Abstract
The effect of the content of an SiO2 additive on the electrochemical reduction of ilmenite concentrate to prepare ferrotitanium is investigated in CaCl2 molten salt. A thermodynamic analysis of the Ti-Fe system and possible reactions has been carried out, and it demonstrated that SiO2 and FeTiO3 can be readily reduced to Si and intermediates (Fe and CaTiO3), respectively. The experimental results demonstrated that the optimal molar ratio of Ti:Fe:Si in the cathode mixtures is 1.2:1:0.2. The porous ferrotitanium with uniform particle sizes were successfully obtained by electrolysis at 1173 K with a cell voltage of 3.2 V for 2 h. The SiO2 additive plays an important role during the formation process of ferrotitanium, which is first reduced to Si and then serves as a reductant to catalyze CaTiO3 and Fe to form FeTi alloys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ferrotitanium, one of the best ferroalloys, has been widely employed as hydrogen storage material or has served as a deoxidizer in the steel-making industry. Generally, ferrotitanium is produced either by aluminothermic reduction of ilmenite concentrate as well as from titanium slag, or by re-melting iron and titanium scraps together at high temperatures.1,2 However, these two methods have obvious disadvantages, such as high residual oxygen content, high impurities in products, and high energy consumption.3,4 In recent decades, many efforts have been made to modify the refinement process for the production of ferrotitanium.
The direct electrolysis of a solid oxide in molten salt, known as the Fray–Farthing–Chen Cambridge (FFC) process with the merits of short-flow course and lower energy consumption, has been applied widely since 1998.5,6 Numerous metals (e.g., Cr,7 Ti,8 Nb,9 Ta,10 Zr11), and alloys (e.g., LaNi,12 TiC/SiC,13 FeTi,14 and TiSi15) have been prepared from corresponding oxides via the FFC method. Panigrahi et al. reported the preparation of ferrotitanium alloys from the direct electrolysis of TiO2 and Fe2O3 mixtures in CaCl2 molten salt.16 However, a relative slower deoxidization rate and higher energy consumption were observed on account of the difficult reduction of TiO2 and the multi-intermediates formed, such as CaTiO3 and TiO. It has become imperative to develop an effective way to promote the reduction process. A great number of endeavors, including lowering the cathode mold pressure, and improving the cathode sintering temperature and cathode porosity, have been put into practice.11,17 As a result, shorter electrolytic times and elevated electrolytic currents have been achieved. However, the intrinsic reduction routes have not been changed. Besides, our previous work suggests that the addition of CaO into CaCl2-based molten salts can accelerate the transmission of O2− and introduce the calciothermic reduction of cathodic oxides, leading to favorable cathodic reaction kinetics.18 The only fly in the ointment could be that the CaO additive will result in the easy formation of intermediate CaTiO3, so that the subsequent reaction is difficult.19
Recently, an acid oxide, SiO2, was introduced in our work to accelerate the reduction of ilmenite. Here, the influence of the content of the SiO2 additive on the electrochemical reduction of ilmenite concentrate to prepare ferrotitanium was investigated. The thermodynamic analysis of the Ti-Fe system and possible reactions were performed by Outokumpu HSC Chemistry. A variety of techniques, including XRD, SEM, and EDS, were utilized to characterize the samples. The roles of the SiO2 additive in the reduction process have also been proposed.
Experimental
Chemicals
The chemicals of analytical grade used in present work were purchased commercially from Aisinaladdin-e.com, Shanghai, China. The x-ray diffraction (XRD) pattern of the ilmenite concentrate sample in this experiment is shown in Fig. 1a, and the main phases are FeTiO3 and TiO2. The chemical composition of the ilmenite concentrate is also given in the inset. The molar ratio of the main elements in ilmenite concentrate is Ti:Fe:Si = 1.2:1:0.076.
Preparation of Cathode
Before electrolysis, the crushed ilmenite concentrate with an average particle size of ~ 50 µm was blended with the SiO2 at different molar ratios of Ti:Fe:Si (1.2:1:0.076, 1.2:1:0.1, 1.2:1:0.2, 1.2:1:0.3, and 1.2:1:0.4). Then, the powders (1.5 g) were mixed with 10 wt.% polyvinyl alcohol and 25 wt.% NH4HCO3, and pressed into a cylindrical pellet (13 mm in diameter and 2.0 mm in thickness) under a pressure of 15 MPa. Finally, the pellets were sintered in an argon inert atmosphere at 1373 K for 4 h to obtain sufficient strength and the desired porosity.
Electrolysis Experiments
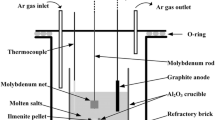
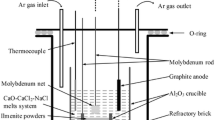
The electrolytic experiments were operated in a crucible furnace. A schematic of the experimental apparatus is shown in Fig. 1b. Before electrolysis, the sintered pellet was assembled by a molybdenum rod (99.95% in purity, 3 mm in diameter and 200 mm in length) to serve as the cathode. The graphite rod (99.9% in purity, 6 mm in diameter and 100 mm in length) tied by a molybdenum rod served as the anode. The electrolytic experiment was performed at 1173 K for 0.5–2.0 h in an Al2O3 crucible (Ф70 mm × 70 mm, 99.9% in purity) and a constant cell voltage of 3.2 V was supplied by a DPS-305BF DC voltage-stabilized power source (Zhejiang Yueqing Yizhan Electronics, China). As the furnace was heated to 1173 K, the electrodes were immersed in the CaCl2 molten salt under an inert atmosphere. After the electrolysis, the cathode pellet was cooled to room temperature in the molten salt, then washed with distilled water to remove excessive molten salt, and dried in vacuum.
Characterization Analysis
The phases of sample were analyzed by XRD (Version D/Max-2200pc model with Cu-Kα radiation) at a scan rate of 8°/min in the range of 10°–90°. The morphology and element constituents of samples were characterized by SEM equipped with EDS (LEO-FEGSEM model).
Results and Discussion
Thermodynamic Analysis
According to existing reports,18,19 the electrochemical reduction of ilmenite concentrate is a complex process which involves the formation of intermediates, such as Fe, CaTiO3, TiO, et al. It can be seen from the Ti-Fe binary phase diagram (Fig. 2a) that ferrotitanium alloys (Fe2Ti and FeTi) exist in this system.20 As a result, Fe2Ti and FeTi can be formed during the electrolysis of FeTiO3. When SiO2 is added into the system, the reduction process will be more complicated. Here, possible reactions during the electrochemical reduction of FeTiO3 and TiO2 are illustrated in Table I. The relationships between the standard Gibbs free energy, \( \Delta G_{T}^{\theta } \), the theoretical decomposition voltage, Eθ, and temperature, T, have been calculated by HSC Chemistry 6.0, as presented in Fig. 2b. Based on the calculation, this demonstrates that the theoretical decomposition voltage, Eθ, of CaCl2 (3.46 V at 1173 K) is the highest, implying that all the oxides can be reduced before the decomposition of the electrolyte. The Gibbs free energy values, \( \Delta G_{T}^{\theta } \), of reactions (3) and (4) are negative at 1173 K, and thus FeTiO3 can be easily reduced to Fe and TiO2 or CaTiO3. However, the Gibbs free energy change of reaction (4) is more negative than that of reaction (3), suggesting that FeTiO3 can be easily reduced to Fe and CaTiO3 via reaction (4).
From the calculation results, the decomposition voltage Eθ of reaction (5) is 0.79 V, indicating that SiO2 can be readily reduced to Si. Moreover, the value of the theoretical decomposition voltage, Eθ, of reaction (5) is much lower than that of reactions (8) and (10), which implies that Si is preferentially formed before the reduction of CaTiO3. Looking further at the thermodynamic calculations, comparing reactions (8–11), CaTiO3 can be reduced to ferrotitanium alloys via reactions (9) and (11) due to their low theoretical decomposition voltages, Eθ. In addition, the theoretical decomposition voltage, Eθ, of reaction (7) is much lower than that of reaction (6), which means that the reduction of TiO2 is significantly promoted by the formation of Si. Therefore, the participant of SiO2 is thermodynamically favorable for the formation of ferrotitanium from ilmenite concentrate.
Time–Current Curves
The current as a function of time was recorded during the electrolysis of ilmenite concentrate with different SiO2 contents, as shown in Fig. 3. All the values of the current decrease remarkably in the initial 30 min and then decay slightly later. The decline of the current value reflects the change of the reaction rate. At the beginning of electrolysis, the rapid reduction rate is put down to the large three-phase reaction interface between the cathodic oxide reactant, the metallic product, and the molten salt. As the reaction is performed inside the pellet, the reduction rate becomes slow because of the lessened three-phase interface.
In addition, from Fig. 3, it can be seen that the increase of SiO2 content has a significant effect on the current values. In the initial 10 min, the current value decreases remarkably when the SiO2 content is high. For example, the current value at 10 min decreases from 1.8 A to 1.76 A, 1.7 A, 1.55 A, and 1.45 A as the Ti:Fe:Si molar ratio varies from 1.2:1:0.076 to 1.2:1:0.1, 1.2:1:0.2, 1.2:1:0.3, and 1.2:1:0.4, respectively. However, in the subsequent electrolysis from 20 min to 120 min, the current values reduce slightly at high SiO2 content. For instance, the current values at 60 min increase from 0.63 A to 0.75 A, 1.0 A, 1.13 A, and 1.83 A, respectively. According to thermodynamic analysis mentioned above, FeTiO3 will be preferentially reduced to Fe and CaTiO3 via reaction (4), and the intermediate CaTiO3 will be deoxidized via reactions (8–11). Thus, the electrochemical reduction of ilmenite concentrate is suggested to be divided into two steps. FeTiO3 is first reduced in the initial period and then CaTiO3 is deoxidized. Moreover, it should be pointed out that the addition of SiO2 into the cathode pellet might reduce the effective interface area of FeTiO3 as the cathode weight is a constant, and the electrolysis of SiO2 will consume more electricity. As a result, the reduction rate of FeTiO3 decreases with an increase in the SiO2 content of the cathode in the initial period. In contrast, with the participation of SiO2, the further reduction of CaTiO3 can be enhanced in thermodynamics, leading to the increase of the current value.
Phase Characterization
The ilmenite concentrate with different SiO2 contents was electrochemically reduced at 3.2 V and 1173 K for 0.5 h, 1 h, and 2 h, respectively. The XRD patterns of the products are illustrated in Fig. 4 and the observed phases are listed in Table II.
As shown in Fig. 4 and Table II, when the samples are electrolyzed for 0.5 h, the FeTiO3 can be completely reduced to form the dominant phases, Fe-Ti-O and CaTiO3, and the minor phases, FeTi, Fe2Ti, and SiO2. This result reveals that FeTiO3 can be preferentially reduced to intermediates Fe-Ti-O and CaTiO3 in the initial period of 0.5 h. Looking further at the results in Fig. 4a and Table II, a small amount of SiO2 can be detected, except in the sample with the Ti:Fe:Si molar ratio of 1.2:1:0.076, indicating that the SiO2 is stable. Therefore, this suggests that the addition of SiO2 has little impact on the initial electrochemical conversion of FeTiO3 to CaTiO3 and Fe via reaction (4). After 1 h (Fig. 4b), Si is observed in the product, which implies that SiO2 can be reduced to Si at the middle stage from 0.5 h to 1 h. Meanwhile, the relative intensity of CaTiO3 decreases with the increase of SiO2 content. When the molar ratio of Si is higher than 0.3, CaTiO3 is completely electrolyzed because the dominant phase is Fe-Ti-O, and the minor phases include FeTi, Fe2Ti, and Si. This result shows that the further reduction process of CaTiO3 can be accelerated by the addition of SiO2, which is in good agreement with the thermodynamic analysis in “Thermodynamic Analysis” section.
When the electrolytic time is prolonged to 2 h (Fig. 4c), a small amount of CaTiO3 is detected in the sample with the Ti:Fe:Si molar ratio of 1.2:1:0.076. However, the residual CaTiO3 has been completely deoxidized to produce the dominant FeTi phase and other minor phases such as Fe2Ti, Fe-Ti-O, TiSi, Ti5Si3, and TiSi2 as the SiO2 content increased. This means that the addition of SiO2 can enhance the reduction process of CaTiO3 and so plays a positive role in the electrolysis process. In addition, a small number of Ti-Si (TiSi, Ti5Si3, and TiSi2) by-products are also formed with the addition of SiO2. This may lead to a higher content of silicon impurities in the ferrotitanium. Thus, it is necessary to optimize the addition of SiO2 content. Based on present research, the optimal Ti:Fe:Si molar ratio in the samples is 1.2:1:0.2, because ilmenite can be completely reduced to ferrotitanium alloys (FeTi, Fe2Ti) for 2 h, as shown in Table II.
Micrograph of Products
The SEM images of the ilmenite concentrate and the products obtained after electrolysis of the samples with different SiO2 contents under constant cell voltage of 3.2 V at 1173 K for 2 h are shown in Fig. 5. The ilmenite concentrate powders are of dense blocky-shaped particles with inhomogeneous structures in a size of about 2–10 μm (Fig. 5a). After electrolysis, products with a porous structure are obtained, as illustrated in Fig. 5b–f. In addition, it can be seen that the size of the newly formed particles decreases with the increase of SiO2 content. Therefore, with the addition of SiO2 into the sample, the ilmenite concentrate can be constantly electrolyzed to produce ferrotitanium powder with a smaller particle size. In addition, the products obtained after electrolysis of the sample with the Ti:Fe:Si molar ratio of 1.2:1:0.2 was further analyzed by EDS, as shown in Fig. 5g. It can be seen that the obtained particles are composed of the enriched elements, Fe and Ti, and that the Si content is much lower. Moreover, the Si is dispersedthroughout these particles, which mainly include FeTi.
Role of SiO2 Additive
Based on the above analysis, the preparation of FeTi from ilmenite concentrate by electrochemical reduction in CaCl2 molten salt is a complex multi-step process. First, FeTiO3 is preferentially reduced to intermediates Fe and CaTiO3 via reaction (4). In the second step, the intermediate CaTiO3 is further reduced to ferrotitanium alloys through reactions (8) and (10). The further reduction of CaTiO3 can be accelerated via reactions (9) and (11) with the addition of SiO2, which demonstrates that the addition of SiO2 into the sample during the electrochemical process can significantly promote the reduction of the intermediate CaTiO3. The detailed roles of SiO2 in the electrochemical reduction process can be proposed, as shown in Fig. 6. SiO2 is first electrolyzed to produce Si, and the newly formed Si will act as the reductant to reduce CaTiO3. Finally, the reduced Ti and Fe form FeTi alloys.
Conclusion
Ferrotitanium alloys were successfully prepared from ilmenite concentrate by electrochemical reduction with the addition of SiO2 in CaCl2 molten salt. SiO2 plays an important role during the reduction process, and the optimal molar ratio in the cathode mixtures is Ti:Fe:Si = 1.2:1:0.2. Thermodynamic analysis of the Ti-Fe system and possible reactions indicate that SiO2 can be readily reduced to Si, and that FeTiO3 can be first transformed into intermediates, such as Fe and CaTiO3. Electrolysis experiments demonstrated that SiO2 is first reduced to Si and then serves as the reductant to participate in the reduction of CaTiO3 and Fe to form FeTi alloys. The further reduction process of CaTiO3 is promoted by the increase of SiO2 content. The micromorphology of the ferrotitanium exhibits a porous structure and uniform particle size with the addition of SiO2 into the ilmenite concentrate.
References
M. Panigrahi, R.K. Paramguru, R.C. Gupta, E. Shibata, and T. Nakamura, High Temp. Mater. Processes 29, 495 (2010).
M. Panigrahi, A. Iizuka, E. Shibata, and T. Nakamura, J. Alloys Compd. 550, 545 (2013).
B. Wang, X. Lan, X. Zhao, and X. Wu, Chin. J. Rare Met. 30, 671 (2006).
G. Shenghui, P. Jinhui, Z. Shiming, F. Xingxiang, and Z. Libo, Light Met. 11, 51 (2002).
G.Z. Chen, D.J. Fray, and T.W. Farthing, Nature 407, 361 (2000).
G.Z. Chen, D.J. Fray, and T.W. Farthing, Metall. Mater. Trans. B 32, 1041 (2001).
Z.W. Liu, H.L. Zhang, L.L. Pei, Y.L. Shi, Z.H. Cai, H.-B. Xu, and Y. Zhang, Trans. Nonferrous Met. Soc. China 28, 376 (2018).
P. Lai, T. Ma, and M. Hu, JOM 71, 1033 (2019).
Q. Song, X. Qian, J. Meng, T. Lou, Z. Ning, Q. Yang, and Y. Kai, J. Alloys Compd. 647, 245 (2015).
Q. Song, Q. Xu, X. Kang, J. Du, and Z. Xi, J. Alloys Compd. 490, 241 (2010).
K.S. Mohandas and D.J. Fray, Metall. Mater. Trans. B 40, 685 (2009).
Z. Yong, D. Wang, M. Meng, X. Hu, X. Jin, and G.Z. Chen, Chem. Commun. 24, 2515 (2007).
K. Chen, Y. Hua, C. Xu, Q. Zhang, C. Qi, and Y. Jie, Ceram. Int. 41, 11428 (2015).
C. Qi, Y. Hua, K. Chen, Y. Jie, Z. Zhou, J. Ru, L. Xiong, and K. Gong, JOM 68, 668 (2016).
K. Zheng, X. Zou, X. Xie, C. Lu, C. Chen, Q. Xu, and X. Lu, JOM 70, 138 (2018).
M. Panigrahi, E. Shibata, A. Iizuka, and T. Nakamura, Electrochim. Acta 93, 143 (2013).
J. Du, Z. Xi, Q. Li, Q. Xu, Y. Tang, and Z. Li, Trans. Nonferrous Met. Soc. China 17, s514 (2007).
L. Xiong, Y. Hua, C. Xu, J. Li, Y. Li, Q. Zhang, Z. Zhou, Y. Zhang, and J. Ru, J. Alloys Compd. 676, 383 (2016).
X. Lu, X. Zou, C. Li, Q. Zhong, W. Ding, and Z. Zhou, Metall. Mater. Trans. B 43, 503 (2012).
Z. Zhou, Y. Zhang, Y. Hua, C. Xu, P. Dong, Q. Zhang, and D. Wang, JOM 70, 575 (2018).
Acknowledgement
The authors acknowledge the financial support of the National Natural Science Foundation of China (Project Nos. 51604136, 51274108).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ai, G., Hua, Y., Xu, C. et al. Roles of SiO2 Additive on Preparation of Ferrotitanium from Ilmenite Concentrate by Electrochemical Reduction in CaCl2 Molten Salt. JOM 72, 3836–3842 (2020). https://doi.org/10.1007/s11837-020-04366-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-020-04366-3