Abstract
Coccinellids (Coccinellidae, commonly referred to as ladybeetles, ladybugs, or ladybirds) are predatory insects that often contribute to the biological control of crop pests. Especially when prey is limited, ladybirds have been reported to consume plant resources such as nectar. However, the importance of nectar consumption to ladybird fitness is not well understood. We performed artificial feeder experiments confirming ladybird consumption of a sugar solution with carbohydrate ratios similar to nectar. Both Harmonia axyridis (harlequin ladybird) and Hippodamia convergens (convergent ladybird) depleted sugar solution in 100% of trials. We also tested the effects of aphid and sugar solution availability on longevity and fecundity of these species. Ladybirds generally died within 10 days if no food was provided but survived for 10 days when either aphids or sugar solution were available. Aphids were required for oviposition. However, when aphids were available, oviposition was 36–90% higher when sugar solution was available as well. We conclude that nectar availability has significant potential to increase ladybird fitness, so may be worth considering in the design of conservation biological control programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arthropod pests represent a significant threat to crop production worldwide. Yield losses may already reach 20% of global annual crop production and are projected to increase with climate change (Deutsch et al. 2018; Sharma et al. 2017; Tonnang et al. 2022). In many cropping systems, arthropod pests are controlled primarily with pesticide applications (Dent and Binks 2020; Tudi et al. 2021). However, pesticide applications can carry significant drawbacks, including hazards to human health, biodiversity, and ecosystem function (Ansari et al. 2014; Chagnon et al. 2015; Thompson et al. 2020). In addition, overreliance on pesticide applications can drive losses in efficacy and unintended negative outcomes, e.g., resistance, resurgence, and replacement (Dutcher 2007; Onstad and Knolhoff 2022). Some of the same issues arise when crops are genetically engineered to produce insecticidal toxins (Tabashnik et al. 2023).
Natural enemies of crop pests, including parasitoids and predators, can make significant contributions to the integrated management of these pests (Barzman et al. 2015; Kogan 1998; Rusch et al. 2010). While natural enemies can be added directly to fields, this approach may be expensive (Collier and Van Steenwyk 2004; van Lenteren 2012). Conservation biological control, i.e., the manipulation of agricultural habitats to enhance the abundance and efficacy of natural enemies, can be an useful alternative in a wide range of cropping systems (Rayl et al. 2018; Shields et al. 2019).
Two potential components of conservation biological control are attraction and facilitation. Attraction involves luring natural enemies to the crop field or other areas infested by pests, whereas facilitation involves increasing the population or fitness of natural enemies (Begg et al. 2017; Jonsson et al. 2008). Different arthropod taxa are attracted and facilitated by different resources, e.g., different forms of shelter, nectar, alternative prey, and pollen (“SNAP”; Gurr et al. 2017). Therefore, habitat created or managed for conservation biological control can be tailored to the needs of natural enemies (Hatt et al. 2020; Holland et al. 2016; Jonsson et al. 2008). For many natural enemy species, these needs are not fully understood.
Many coccinellids (Coccinellidae, commonly known as ladybugs, ladybeetles, or ladybirds) are predatory insects that are frequently abundant in agroecosystems (Obrycki and Kring 1998). Some species, such as Harmonia axyridis Pallas 1773 (harlequin ladybird), are considered invasive in all regions outside their native range in Asia (Soares et al. 2023, Hodek et al. 2012). Other species, such as Hippodamia convergens Guérin-Méneville 1842 (convergent ladybird), are native and have a long history of use as biological control agents (Hodek et al. 2012). These desirable ladybirds can help control populations of aphids (Aphididae) and other crop pests (Kundoo and Khan 2017; Obrycki et al. 2009). Both augmentation and conservation biological control have the potential to enhance predation by ladybirds (Obrycki and Kring 1998).
Conservation biological control programs intended to promote ladybird predation of crop pests can both attract and facilitate ladybird populations. More information is available about attraction, relative to facilitation. Previous studies have confirmed that coccinellids are attracted to plants in the Apiaceae (carrot) family (Hatt et al. 2019b; Losey et al. 2022). Plants in this family may have attractive traits such as yellow flowers and specific volatiles, as well as accessible nectar (Adedipe and Park 2010; Campbell et al. 2017; Losey et al. 2022; Togni et al. 2016; Venjakob et al. 2022). While color and scent only serve as signals to ladybirds, nectar is a potential food source. Like other short-tongued beneficial arthropods, coccinellids can only reach nectar when plants have shallow nectary depths or extrafloral nectaries (Hatt et al. 2019a; Lundgren 2009a). In other words, accessible nectar may contribute to the facilitation component of conservation biological control.
Ladybirds have been reported to consume a variety of non-prey foods, including nectar (Hodek et al. 2012; Lundgren 2009a, b). It is possible for nectar (or sugar solution) consumption to increase ladybird fitness (Evans and Gunther 2013; He and Sigsgaard 2019; Lundgren and Seagraves 2011; Mathews et al. 2016; Wolf et al. 2018). There is more evidence for feeding on extrafloral nectar, relative to floral nectar. Extrafloral nectar is frequently more accessible to these short-tongued insects, may be less strongly defended, and is often available for longer time periods (Lundgren 2009a). However, extrafloral nectaries are absent in many plants, including all members of the Apiaceae family (Keeler et al. 2023; Weber and Keeler 2013).
There is more limited evidence for ladybird feeding from floral nectaries (Bugg 1987; Nalepa et al. 1992; Patt et al. 1997). While ladybirds do consume floral resources, they may feed on pollen more than nectar (Wäckers and van Rijn 2012). Some studies have demonstrated effects of flower availability on ladybirds without distinguishing between pollen and nectar provision. For example, Calendula officinalis L. (pot marigold) flowers decreased intraguild predation involving H. axyridis and Propylea japonica Thunberg 1781 (Liang et al. 2022). Perilla frutescens (L.) Britton (beefsteak mint) flowers increased H. axyridis longevity, relative to a no-food control (Hatt and Osawa 2019).
To summarize, the existing literature suggests that several ladybird species can consume nectar. However, it is not clear how often they do so. In addition, the functional importance of nectar resources in supporting coccinellid fitness is not well understood. Such knowledge can be used to design effective biological control programs. In this study, we addressed the following questions:
-
1.
Do H. axyridis and H. convergens consume a sugar solution with carbohydrate ratios similar to Apiaceae nectar?
-
2.
Does the sugar solution increase survival when prey levels are low?
-
3.
Does the sugar solution increase reproduction when prey levels are low?
Materials and methods
Ladybird rearing
Two ladybird species, Harmonia axyridis and Hippodamia convergens, were taken from a laboratory stock colony at Cornell University. Beetles were maintained in a reach-in environmental incubator at 25 ± 2 °C with a 16:8 h (L:D) light cycle. Adult beetles of each species were maintained singly in plastic cups that contained a single piece of paper towel. We provided beetles with an ad libitum diet of Acyrthosiphum pisum Harris 1776 (pea aphid). Aphids had been produced on and collected from Vicia faba L. (fava bean) plants. Cups were monitored daily for the production of eggs, which were separated from their mothers within 24 h. Larvae were fed excess diet through eclosion. Upon eclosion, adult females were placed in a new cup.
Aphid rearing
Vicia faba seeds were sown in pots and maintained in a greenhouse at 23 ± 2 °C with ambient relative humidity and a 16:8 hr L:D cycle. Germinated seedlings (2 days old) were then moved to an environmental incubator at 21 ± 2 °C with a 16:8 hr L:D cycle. Fifty to one hundred mixed-age aphids were added to each pot. Aphids were collected from the plants 7–10 days later and used to rear ladybirds and for experiments.
Sugar solution
Plant nectar is largely composed of glucose, fructose, and sucrose (Baker and Baker 1983). As ladybirds may respond differently to these different sugars (He and Sigsgaard 2019), we formulated our “nectar” solution to match the composition of Pastinaca sativa L. (wild parsnip), a common species in the Apiaceae family that has been reported to harbor ladybirds and aphids (Losey et al. 2022). Nectar in this species contains a total of 97.45 mg/mL carbohydrates, including 31.71 mg/ml glucose, 36.58 mg/ml fructose, and 29.16 mg/ml sucrose (Venjakob et al. 2022). Our sugar solution was therefore prepared according to these proportions.
Experiment 1: consumption of sugar solution
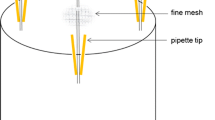
We tracked consumption of the sugar solution by measuring depletion from feeder tubes placed in arenas with and without adult ladybirds (Fig. 1). The no-beetle control enabled us to distinguish sugar consumption by beetles from any effects of evaporation or dripping. Feeder tubes were filled with sugar solution to the same height in all arenas. After 24 h, the height of sugar solution in tubes was compared between beetle and control arenas. The amount of depletion (reduction in height) was recorded as Beetle > Control or Beetle ≤ Control. Comparisons were made for 40 H. axyridis and 60 H. convergens with each beetle tested twice during the study, yielding 80 and 120 total comparisons, respectively.
Experiment 2: survival and reproduction
For each ladybird species, newly mated females were placed singly in plastic cups provisioned with a piece of paper towel and different diets. Diets included mixed-age high, low, or no aphids, with or without sugar solution. The high aphid rate was 0.1 mg, the low aphid rate was 0.02 mg, and the sugar solution rate was 40 μl. The sugar solution and feeding tubes were as in Experiment 1. Each day for 10 days, we recorded survival and number of eggs, then moved females to new cups and added freshly collected aphids. There were two replications per species blocked by time. For H. axyridis, sample sizes were N = 6 for the first replication and N = 7 for the second replication. For H. convergens, sample sizes were N = 10 for both replications.
Statistical analyses
Data analysis was performed in R version 4.3.1 (R Core Team 2023). All analyses were performed separately for the two species. Results from the sugar consumption study were analyzed using a one-sided exact binomial test of the alternative hypothesis that sugar solution was more depleted in the ladybird arena, relative to the no-beetle control, in greater than 50% of all trials (package “stats”). We evaluated effect size as Fei, an adjusted version of Cohen’s ω (package “effectsize”, Ben-Shachar et al. 2023). Fei is bounded between 0 and 1, with 0 representing a perfect fit to the expected distribution and 1 representing maximum deviation (Ben-Shachar et al. 2023).
For survival data, we used the commands prop.test and pairwise.prop.test (package “stats” with Holm P value adjustment) to evaluate the alternative hypothesis that the probability of survival for the entire 10-day period differed by feeding treatment. The total number of eggs laid over 10 days was analyzed using linear mixed models with aphid availability, sugar availability, and their interaction as fixed effects, and replication as a random effect (package “lme4”). The random effect of replication was not significant for either species (command ranova, package “lmerTest”). Square root and ln(x + 1) transformations were applied to the total number of eggs for H. axyridis and H. convergens, respectively. ANOVA and post-hoc tests (package “emmeans” with Tukey method for P value adjustment) were performed on the transformed scales. Post-hoc tests were performed for all combinations of aphid and sugar availability, and for levels of sugar availability within levels of aphid availability. Oviposition graphs show raw means and standard errors alongside results from these post-hoc analyses (Excel, Microsoft Corporation, Redmond, WA).
Results
Experiment 1: consumption of sugar solution
In 100% of comparisons (N = 80 for H. axyridis and N = 120 for H. convergens), more sugar solution was depleted in the ladybird beetle treatment compared with the no-beetle control (Table 1). Exact binomial tests indicated that these results were highly significant for both species, i.e., that depletion in the ladybird treatment exceeded depletion in the control treatment more often than expected according to a null hypothesis of no difference (P < 0.0001). The effect size Fei was maximized at 1.0 for both species, with one-sided 95% confidence intervals beginning at 0.82 (H. axyridis) or 0.85 (H. convergens).
Based on these results, we confirm that both species did consume the sugar solution from our feeder tubes. Although we did not quantify the amount of solution consumed, we estimate that consumption was between 10 and 15 μl per individual over 24-h period, i.e., 25 to 38% of the initial volume.
Experiment 2: survival
All H. axyridis beetles provided with aphids or sugar solution survived for the full 10 days, in both replications of the study (Table 2). In contrast, H. axyridis beetles provided with neither aphids nor sugar solution never survived for the full 10 days. In this no-food treatment, survival time ranged from 3 to 8 days with a mean of 4.92 ± 0.45 days. The likelihood of survival for the entire 10-day period differed by feeding treatment, i.e., death was much more likely when ladybirds were not fed (P < 0.0001).
All H. convergens beetles provided with either the high rate of aphids (0.1 mg) or sugar solution (40 μl) survived for the full 10 days, in both replications of the study (Table 2). Among the 20 beetles provided with the low aphid rate (0.02 mg) and no sugar solution, 19 beetles survived for the full 10 days and the remaining beetle survived for 9 days. However, among the 20 beetles provided with neither aphids nor sugar solution, only 2 beetles survived for the full 10 days. In this no-food treatment, survival time ranged from 4 to 10 days with a mean of 7.00 ± 0.40 days. The likelihood of death during the 10 days differed by feeding treatment (P < 0.0001). Survival was lower when beetles were not fed than in other treatments; however, there was no significant difference between the low aphid rate/no sugar treatment and other feeding treatments.
Experiment 2: oviposition
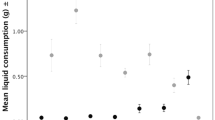
The total number of eggs laid by H. axyridis was influenced by aphid availability (P < 0.0001) and the interaction between aphid and sugar availability (P = 0.0012). Eggs were not laid in treatments without aphids (Fig. 2). At the low level of aphid availability (0.02 mg), the availability of 40 μl sugar increased oviposition from 97 ± 10 to 137 ± 12 eggs. At the high level of aphid availability (0.1 mg), the availability of 40 μl sugar increased oviposition from 195 ± 14 to 305 ± 25 eggs.
Effects of feeding treatments on total number of eggs oviposited by Harmonia axyridis over 10 days (mean ± SE, N = 13). Similar letters indicate no significant differences between treatments (Tukey’s HSD, α = 0.05). Black letters show pairwise comparisons for all combinations of aphid and sugar availability. Red letters show comparisons between levels of sugar availability within levels of aphid availability (red vertical lines)
Similarly, the total number of eggs oviposited by H. convergens was influenced by aphid availability (P < 0.0001) and the interaction between aphid and sugar availability (P < 0.0001). Eggs were not laid in treatments without aphids (Fig. 3). At the low level of aphid availability (0.02 mg), the availability of 40 μl sugar increased oviposition from 92 ± 4 to 175 ± 4 eggs. At the high level of aphid availability (0.1 mg), the availability of 40 μl sugar increased oviposition from 234 ± 6 to 319 ± 11 eggs.
Effects of feeding treatments on total number of eggs oviposited by Hippodamia convergens over 10 days (mean ± SE, N = 20). Similar letters indicate no significant differences between treatments (Tukey’s HSD, α = 0.05). This post-hoc analysis was performed for all pairwise combinations of aphid and sugar availability
Discussion
This research demonstrated that Harmonia axyridis and Hippodamia convergens both consume a sugar solution that is similar to Apiaceae nectar in its carbohydrate composition. This solution was consumed in substantial quantities (> 10 μl) per individual within a 24-h period. The sugar solution was provided through a feeder consisting of a narrow tube, so that adhesion balanced the force of gravity. Ladybirds pulled solution down through the tube by capillary action. From a methodological standpoint, this feeder system proved to be an effective way to provide precise quantities of sugar solutions to coccinellids. It could also be used to track consumption of various solution types with a high level of precision.
In the second experiment, availability of sugar solution prevented mortality when aphids were unavailable. Although sugar solution alone (without aphids) did not enable oviposition, sugar solution did increase oviposition when aphids were present. The increase in oviposition was approximately 40–110 eggs over the 10-day period, corresponding to 36–90% increases. Although we did not statistically compare the two ladybird species, we note that H. convergens appeared to survive longer without food and respond more strongly to the presence of sugar solution at the low level of aphid availability.
Our results are consistent with previous research showing that nectar can function as an alternative food source for ladybirds, potentially increasing performance. Access to extrafloral nectar can increase survival and fecundity when other food is unavailable (Lundgren and Seagraves 2011; Mathews et al. 2016). Ladybirds may consume extrafloral nectar even when prey is available, potentially reducing predation rates (Choate and Lundgren 2013; Mathews et al. 2016). Spellman et al. (2006) reported that the presence of extrafloral nectaries, but not floral resources, reduced H. axyridis feeding on aphid prey. However, the prevalence of ladybirds on species that have accessible floral nectaries but no extrafloral nectar (e.g., Apiaceae), combined with our finding that coccinellids readily consume large amounts of sugar from capillary feeders, suggests that floral nectar consumption may be important as well. In a field cage experiment, Wolf et al. (2018) found that a species with accessible floral nectar (Fagopyrum esculentum Moench, buckwheat), in addition to a species with extrafloral nectaries (Centaurea cyanus L., cornflower), increased H. axyridis performance relative to a species with less accessible nectar (Calendula arvensis L., field marigold).
Studies that have previously evaluated consumption of sugar solutions by ladybirds in controlled environments reported fitness benefits. Evans and Gunther (2013) found that H. axyridis oviposition was possible when diets included both sugar (sucrose) solution and Hypera postica L. Gyllenhal 1813 (alfalfa weevil) but not either resource alone. He and Sigsgaard (2019) found that sugar diets (glucose, fructose, or sucrose solutions) promoted Adalia bipunctata Linnaeus 1758 longevity but did not enable molting or reproduction. Larvae survived longer when provided with sugar diets or entire F. esculentum flowers, relative to other flowers or pollen diets (He and Sigsgaard 2019). Similarly, adults survived longer when provided with sugar diets, relative to entire flowers or pollen diets (He and Sigsgaard 2019). Among entire flowers, F. esculentum and Anethum graveolens L. (dill) outperformed Sinapis alba L. (white mustard; He and Sigsgaard 2019). These findings could reflect factors including the high levels of fructose and glucose in F. esculentum and A. graveolens nectar, lower nectar accessibility in S. alba, or the presence of toxic secondary compounds in S. alba (He and Sigsgaard 2019). These findings indicate that additional experiments should consider comparing actual nectars with artificial sugar solutions to understand the roles of non-carbohydrate components in driving ladybird consumption and performance. Artificial feeder trials should also be complemented by trials using entire flowers.
Overall, we conclude that nectar may be an important resource for ladybirds, especially when prey availability is limited. This resource may be underrecognized especially in taxa without extrafloral nectar. Flowering plants with accessible floral morphology and nectar containing an optimal profile of sugar and other constituents has the potential to enhance the effectiveness of conservation biological control.
References
Adedipe F, Park Y-L (2010) Visual and olfactory preference of Harmonia axyridis (Coleoptera: Coccinellidae) adults to various companion plants. J Asia-Pac Entomol 13:319–323. https://doi.org/10.1016/j.aspen.2010.07.004
Ansari MS, Moraiet MA, Ahmad S (2014) Insecticides: impact on the environment and human health. In: Malik A, Grohmann E, Akhtar R (eds) Environmental deterioration and human health: natural and anthropogenic determinants. Springer Netherlands, Dordrecht, pp 99–123. https://doi.org/10.1007/978-94-007-7890-0_6
Baker HG, Baker I (1983) Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold Company, New York, pp 117–141
Barzman M, Bàrberi P, Birch ANE, Boonekamp P, Dachbrodt-Saaydeh S, Graf B, Hommel B, Jensen JE, Kiss J, Kudsk P, Lamichhane JR, Messéan A, Moonen A-C, Ratnadass A, Ricci P, Sarah J-L, Sattin M (2015) Eight principles of integrated pest management. Agron Sustain Dev 35:1199–1215. https://doi.org/10.1007/s13593-015-0327-9
Begg GS, Cook SM, Dye R, Ferrante M, Franck P, Lavigne C, Lövei GL, Mansion-Vaquie A, Pell JK, Petit S, Quesada N, Ricci B, Wratten SD, Birch ANE (2017) A functional overview of conservation biological control. Crop Prot 97:145–158. https://doi.org/10.1016/j.cropro.2016.11.008
Ben-Shachar MS, Patil I, Thériault R, Wiernik BM, Lüdecke D (2023) Phi, fei, fo, fum: effect sizes for categorical data that use the chi-squared statistic. Mathematics 11:1982. https://doi.org/10.3390/math11091982
Bugg RL (1987) Observations on insects associated with a nectar-bearing Chilean tree, Quillaja saponaria Molina (Rosaceae) (Abstract only). Pan-Pacific Entomol 63:60–64
Campbell AJ, Wilby A, Sutton P, Wäckers F (2017) Getting more power from your flowers: multi-functional flower strips enhance pollinators and pest control agents in apple orchards. InSects 8:101. https://doi.org/10.3390/insects8030101
Chagnon M, Kreutzweiser D, Mitchell EAD, Morrissey CA, Noome DA, Van der Sluijs JP (2015) Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ Sci Pollut Res 22:119–134. https://doi.org/10.1007/s11356-014-3277-x
Choate BA, Lundgren JG (2013) Why eat extrafloral nectar? Understanding food selection by Coleomegilla maculata (Coleoptera: Coccinellidae). Biocontrol 58:359–367. https://doi.org/10.1007/s10526-012-9501-z
Collier T, Van Steenwyk R (2004) A critical evaluation of augmentative biological control. Biol Control 31:245–256. https://doi.org/10.1016/j.biocontrol.2004.05.001
Dent D, Binks RH (2020) Insect pest management, 3rd edn. CAB International, Wallingford
Deutsch CA, Tewksbury JJ, Tigchelaar M, Battisti DS, Merrill SC, Huey RB, Naylor RL (2018) Increase in crop losses to insect pests in a warming climate. Science 361:916–919. https://doi.org/10.1126/science.aat3466
Dutcher JD (2007) A review of resurgence and replacement causing pest outbreaks in IPM. In: Ciancio A, Mukerji KG (eds) General concepts in integrated pest and disease management. Springer Netherlands, Dordrecht, pp 27–43. https://doi.org/10.1007/978-1-4020-6061-8_2
Evans EW, Gunther DI (2013) The link between food and reproduction in aphidophagous predators: a case study with Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 102:423–430. https://doi.org/10.14411/eje.2005.060
Gurr GM, Wratten SD, Landis DA, You M (2017) Habitat management to suppress pest populations: progress and prospects. Annu Rev Entomol 62:91–109. https://doi.org/10.1146/annurev-ento-031616-035050
Hatt S, Francis F, Xu Q, Wang S, Osawa N (2020) Perennial flowering strips for conservation biological control of insect pests: from picking and mixing flowers to tailored functional diversity. In: Gao Y, Hokkanen HMT, Menzler-Hokkanen I (eds) Integrative biological control: ecostacking for enhanced ecosystem services. Springer, Cham, pp 57–71. https://doi.org/10.1007/978-3-030-44838-7_4
Hatt S, Osawa N (2019) The role of Perilla frutescens flowers on fitness traits of the ladybird beetle Harmonia axyridis. Biocontrol 64:381–390. https://doi.org/10.1007/s10526-019-09937-1
Hatt S, Uytenbroeck R, Lopes T, Mouchon P, Osawa N, Piqueray J, Monty A, Francis F (2019a) Identification of flower functional traits affecting abundance of generalist predators in perennial multiple species wildflower strips. Arthropod-Plant Interact 13:127–137. https://doi.org/10.1007/s11829-018-9652-7
Hatt S, Xu Q, Francis F, Osawa N (2019b) Aromatic plants of East Asia to enhance natural enemies towards biological control of insect pests. A review. Entomol Gen 38:275–315. https://doi.org/10.1127/entomologia/2019/0625
He X, Sigsgaard L (2019) A floral diet increases the longevity of the coccinellid Adalia bipunctata but does not allow molting or reproduction. Front Ecol Evol 7:6. https://doi.org/10.3389/fevo.2019.00006
Hodek I, Van Emden HF, Honěk A (eds) (2012) Ecology and behaviour of the ladybird beetles (Coccinellidae), 1st edn. Wiley, West Sussex. https://doi.org/10.1002/9781118223208
Holland JM, Bianchi FJ, Entling MH, Moonen A-C, Smith BM, Jeanneret P (2016) Structure, function and management of semi-natural habitats for conservation biological control: a review of European studies. Pest Manag Sci 72:1638–1651. https://doi.org/10.1002/ps.4318
Jonsson M, Wratten SD, Landis DA, Gurr GM (2008) Recent advances in conservation biological control of arthropods by arthropods. Biol Control 45:172–175. https://doi.org/10.1016/j.biocontrol.2008.01.006
Keeler KH, Porturas LD, Weber MG (2023) World list of plants with extrafloral nectaries. http://www.extrafloralnectaries.org. Accessed 14 May 2024
Kogan M (1998) Integrated pest management: historical perspectives and contemporary developments. Annu Rev Entomol 43:243–270. https://doi.org/10.1146/annurev.ento.43.1.243
Kundoo AA, Khan AA (2017) Coccinellids as biological control agents of soft bodied insects: a review. J Entomol Zool Stud 5:1362–1373
Liang Y, Chen X, Dai H, Wang J, Guo X, Wang S, Jaworski CC (2022) Flower provision reduces intraguild predation between predators and increases aphid biocontrol in tomato. J Pest Sci 95:461–472. https://doi.org/10.1007/s10340-021-01396-x
Losey J, Allee L, Gill H, Morris S, Smyth R, Wolleman D, Westbrook A, DiTommaso A (2022) Predicting plant attractiveness to coccinellids with plant trait profiling, citizen science, and common garden surveys. Biol Control 176:105063. https://doi.org/10.1016/j.biocontrol.2022.105063
Lundgren JG (2009a) Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol Control 51:294–305. https://doi.org/10.1016/j.biocontrol.2009.05.016
Lundgren JG (2009b) Relationships of natural enemies and non-prey foods. Springer Netherlands, Dordrecht
Lundgren JG, Seagraves MP (2011) Physiological benefits of nectar feeding by a predatory beetle. Biol J Linn Soc 104:661–669. https://doi.org/10.1111/j.1095-8312.2011.01729.x
Mathews CR, Brown MW, Wäckers FL (2016) Comparison of peach cultivars for provision of extrafloral nectar resources to Harmonia axyridis (Coleoptera: Coccinellidae). Environ Entomol 45:649–657. https://doi.org/10.1093/ee/nvw035
Nalepa CA, Bambara SB, Burroughs AM (1992) Pollen and nectar feeding by Chilocorus kuwanae (Silvestri) (Coleoptera: Coccinellidae) (Abstract only). Proc Entomol Soc Wash 94:596–597
Obrycki JJ, Harwood JD, Kring TJ, O’Neil RJ (2009) Aphidophagy by Coccinellidae: application of biological control in agroecosystems. Biol Control 51:244–254. https://doi.org/10.1016/j.biocontrol.2009.05.009
Obrycki JJ, Kring T (1998) Predaceous Coccinellidae in biological control. Annu Rev Entomol 43:295–321. https://doi.org/10.1146/annurev.ento.43.1.295
Onstad DW, Knolhoff LM (2022) Insect resistance management: biology, economics, and prediction, 3rd edn. Academic Press, London
Patt JM, Hamilton GC, Lashomb JH (1997) Impact of strip-insectary intercropping with flowers on conservation biological control of the Colorado potato beetle. Adv Hortic Sci 11:175–181
R Core Team (2023) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Rayl RJ, Shields MW, Tiwari S, Wratten SD (2018) Conservation biological control of insect pests. In: Gaba S, Smith B, Lichtfouse E (eds) Sustainable agriculture reviews 28: ecology for agriculture. Springer, Cham, pp 103–124. https://doi.org/10.1007/978-3-319-90309-5_3
Rusch A, Valantin-Morison M, Sarthou J-P, Roger-Estrade J (2010) Biological control of insect pests in agroecosystems: effects of crop management, farming systems, and seminatural habitats at the landscape scale: a review. Adv Agron 109:219–259. https://doi.org/10.1016/B978-0-12-385040-9.00006-2
Sharma S, Kooner R, Arora R (2017) Insect pests and crop losses. In: Arora R, Sandhu S (eds) Breeding insect resistant crops for sustainable agriculture. Springer, Singapore, pp 45–66. https://doi.org/10.1007/978-981-10-6056-4_2
Shields MW, Johnson AC, Pandey S, Cullen R, González- Chang M, Wratten SD, Gurr GM (2019) History, current situation and challenges for conservation biological control. Biol Control 131:25–35. https://doi.org/10.1016/j.biocontrol.2018.12.010
Soares AO, Haelewaters D, Ameixa OMCC, Borges I, Brown PMJ, Cardoso P, de Groot MD, Evans EW, Grez AA, Hochkirch A, Holecová M, Honěk A, Kulfan J, Lillebø AI, Martinková Z, Michaud JP, Nedvěd O, Omkar HE, Roy S, Saxena A, Shandilya A, Sentis J, Skuhrovec S, Viglášová P, Zach TZ, Losey JE (2023) A roadmap for ladybird conservation and recovery. Conserv Biol 37(1):e13965
Spellman B, Brown MW, Mathews CR (2006) Effect of floral and extrafloral resources on predation of Aphis spiraecola by Harmonia axyridis on apple. Biocontrol 51:715–724. https://doi.org/10.1007/s10526-005-5252-4
Tabashnik BE, Fabrick JA, Carrière Y (2023) Global patterns of insect resistance to transgenic Bt crops: the first 25 years. J Econ Entomol 116:297–309. https://doi.org/10.1093/jee/toac183
Thompson DA, Lehmler H-J, Kolpin DW, Hladik ML, Vargo JD, Schilling KE, LeFevre GH, Peeples TL, Poch MC, LaDuca LE, Cwiertny DM, William Field R (2020) A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environ Sci Process Impacts 22:1315–1346. https://doi.org/10.1039/C9EM00586B
Togni PHB, Venzon M, Muniz CA, Martins EF, Pallini A, Sujii ER (2016) Mechanisms underlying the innate attraction of an aphidophagous coccinellid to coriander plants: implications for conservation biological control. Biol Control 92:77–84. https://doi.org/10.1016/j.biocontrol.2015.10.002
Tonnang HE, Sokame BM, Abdel-Rahman EM, Dubois T (2022) Measuring and modelling crop yield losses due to invasive insect pests under climate change. Curr Opin Insect Sci 50:100873. https://doi.org/10.1016/j.cois.2022.100873
Tudi M, Daniel Ruan H, Wang L, Lyu J, Sadler R, Connell D, Chu C, Phung DT (2021) Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health 18:1112. https://doi.org/10.3390/ijerph18031112
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20. https://doi.org/10.1007/s10526-011-9395-1
Venjakob C, Ruedenauer FA, Klein A-M, Leonhardt SD (2022) Variation in nectar quality across 34 grassland plant species. Plant Biol 24:134–144. https://doi.org/10.1111/plb.13343
Wäckers FL, van Rijn PCJ (2012) Pick and mix: selecting flowering plants to meet the requirements of target biological control insects. In: Biodiversity and insect pests: key issues for sustainable management. Wiley, West Sussex, pp 139–165. https://doi.org/10.1002/9781118231838.ch9
Weber MG, Keeler KH (2013) The phylogenetic distribution of extrafloral nectaries in plants. Ann Bot 111:1251–1261. https://doi.org/10.1093/aob/mcs225
Wolf S, Romeis J, Collatz J (2018) Utilization of plant-derived food sources from annual flower strips by the invasive harlequin ladybird Harmonia axyridis. Biol Control 122:118–126. https://doi.org/10.1016/j.biocontrol.2018.04.008
Acknowledgements
O. Hameed was supported to undertake this research at Cornell University by a grant from the International Research Support Program (IRSIP) through the Higher Education Commission of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Danny Haelewaters and Heikki Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hameed, O., Ugine, T., Westbrook, A. et al. Consumption of nectar-like sugar solutions promotes longevity and fecundity in the ladybird beetles Harmonia axyridis and Hippodamia convergens. Arthropod-Plant Interactions 18, 763–770 (2024). https://doi.org/10.1007/s11829-024-10086-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-024-10086-1