Abstract
Ferrotitanium alloy is prepared by electrochemical reduction from ilmenite in LiCl-KCl and LiCl-KCl-CaCl2 molten salts, respectively. The products prepared are observed by x-ray diffraction (XRD). It is shown that Fe2Ti can be prepared from ilmenite in LiCl–KCl molten salt at 1073 K with a cell voltage of 3.2 V. Ilmenite can be electrochemically reduced to FeTi in LiCl-KCl-CaCl2 molten salt under the same condition. It is indicated that CaCl2 can promote the reaction and is favors the deoxidization of the FeTiO3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ferrotitanium alloy is not only a significant material in steel smelting which can improve the quality of steel but also an important magnetic material and one of the most promising hydrogen storage materials. It is used widely in various industries. Nowadays, the ferrotitanium alloy is mainly produced by the aluminothermic reduction method and the remelting process. But with the former method, the ferrotitanium alloy has a high content of oxygen and impurities. Although the content of oxygen in the alloy can be controlled effectively by the remelting process, its raw materials are limited and energy consumption is high. Therefore, it is urgently required to develop a new process to produce ferrotitanium alloy with energy saving and low cost.
A new method, the cathodic electrochemical reduction of TiO2 to Ti, which is also called the FFC (Fray–Farthing–Chen) Cambridge process,1,2 is a simple process with low energy consumption. Based on the FFC Cambridge process, various reactive metals (e.g., Ti, Si, Cr, Nb, Dy)2–6 and many functional alloys (e.g., Fe-Ti, La-Ni, Ce-Ni-Cu, Zr-Cr-Ni)7–11 have been extracted from their oxides. The reduction of metal oxides in situ has been studied in various molten salts, e.g., CaCl2, CaCl2–NaCl, LiCl–KCl and LiCl–KCl–CaCl2 5,9,12–17 molten salts. The ferrotitanium alloy has been successfully obtained by the electro-reduction of ilmenite in CaCl2 and CaCl2–NaCl molten salt. However, the electro-reduction of ilmenite in LiCl–KCl has not been reported. In the present study, we used LiCl–KCl as the supporting electrolyte, the eutectic point (625 K) of which is lower than those of CaCl2 (1045 K) and CaCl2–NaCl (777 K), to prepare ferrotitanium alloy from ilmenite.
The electrolysis experiments were performed with a two-electrode system consisting of an ilmenite cathode and a graphite anode. A constant voltage applied between the cathode and anode was lower than the theoretical decomposition potential of LiCl–KCl but higher than the theoretical decomposition potential of ilmenite. This work demonstrates that the electro-reduction of ilmenite in LiCl–KCl is available. The electro-reduction process of ilmenite was studied by constant voltage electrolysis. For comparison, some CaCl2 was added to LiCl–KCl molten salt. It is shown that the electro-reduction of ilmenite can be accelerated by the addition of CaCl2 into LiCl–KCl molten salt.
Experimental
Chemical Reagents
According to the procedures reported in the literature,18 the ilmenite (FeTiO3) powder used in this work was synthesized by the co-precipitation method using Ti(O-Bu)4 (99.9%; Aldrich), FeCl2·4H2O (99.9%; Aldrich) and Na2CO3 (99.9%; Aldrich) as raw materials.
Anhydrous LiCl (AR) and KCl (AR) were supplied by Sinopharm Chemical Reagent, China. The LiCl and KCl were dehydrated under 573 K for 24 h and then kept in an argon glove box prior to use. The electrolyte (the molar ratio of LiCl to KCl was 0.6:0.4) was prepared by mixing appropriate amounts of LiCl and KCl.
Preparation of FeTiO3 Electrodes
The FeTiO3 powder was manually pressed (20 MPa) into small cylindrical pellets (13 mm in diameter with thickness ranging from 2.0 mm to 3.0 mm) mixed with 10 wt.% polyvinyl alcohol (PVA) and 15 wt.% NH4HCO3. The FeTiO3 pellets were sintered at 1073 K for 3 h in an argon flow to gain sufficient strength and high porosity. A typical program used in this work was as follows: (1) heating from room temperature to 573 K at 5 K/min and holding at 573 K for 1 h, (2) heating from 573 K to 1123 K at 6 K/min and holding at 1123 K for 3 h. The programmed heating is aimed at promoting dehydration and pore-forming. The porosity of the sintered pellets ranged from 30% to 40% by volume. The sintered ilmenite pellets were used as cathodes, which were wrapped tightly with molybdenum wire and connected to a DC power source.
Electrochemical Experiments
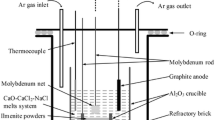
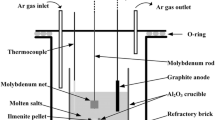
Electrolysis experiments were performed in a crucible furnace with a standard two-electrode system. The experimental apparatus is shown in Fig. 1.
A molybdenum bar loaded with a sintered FeTiO3 pellet acted as the cathode and a graphite rod (Φ5 × 80 mm, 99.9%) served as the anode. The electrolysis was conducted in an alumina crucible (Φ60 × 60 mm, 99.9%) at a constant cell voltage of 3.2 V in the temperature range of 673 K to 1073 K under Ar atmosphere. The distance of the inter-electrode was 2 cm. The constant cell voltage was controlled by a DC power source (Zhejiang Yueqing Yizhan Electronics, China). When the temperature reached the melting point of the electrolyte used, the electrodes (cathode and anode) were lowered into the molten salt. After electrolysis, the pellet was raised and cooled in Ar flow. All the electrochemical experiments were operated under dry high-purity argon atmosphere with a flow of 200 ml/min. The reduced pellets were manually ground into powders and then cleaned with distilled water in an ultrasonic cleaner to remove the residual molten salt in the reduced samples. The samples were analyzed after dried in vacuum at 353 K.
The surface morphology and the phase composition of the reduced pellets were observed with a scanning electron microscope (SEM) and x-ray diffraction (XRD), respectively. The samples were analyzed by XRD (D/Max-2200 model) with Cu-Kα radiation at a scan rate of 10°/min in the range of 10°–90°. SEM (XL 30 ESEM TMP model) was used to characterize the morphology.
Results and Discussion
Theoretical Considerations
To determine the thermodynamics of the electrolysis process, the theoretical decomposition potentials of the possible reactions are calculated from Eq. 1.
\( \Delta E_{{_{\text{T}} }}^{\theta } \) theoretical decomposition potential;
\( \Delta G_{{_{\text{T}} }}^{\theta } \) standard Gibbs free energy;
n transferred number of electrons;
F Faraday constant.
\( \Delta G_{{_{\text{T}} }}^{\theta } \) is obtained from the known thermodynamic data at different temperature in HSC chemistry 6.0. The Gibbs free energy \( \Delta G_{{_{\text{T}} }}^{\theta } \) of the possible reactions is shown in Table I. According to Table I and Eq. 1, the theoretical decomposition potentials of pure LiCl, KCl and CaCl2, and the theoretical electro-reduction potentials of the possible reactions to produce metal or ferrotitanium alloy at cathode and CO gas at graphite anode were calculated, as shown in Fig. 2.
Table I reveals that the potentials and decrease gradually with temperature. For each reaction, the electrochemical potentials are lower by 0.2–0.4 V at 1073 K than those at 673 K. This suggests that the reaction becomes easier at a higher temperature.
From Fig. 2, it can be seen that the theoretical decomposition potential of CaCl2 is lower than that of LiCl and KCl, and that the theoretical decomposition potentials of the molten salt electrolytes are much higher than those of the metal oxides.
Both in LiCl-KCl and LiCl-KCl-CaCl2 molten salts, the potential for the reduction of ilmenite to metallic Fe is the lowest. This is in agreement with the previous study that metallic Fe is the easiest to obtain from FeTiO3. 19,20 From the Gibbs free energy changes for reactions (14), (15) and (16), it can be seen that the reduction of iron from ilmenite with participation of Li2O and CaO is thermodynamically more favorable than without them because reactions (15) and (16) require lower potentials compared to reaction (14). Li2O and CaO are normally formed by Ca2+ and Li+ with O2− dissolved in the molten salt during the deoxidation of oxides. In addition, reactions (15) and (16) can proceed spontaneously at 673 K, while reaction (14) cannot be achieved until the temperature rises to 1073 K.
According to the theoretical electrochemical potentials of reactions (7), (9), (10) and (12) in Fig. 2, it is revealed that the formation of Fe2Ti requires a lower potential than that of FeTi. Hence, Fe2Ti rather than FeTi would be preferentially formed at low temperatures.
No titanium oxides were observed in the products in the present experiments. Li2TiO3 and CaTiO3 are the major oxide phases formed during the electrolysis. Reactions (15) and (16) are responsible for the formation of CaTiO3 and Li2TiO3. The electro-reduction potential of CaTiO3 is 0.25 V lower than that of Li2TiO3. This indicates that CaTiO3 is easier to reduce than Li2TiO3. Therefore, the deoxidation of ilmenite in LiCl-KCl-CaCl2 ternary molten salt is easier than that in the LiCl-KCl binary system.
Influence of Temperature
To confirm the influence of the temperature on the phase changes occurring during the electro-reduction process in LiCl-KCl molten salt, experiments were performed at different temperatures (673 K, 873 K and 1073 K) with a constant cell voltage of 3.2 V.
The current–time plots during the reduction process of ilmenite pellets at different temperatures for 28 h are illustrated in Fig. 3. This indicates that a higher temperature can contribute to a faster reduction rate. In addition, it is shown that all the current curves have the similar features. The current rises rapidly in the first 2–3 min to a peak. After 0.4 h electrolysis, the current declines gradually to a stable level until the electrolysis is terminated.
The XRD patterns of synthetic FeTiO3 and partially reduced samples obtained from electrolysis at different temperatures for 20 h are shown in Fig. 4 and the phase compositions detected are shown in Table II, and are arranged in decreasing order of relative diffraction peak intensities.
It can be seen from Fig. 4 and Table II that Fe and Li2TiO3 are the main phases in the products at 673 K, which indicates that metallic Fe can be easily reduced from FeTiO3. Li2TiO3 is the only oxide phase, whereas phases of TiO2, titanium sub-oxides and titanium metal are not detected in the products at 673 K and 873 K. Chemical reaction (16) in Table I may be responsible for the formation of Li2TiO3.
The main phases have no significant changes at 873 K compared with that at 673 K. No ferrotitanium alloy is observed at 673 K and 873 K, which implies that the ferrotitanium alloy cannot be obtained from FeTiO3 at low temperatures. When the temperature rises to 1073 K, Fe2Ti and TiO are the main phases. This suggests that some Li2TiO3 are reduced to TiO. Thus, the current is higher than those at 673 K and 873 K. Ferrotitanium alloy cannot be prepared from FeTiO3 by electro-reduction until the temperature is as high as 1073 K. There is no metallic Ti observed in the products, since metallic Fe can combine with Ti to form ferrotitanium alloy (Fe2Ti) as soon as the metallic Ti is reduced from TiO.
The experiments suggest that Li2TiO3 cannot be reduced to titanium sub-oxide or metallic Ti when the temperature is low. High temperature favors the reduction of Li2TiO3. From the experiments, we can conjecture that the reduction of Li2TiO3 and TiO is the limiting step for the forming of ferrotitanium alloy in LiCl-KCl.
According to Figs. 3 and 4, it can be predicted that the reduction stages are probably explained as follows: (1) Fe is firstly produced; thus, the electrical conductivity of the whole pellet was improved and the current increases to a peak; and (2) with the time increasing, the current declines gradually to a stable level. This indicates that the reactions became difficult.12,21–23 This is in agreement with the fact that Li2TiO3 and TiO are difficult to reduce, as shown in Fig. 2.
The Influence of Reaction Time
The measured XRD patterns of the synthetic FeTiO3 and partially reduced samples are illustrated in Fig. 5b–e and the corresponding phase compositions are shown in Table III.
As shown in Fig. 5b and Table III, the sample is mainly composed of Fe and Li2TiO3 after being electrolyzed for 20 min. This indicates that the reduction rate of iron from ilmenite is rapid. The sample electrolyzed after 3 h is composed of Fe and Li2TiO3. With the time increasing to 12 h, Li2TiO3 can be reduced to TiO and Fe2Ti is obtained. There is still an amount of metallic Fe in the products. After 20 h, TiO and Fe2Ti are the prevailing phases.
For all the products obtained from the interrupted experiments, the electro-reduction of ilmenite can be divided into the following steps: Fe is firstly produced with the removal of amounts of oxygen, which makes the FeTiO3 pellet become a highly conducting cathode. Secondly, the oxygen removed from FeTiO3 pellet dissolves in the molten salt. As more and more O2− dissolves in the molten salt, it may react with Li+ and TiO2 to form Li2TiO3. In the further electrolysis process, Li2TiO3 will be deoxidized to form titanium sub-oxide, TiO. At the same time, Li+ ions are liberated from the Li2TiO3. Finally, metallic titanium is reduced from the TiO and combines with metallic Fe to form ferrotitanium alloy.
The Influence of Ca2+
There is no FeTi obtained in the KCl-LiCl molten salt; however, FeTi has been successfully prepared in CaCl2-containing molten salts (CaCl2 and CaCl2-NaCl molten salts). To investigate the influence of the Ca2+ on the electro-reduction of ilmenite, some CaCl2 was added into the KCl-LiCl molten salt. The synthetic FeTiO3 was electrolyzed in KCl-LiCl-CaCl2 (0.45:0.30:0.25) molten salt for various times. XRD patterns of the synthetic FeTiO3 and the products reduced by electrolysis in KCl-LiCl-CaCl2 molten salt with different times at 3.2 V are shown in Fig. 6. The main phase compositions are showed in Table IV, and are arranged in decreasing order of relative diffraction peak intensities. The experiments indicate that the deoxidation rate of oxides in KCl-LiCl-CaCl2 molten salt is faster than that without CaCl2, suggesting that CaCl2 plays an important role in the electro-reduction.
As shown in Fig. 6 and Table IV, Fe is first obtained in 10 min. CaTiO3 and Li2TiO3 are intermediate products during the electrolysis. In addition, CaCO3 is also detected in the products. This may be attributed to the fact that CO2 can dissolve in KCl-LiCl-CaCl2 molten salt as CO3 2−, which can react with the Ca2+ present in the molten salt to form CaCO3.
With time increasing to 1 h, the phases observed in the sample includes Li2TiO3, CaCO3, CaTiO3 and TiO, as shown in Fig. 6b. As compared with Fig. 6a, the relative diffraction peak intensity of Li2TiO3 and CaCO3 in Fig. 6b increases and that of CaTiO3 decreases. This indicates that Li2TiO3 is more difficult to reduce than CaTiO3. TiO observed in the sample may result from the reduction of CaTiO3.
After 5 h, FeTi and Fe2Ti are present in the samples. This result indicates that TiO has been reduced to titanium metal, which reacts with metallic Fe to form ferrotitanium alloy. In addition, the relative diffraction peak intensity of CaCO3 increases.
By 12 h, FeTi and Fe2Ti are the main phases and Li2TiO3 disappears. There is a little TiO and CaCO3. The XRD analysis shows that the FeTiO3 is almost completely reduced to FeTi after electrolysis for 20 h in KCl-LiCl-CaCl2 molten salt.
The influence of Ca2+ on the electrochemical reduction process of ilmenite is related to its effect on the solubility of O2− in the chloride molten salts. The solubility of O2− in different molten salts is given in Table V, where T is the absolute temperature and \( X_{{{\text{O}}^{{2\text{ - }}} }} \) is the mol fraction of O2− in molten salt.
It can be seen from Table V that the solubility of O2− in LiCl-KCl molten salt is much lower than that of CaCl2-containing ones. The solubility of O2− appreciably increases with an increase in the CaCl2 mol fraction of the molten salts. Hence, the addition of CaCl2 into LiCl-KCl molten salt will result in a high concentration of O2− in the salt, which is beneficial to the transportation and discharge of O2−. Accordingly, the electrochemical reduction rate of ilmenite will be accelerated due to the presence of CaCl2 in LiCl-KCl molten salt.
In addition, the metallic Ca may be deposited from Ca2+ in the electrolyte on the cathode and dissolved in the electrolyte during the electrolysis process. The metallic calcium dissolved may also reduce the titanium oxides in the cathode and promote the overall electrochemical reduction of the ilmenite.
Micrograph Analysis
The micrograph of reduced sample obtained after electrolysis for 20 h in KCl-LiCl-CaCl2 molten salt is shown in Fig. 7. It can be seen that the particles of the reduced product are uniformly shaped. This shows that the reduced product is porous.
Conclusion
In the current work, a study was carried out on the electro-reduction of synthetic FeTiO3 in LiCl-KCl and LiCl-KCl-CaCl2 molten salts at a voltage of 3.2 V. The study has shown that metallic Fe is obtained at low temperatures and Fe2Ti is obtained at 1073 K. FeTi can be prepared in LiCl-KCl-CaCl2 molten salt under the same conditions.
From the interrupt experiments both in LiCl-KCl and LiCl-KCl-CaCl2 molten salts, the electro-reduction process can be included as follows. According to XRD patterns, Fe(II) in ilmenite is firstly reduced to metallic Fe. Then, the intermediate Li2TiO3 or CaTiO3 is reduced to TiO. Finally, metallic Fe combines with Ti partially reduced from TiO to form Fe2Ti or FeTi.
The electrochemical reduction rate of ilmenite in LiCl-KCl-CaCl2 molten salt is faster than that in LiCl-KCl, which indicates that the Ca2+ in the electrolyte favors the electro-reduction of ilmenite. The influence of Ca2+ can be drawn from the present work: (1) the electro-reduction of intermediate CaTiO3 is much easier than that of Li2TiO3; (2) Ca2+ contributes to the dissolution of O2− in molten slat and is beneficial to the removal of O2−; and (3) Ca2+ may be reduced to metallic Ca, which will cause the calcium thermal reduction of oxides and thus promote the electro-reduction of the ilmenite.
References
D.J. Fray, T.W. Farthing, G.Z. Chen, World Patent WO9964638.
G.Z. Chen, D.J. Fray, and T.M. Farthing, Nature 407, 361 (2000).
T. Nohira, K. Yasuda, and Y. Ito, Nat. Mater. 2, 397 (2003).
G.Z. Chen, E. Gordo, and D.J. Fray, Metall. Mater. Trans. B 35, 223 (2004).
X.Y. Yan and D.J. Fray, Metall. Mater. Trans. B 33, 685 (2002).
P. Kim, H.W. Xie, Y.H. Zhai, X.Y. Zou, and X.C. Lang, Appl. Electrochem. 42, 257 (2012).
M. Panigrahi, R. Paramguru, R. Gupta, E. Shibata, and T. Nakamura, High Temp. Mater. Process. 29, 495 (2010).
M. Panigrahi, E. Shibata, A. Iizuka, and T. Nakamura, Electrochim. Acta 93, 143 (2013).
Y. Zhu, D.H. Wang, X.H. Hu, X.B. Jin, and G.Z. Chen, Chem. Commun. 24, 2515 (2007).
X. Kang, Q. Xu, X.M. Yang, and Q.S. Song, Mater. Lett. 64, 2258 (2010).
J.J. Peng, Y. Zhu, D.H. Wang, X.B. Jin, and G.Z. Chen, J. Mater. Chem. 19, 2803 (2009).
W. Bin, K.R. Liu, and J.S. Chen, Trans. Nonferrous Met. Soc. China 21, 2327 (2011).
M. Ma, D.H. Wang, W.G. Wang, X.H. Hu, X.B. Jin, and G.Z. Chen, Alloys Compd. 420, 37 (2006).
K. Yasuda, T. Nohira, Y.H. Ogata, and Y. Ito, Electrochem. Soc. 152, D208 (2005).
Y.C. Zhai, P. Kim, H.W. Xie, and S.H. Ji, Adv. Mater. Res. 284, 2114 (2011).
Y. Katayama, B. Friedrich, in Proceedings-206th Meeting of The Electrochemical Society, Honolulu, American (2004).
K. Yasuda, T. Nohira, R. Hagiwara, and Y.H. Ogata, Electrochim. Acta 53, 106 (2007).
J.J. Ru, Y.X. Hua, C.Y. Xu, J. Li, Y. Li, D. Wang, K. Gong, R. Wang, and Z.R. Zhou, Ceram. Int. 40, 6799 (2014).
A.M. Abdelkader, K.T. Kilby, A. Cox, and D.J. Fray, Chem. Rev. 113, 2863 (2013).
M. Ma, D.H. Wang, W.G. Wang, X.H. Hu, X.B. Jin, and G.Z. Chen, Chemistry 12, 5075 (2006).
S.L. Wang and F.S. Zhang, J. Northeast. Univ. 31, 92 (2010).
M. Panigrahi, A. Lizuka, E. Shibata, and T. Nakamura, Alloys Compd. 550, 545 (2013).
P.K. Tripathy, M. Gauthier, and D.J. Fray, Metall. Mater. Trans. B 38, 893 (2007).
Y. Kado, T. Goto, and R. Hagiwara, J. Chem. Eng. Data 53, 2816 (2008).
T. Goto, Y. Kado, R. Hagiwara. Molten Salts Chem., p.187 (2013).
Acknowledgements
The authors acknowledge gratefully the financial support from the National Natural Science Foundation of China (Grant Nos. 51274108, 21263007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, Cc., Hua, Yx., Chen, Kh. et al. Preparation of Ferrotitanium Alloy from Ilmenite by Electrochemical Reduction in Chloride Molten Salts. JOM 68, 668–674 (2016). https://doi.org/10.1007/s11837-015-1710-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1710-3