Curves
Current–time curves for the electrolysis-assisted calciothermic reduction of ilmenite at different cell voltages are shown in Fig. 3. It is obvious from this figure that the current of electrolysis is considerably increased with increasing cell voltage from 3.2 V to 4.4 V, suggesting that higher cell voltages can accelerate the electrolysis process and, hence, promote the formation of calcium metal. When the cell voltage applied is 3.2 V, which is higher than the theoretical decomposition potential of CaO and lower than those of CaCl2 and NaCl, the source of calcium metal mainly comes from the decomposition of CaO through reactions 1–3. At a cell voltage of 3.2 V, the current increased in the initial 30 min and then decreased gradually, as shown in Fig. 3. This trend of current decayed with time indicates that the formation rate of calcium metal is decreased during electrolysis. The reason could be attributed to the progressive decrease in the regeneration rate of CaO through reactions 8–10. The slower regeneration rate of CaO will reduce the formation rate of calcium metal, which leads to a decrease in Ca concentration or activity of the molten salt and, hence, affect the calciothermic reduction rate of ilmenite. When the cell voltage applied is higher than 3.6 V, which is over the theoretical decomposition potentials of CaCl2 and NaCl as well as CaO, however, the source of calcium metal may come from the decomposition of both CaO and CaCl2. As shown in Fig. 3, the current increased with time during electrolysis at cell voltages higher than 3.6 V; that is, a higher cell voltage can maintain a faster rate for the formation of calcium metal because apart from CaO there is enough CaCl2 in the molten salt as a source of Ca during the reaction process. As a result, higher cell voltages will increase the concentration or activity of calcium metal in CaCl2-NaCl molten salt. This is favorable for accelerating the calciothermic reduction of ilmenite.
It should be pointed out that NaCl presented in the molten salt may also be decomposed to Na metal during the electrolysis process when the cell voltage is higher than 3.33 V and then is inclined to act as a reductant to reduce the ilmenite. However, the contribution of Na to the reduction of ilmenite could be ignored because no the sodium compounds combined with titanium oxides were detected by XRD analysis and EDS.
Effect of Cell Voltages
The product samples that were prepared by electrolysis-assisted calciothermic reduction for 6 h at 973 K and different cell voltages were identified by XRD, as shown in Fig. 4. According to the XRD patterns obtained, the relative abundances of CaTiO3, Fe, Fe2Ti, and FeTi in the reduced mixtures are estimated semiquantitatively by using the reference intensity ratio (RIR) method. Assuming the recrystallization degree of the reduced pellet is 100%, the detailed data are listed in Table III.
It can be observed from Fig. 4 and Table III that at 3.2 and 3.6 V, the ferrotitanium was not observed in the reduced mixture and the major phases detected were Fe and CaTiO3. This result indicates that FeTiO3 is preferentially reduced to Fe and CaTiO3 by Ca through reaction 6 when the cell voltage applied is low; i.e., the concentration of Ca in CaCl2-NaCl molten salt is low. As the cell voltages increase over 4.0 V, the products are dominated by the FeTi, Fe2Ti, and CaTiO3, indicating that ferrotitanium alloy can be produced through reactions 8–10 at higher cell voltages. This is reasonable because a higher cell voltage can give rise to a higher Ca concentration or activity in the molten salt and promote the further reduction of CaTiO3 and the formation of ferrotitanium alloy via reactions 8–10. These results are in good agreement with the prediction of thermodynamics.
However, the presence of intermediate product CaTiO3 in the reduced mixtures shows that the electrolysis-assisted calciothermic reduction of ilmenite is a complex process. Ilmenite is not directly reduced to ferrotitanium alloy via a single reaction 7. It is believed that the ferrotitanium alloy is produced from ilmenite by a stepwise process including reactions 6, 8, 9, and 10. Therefore, the intermediate products such as Fe2Ti, CaTiO3, and Fe remain in the reduced mixtures and even higher cell voltages of 4.0 and 4.4 V are applied for 6 h.
Effect of Reaction Time
To understand the electrolysis-assisted calciothermic reduction process of ilmenite better, experiments were conducted at 973 K with a cell voltage of 4.4 V for 3 h, 6 h, 12 h, and 24 h, respectively. XRD patterns of the reduced mixtures for these experiments are shown in Fig. 5. The figure shows clearly that the major phases were Fe and CaTiO3 in the mixture reduced for 3 h, suggesting that FeTiO3 was first reduced to intermediates Fe and CaTiO3 according to reaction 6 in the initial period of time when the cell voltage was as high as 4.4 V. In the mixtures reduced for 6 and 12 h, CaTiO3, Fe, Fe2Ti, and FeTi were detected. This result demonstrates that the intermediate CaTiO3 formed in the initial period was further reduced by calcium metal and simultaneously reacted with Fe to form Fe2Ti and FeTi via reactions 8 and 9. In this period, CaTiO3 could be also reduced by Ca and combined with Fe2Ti to form FeTi through reaction 10. When reaction time reached 24 h, the major phase was FeTi and the minor phase was Fe2Ti. This shows that at 973 K and definite cell voltages of greater than 3.2 V, the ferrotitanium alloys can be obtained from ilmenite by the electrolysis-assisted calciothermic reduction process if the reaction time is long enough.
The by-product CaO produced in reactions 8, 9, and 10 was not detected in all the reduced mixtures. The reason is that CaO produced during the reduction process would be re-dissolved in the CaCl2-NaCl molten salt and then electrochemically reduced to calcium metal as a reductant for reactions 8–10 circularly. In addition, Na containing oxides was also not observed in all the reduced mixtures, implying that sodium had no contributions to the electrolysis-assisted calciothermic reduction process of ilmenite.
Backscatter Electron Morphology
The mixtures partially reduced with cell voltages of 3.6 and 4.4 V for 6 h at 973 K were polished and then examined with SEM and EDS. The backscatter electron (BSE) images and EDS identified phases of the samples are shown in Fig. 6, where a1, a2, and a3 represent BSE images of the sample with 3.6 V and b1, b2, and b3 represent those with 4.4 V. As shown in Fig. 6, the mixtures partially reduced were mainly composed of two phases, i.e., Fe- and CaTiO3-enriched phases. In the mixtures reduced with 3.6 V for 6 h at 973 K, the composition of the Fe-enriched phase (white) was 75.0 mass% Fe, 14.9 mass% Ti, and 10.1 mass% Ca, and that of CaTiO3-enriched phase (light gray) was 4.1 mass% Fe, 33.9 mass% Ti, 31.5 mass% Ca, and 30.5 mass% O in accordance with EDS analysis. In the mixtures reduced with 4.4 V for 6 h at 973 K, the composition of the Fe-enriched phase was 97.6 mass% Fe, 1.3 mass% Ti, and 1.1 mass% Ca, whereas that of the CaTiO3-enriched phase was 2.6 mass% Fe, 35.5 mass% Ti, 31.7 mass% Ca, and 30.2 mass% O. When the composition of the two samples was compared, it was found that the mass fraction of Fe and Ti in the mixture reduced with 4.4 V was higher than that with 3.6 V, which further indicated that higher cell voltages were favored to the electrolysis-assisted calciothermic reduction process of ilmenite.
Figure 6 also showed that the Fe-enriched phase with a reticular structure was distributed in the CaTiO3 matrix, which had a cubic microstructure (as shown in Fig. 6a3). In addition, an amount of pores (black) and some residual CaCl2 (Fig. 6b3) were also observed in the BSE images. These pores play an important role in the transport of [Ca] and [CaO] within the pellets during the reduction process. In the calciothermic reduction process of ilmenite, the CaCl2-NaCl molten salt could penetrate into the pellets through these pores and the calcium metal dissolved in the molten salt would be able to react with FeTiO3 or CaTiO3 throughout the pellets. In the initial period of reaction, FeTiO3 is preferentially reduced to Fe and CaTiO3. As a result, an Fe-enriched phase with a reticular structure and a CaTiO3-enriched phase as a matrix were formed in the whole pellets. With the proceeding of the calciothermic reduction process, CaTiO3 within the pellets was further reduced by Ca to form ferrotitanium alloys in the presence of an Fe-enriched phase according to heterogeneous reactions 8–10. At the same time, the by-product CaO produced in reactions 8–10 returned from the reaction interface to the bulk molten salt through these pores.
Theoretical Power Consumption Comparison of the Calciothermic Reduction with the Aluminothermic Reduction
The heat to maintain the reaction bath at the desired temperature is supplied by the energy released in the electrolytic zone in both the calciothermic and aluminothermic processes. However, in the calciothermic process, as the electrolytic zone and the reduction zone are designed in combination in a single bath, the energy released from the exothermic reduction of FeTiO3 can produce additional heat. The carbon anode consumption and CO2 emission must also be considered in the energetic balance. According to this analysis, the theoretical electric power requirement in the calciothermic process is lower than that in the aluminothermic process, as shown in Table IV.
Conclusion
-
(1)
Ferrotitanium alloys have been successfully prepared from ilmenite pellets by the electrolysis-assisted calciothermic reduction process with equal-molar CaCl2-NaCl molten salt as electrolyte, molybdenum rod as cathode, and graphite as anode at 973 K with cell voltages of 3.2 V to 4.4 V under inert atmosphere.
-
(2)
The dissolution-electrodeposition process of CaO offers a closed cycle for the production of metallic calcium during the electrolysis-assisted calciothermic reduction process of ilmenite. CaO as one of the reduction products can be dissolved in CaCl2-NaCl molten salt and then electrochemically reduced to calcium metal, which also dissolves in the molten salt and acts as a reductant in the reduction process of ilmenite. When the cell voltages applied are higher than the theoretical decomposition potential 3.36 V of CaCl2, however, calcium metal may also be generated by the decomposition of CaCl2. A higher cell voltage can accelerate the formation of calcium metal through electrolysis and, hence, promote the reduction of ilmenite.
-
(3)
From thermodynamics, FeTiO3 can be directly reduced by calcium metal to FeTi. However, the calciothermic reduction of FeTiO3 is a stepwise process rather than a single one. During the reduction process, FeTiO3 is first reduced by Ca to Fe and CaTiO3. The intermediate CaTiO3 is further reduced by Ca in the presence of solid Fe to form ferrotitanium alloys.
-
(4)
The pores within the ilmenite pellets play an important role in the electrolysis-assisted calciothermic reduction process because these pores provide Ca and CaO with transport path within the pellets.
-
(5)
In the calciothermic process, the single chamber elaboration for the reduction of ilmenite, in which the electrolysis and reduction process are combined homogeneously, opens the way to benefit both environment and energy consumption. This economic process provides a promising refining path for production of FeTi.
References
M. Panigrahi, P.K. Paramguru, R.C. Gupta, E. Shibata, and T. Nakamura, High Temp. Mat. Process. -Isr. 29, 495 (2010).
B. Sakintuna, F. Lamari-Darkrim, and M. Hirscher, Int. J. Hydrog. Energy 32, 1121 (2007).
M. Panigrahi, E. Shibata, A. Iizuka, and T. Nakamura, Electrochim. Acta 93, 143 (2013).
M. Panigrahi, A. Iizuka, E. Shibata, and T. Nakamura, J. Alloys Compd. 550, 545 (2013).
A.A. Francis and A.A. EI-Midany, J. Mater. Process. Technol. 199, 279 (2008).
N.J. Welham, Miner. Eng. 9, 1189 (1996).
V.M. Sokolov, V.D. Babyuk, Y.A. Zhydkov, and Y.Y. Skok, Miner. Eng. 21, 143 (2008).
G.Z. Chen, Miner. Process. Extr. Metall. (Trans. Inst. Min. Metall. C) 124, 106 (2014).
M. Hu, C. Bai, X. Liu, X.I. Lv, and J. Du, J. Min. Metall. B 47, 193 (2011).
X. Liu, M. Hu, C. Bai, and X. Lv, High Temp. Mater. Process. -Isr. 33, 377 (2014).
G.Z. Chen, D.J. Fray, and T.W. Farthing, Nature 407, 361 (2000).
R.O. Suzuki, J. Phys. Chem. Solids 66, 461 (2005).
K. Ono and R.O. Suzuki, JOM 54, 59 (2002).
T.H. Okabe, R.O. Suzuki, T. Oishi, and K. Ono, Mater. Trans. JIM 32, 485 (1991).
R.O. Suzuki and S. Fukui, Mater. Trans. 45, 1665 (2004).
A.M. Abdelkader, K.T. Kilby, A. Cox, and D.J. Fray, Chem. Rev. 113, 2863 (2013).
R.O. Suzuki and S. Inous, Metall. Mater. Trans. B 34, 277 (2003).
R.O. Suzuki, K. One, and K. Teranuma, Metall. Mater. Trans. B 34, 287 (2003).
R.O. Suzuki, JOM 59, 68 (2007).
R.O. Suzuki, M. Aizawa, and K. Ono, J. Alloys Compd. 288, 173 (1999).
M. Baba, Y. Ono, and R.O. Suzuki, J. Phys. Chem. Solids 66, 466 (2005).
T. Kikuchi, M. Yoshida, S. Matsuura, S. Natsui, E. Tsuji, H. Habazaki, and R.O. Suzuki, J. Phys. Chem. Solids 75, 1041 (2014).
J. Jia, B. Xu, B. Yang, D. Wang, and D. Liu, JOM 65, 630 (2013).
M. Peretti, JOM 61, 44 (2009).
A. Martin, D. Lambertin, J.C. Poignet, M. Allibert, G. Bourges, L. Pescayre, and J. Fouletier, JOM 55, 52 (2003).
D.A. Wenz, I. Johnson, and R.D. Wolson, J. Chem. Eng. Data 14, 250 (1969).
K.M. Axler and G.L. DePoorter, Mater. Sci. Forum 73, 19 (1991).
Acknowledgement
The authors acknowledge the financial support of the National Natural Science Foundation of China (Project Nos. 51274108 and 21263007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Hua, Y., Xu, C. et al. Preparation of Ferrotitanium from Ilmenite by Electrolysis-Assisted Calciothermic Reduction in CaCl2-NaCl Molten Salt. JOM 68, 532–539 (2016). https://doi.org/10.1007/s11837-015-1723-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1723-y


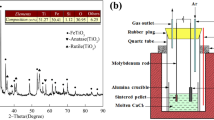




 FeTiO3
FeTiO3
 CaTiO3
CaTiO3
 Fe
Fe FeTi
FeTi Fe2Ti
Fe2Ti Fe3Ti3O)
Fe3Ti3O)
