Abstract
Intraguild predation (IGP)–the predation of a natural enemy species upon another one sharing a prey species–is relatively frequent in both natural and agroecosystems. This may reduce pest control and the establishment of predator populations during mass release of biological control agents or in multi-predator systems due to increased mortality of predators. IGP is exacerbated in isolated and space-limited systems such as greenhouses, due to reduced food resources and movement. Therefore, adding food resources as an alternative to the main prey, such as floral resources, could reduce IGP between natural enemies in these systems. In the present study we investigated the role of supplemental floral resources to help reduce intra- and interspecific IGP involving Harmonia axyridis and Propylea japonica (Coleoptera: Coccinellidae) in laboratory conditions, and we tested its application in a greenhouse setup. We found a significant reduction in intra- and interspecific IGP in laboratory conditions when floral resources were abundant. At a greenhouse scale, abundances of both ladybird species increased when floral resources were abundant, potentially through a combination of enhanced fertility and reduced IGP. This resulted in reduced abundances of aphid pest populations on tomato crops. Our study demonstrates that companion plants in greenhouses can improve pest control in systems with multi-species biological control agent releases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Intraguild predation (IGP) is ubiquitous in natural and agroecosystems.
-
We tested the impact of flowers on IGP between ladybirds in laboratory and greenhouse.
-
IGP was reduced by the presence of floral resources resulting in higher larvae survival rate.
-
Flower provision induced higher ladybird densities and enhanced pest control in greenhouses.
Introduction
Intraguild predation (IGP) occurs when a predator preys on another natural enemy—the IGP prey—in addition to preying on their shared prey (Rosenheim et al. 1995; Chailleux et al. 2013; Mohammadpour et al. 2020). IGP is exacerbated when primary prey are scarce and negatively affects the development, colonization and distribution of the IGP prey species (van Veen et al. 2006). In particular, exotic predatory species have the potential to negatively affect populations of native predators, potentially disrupting pest control (Polis and Myers 1989; Polis and Holt 1992; Snyder and Evans 2006; Lamichhane et al. 2015). Despite ubiquitous cases of IGP in natural or agroecosystems (in absence of biological control agent release), laboratory and field studies showed that the disruption of pest control by one of the predator involved in IGP did not necessarily result in higher pest densities (Rosenheim et al. 1993; 1995; Gagnon et al. 2015; Aparicio et al. 2020; Ortiz-Martínez et al. 2020).
Yet many applications of biological control agents occur in more isolated systems, such as greenhouses, with more limited resources and arthropod movement, but studies investigating the potential for IGP to disrupt the biological pest control in such systems are scarce. In addition, applications of multiple biological control agents specialized on distinct target pests are commonly used over short periods of time and preferably at early stages of pest outbreaks in multi-pest systems (Albajes et al. 2000; Liu et al. 2012; Tan et al. 2016; Sanchez-Hernandez et al. 2021). These low-prey, multi-predator species conditions are ideal for IGP to occur among biological control agents (Chailleux et al. 2013). Thus, enhancing food provision in such systems could help reduce the intensity of IGP and thereby avoid the disruption of biological pest control (Lu et al. 2014).
Companion plants—non-crop plants providing ecosystem services in agroecosystems—have been used to provide alternative food resources and/or shelter in many conservation biological control applications (Balzan et al. 2016; Biondi et al. 2106; Gurr et al. 2017; Perovic et al. 2018; Foti et al. 2019; Snyder 2019). Companion plants may also be integrated and managed at a field scale in attract-and-reward strategies (Gardarin et al. 2018; Jaworski et al. 2019; Cai et al. 2020, 2021). Flowering companion plants may attract arthropod natural enemies of crop pests via olfactory and visual floral signals and reward them with floral nectar and pollen resources (Barbosa 1998; Damien et al. 2017, 2020; Chailleux et al. 2019; Wang et al. 2020). Such floral food resources may enhance natural enemy populations especially when prey density is low (Li et al. 2015; Zhao et al. 2017). Companion plants may also create habitat separation or provide micro-habitats in agroecosystems. There, arthropod natural enemies of crop pests may find shelter from their own natural enemies such as birds, or from environmental pollutants such as chemical pesticides that could negatively impact them (Bommarco and Ekbom 2000; Desneux et al. 2007). However, the role of companion plants in reducing IGP has been poorly documented so far.
Despite more and more abundant literature on habitat management to benefit natural enemies of crop pests in large, open fields (Albrecht et al. 2020), using similar ecological regulation remains limited in more isolated and space-limited systems, such as greenhouses (but see Li et al. 2021a; Xu et al. 2020). The use of companion plants providing alternative resources to biological control agents could be convenient in greenhouses, especially in multi-predator species where food resources could be limited, increasing the risk for IGP (Parolin et al. 2012; Sun and Song 2019).
Predatory ladybirds are a well-known group of very effective biological control agents and with strong colonization and spread capacity, wide food breadth and high prey consumption (Hodek et al. 2012; Lu et al. 2012; Thomine et al. 2020). These predatory ladybirds are top consumers in arthropod communities and play a key role in the structure and stability of food webs in natural habitats (Hodek and Michaud 2008; Ragsdale et al. 2011; Hodek et al. 2012). Many studies have shown that IGP was frequently occurring among predatory ladybirds, both among conspecific (intraspecific IGP) or between species (interspecific IGP) and especially under insufficient food supply (Fedriani et al. 2000; Ware and Majerus 2008; Pervez and Gupta 2010). IGP has been described in ladybirds between larvae, or by larvae on eggs, by adults on larvae, or between adults (Michaud 2010; Osawa 2015; Ovchinnikov et al. 2019).
Remarkably, Harmonia axyridis Pallas, 1773 (Coleoptera: Coccinellidae), has been widely characterized both as a very efficient biological control agent in its native territory in Asia and as a worldwide invasive predator causing severe ecological risks in decreasing biodiversity and destroying ecological balance in introduced areas notably via IGP and resource competition (Koch and Galvan 2008; Pell et al. 2008; Li et al. 2021b). Harmonia axyridis reduces populations of ladybird species under IGP by attacking their larvae and eggs (Burgio et al. 2002; Mirande et al. 2015). Wang et al. (2012) showed in a field survey that H. axyridis was a dominant competitor over two other predatory ladybird species common in China, Hippodamia variegata Goeze, 1777, and Propylea japonica Thunberg, 1780 (Coleoptera: Coccinellidae): H. axyridis preyed on more eggs, mostly heterospecific, had the highest survival rate and the lowest rate of IGP victims. Also, H. axyridis had a niche breadth twice as big as the other two species, but their niche overlap with that of H. axyridis was extremely high. Finally, P. japonica is more tolerant and a more efficient biocontrol agent at higher temperature (Lei et al. 1988). For these complementary features, H. axyridis and P. japonica are often released together to control aphid populations (Gao et al. 2016) and especially in greenhouses (Vuong et al. 2001; Yang et al. 2014; Kuroda and Miura 2003). In particular, they are used against the generalist aphid species Myzus persicae Sulzer, 1776 (Hemiptera: Aphididae), which is a major pest in a diversity of vegetable crops including tomato crops in China (Li 2013).
Many ladybird species may feed on floral resources (Wäckers and van Rijin 2012; Hatt et al. 2019). A field study showed that H. axyridis used floral resources all year long even in presence of aphids (Berkvens et al. 2010). Harmonia axyridis used floral resources of Vicia sativa, Fagopyrum esculentum, Coriandrum sativum and Calendula officinalis in laboratory conditions (Wang et al. 2020). While Calendula officinalis had a negative impact on H. axyridis fecundity and predation activity (Wang et al. 2020), it increased the fecundity and longevity of P. japonica females (Jaworski et al. 2019). Besides, C. officinalis is commonly used in habitat management programs in China to enhance biological pest control (Zhao et al. 2017; Jaworski et al. 2019).
In the present study, we conducted two laboratory experiments to evaluate the intraspecific IGP in H. axyridis and interspecific IGP between H. axyridis and P. japonica, and the impact of flowering potted plants of marigold C. officinalis on the intensity of IGP. In addition, we conducted a one-year greenhouse experiment to assess the practical impact of the abundance of marigold flowers on the population dynamics of a mixed community of H. axyridis and P. japonica, and on the efficacy of the biological control of the aphid pest M. persicae in tomato crops. We investigated the impact of the abundance of marigold flowers on (i) the intensity of intraspecific IGP in H. axyridis, (ii) the intensity of interspecific IGP between H. axyridis and P. japonica, and (iii) the population dynamics of the two predatory ladybirds and the regulation of aphid pest populations.
Materials and methods
Ladybirds and plants
Experimental colonies of ladybirds were established in the insectary of the Institute of Plant and Environment Protection (IPEP), BAAFS, from live specimens collected in an alfalfa field in the campus of Weifang University of Science and Technology (GPS: E118.78, N36.89) in June 2018 (H. axyridis: 330 adults; P. Japonica: 424 adults). The two species were reared in different cages with around 30–35 pairs of ladybirds per cage (35.0 cm3 plastic frame covered with 40-mesh net). Fifty fresh artificial diet microcapsules similar to those used in industrial productions were supplied daily in each cage to maintain the experimental colonies, as described in Tan et al. (2015): 1.0% Ca‐alginate, 1.6% chitosan and shell:core = 1:2, size 2.0–2.5 mm. After about five reared generations since the collection of wild specimens, five paper strips (3.0 × 10.0 cm each) were placed in each cage as oviposition substrates to collect fresh eggs. Each strip was removed and placed in a Petri dish (D = 12.0 cm) after five days. Newly hatched 1st instar larvae were collected every other day and placed in a new plastic Petri dish with 10 larvae per dish. The larvae were provided with 10 daily supplied fresh artificial diet microcapsules until they developed to 4th instar, after what the density was reduced to three larvae per dish. More than 5000 4th instar larvae for each ladybird species were prepared for the laboratory and greenhouse experiments. Environmental conditions inside the insectary were automatically regulated (T = 25 ± 1 ℃; RH = 60 ± 5%; photoperiod: 16:8 h L:D 500 lx; automatic regulation systems Est100, JiangNan, Ningbo, China).
Experimental tomato Solanum lycopersicum cv. Beryl (Jingyan Yinong Seed Sci-tech Co., Ltd.) seedlings were grown in plastic trays (56 × 25 × 20 cm, 12 plants per tray). Once they reached 15 cm height, we transplanted them individually in plastic flower pots (H = 25 cm, D = 15 cm, 1 plant per pot). We used standard growing soil (Miracle Gro). All tomato plants were maintained in artificial climatic chambers (MH-351, Sanyo, Nagoya, Japan). The environmental conditions were set as T = 27 ± 1 ℃, RH = 60 ± 5% RH and a 14:10 h L:D photoperiod. Over 1000 tomato plants were prepared and used for the laboratory and greenhouse experiments when they reached 30–35 cm height with 5–7 fully expanded true leaves.
Plants of marigold, Calendula officinalis Linnaeus, 1753 (Asterales: Asteraceae) var. Kablouna (Sinic Horticulture and Flower Co. Ltd, Beijing, China), were bought from the Yajie flower market (Changping, Beijing) at the two true leaves stage (~ 10–15 cm height), and grown in plastic trays as above mentioned. They were prepared specifically for our study and grown according to our specifications, and without pesticide application. Marigold is a common plant species used as an alternative floral resource for ladybirds (P. japonica, Jaworski et al. 2019) and easy to get. We transplanted the plants in plastic flower pots when they reached 3–4 true leaves (H = 25 cm, D = 15 cm, 1 plant per pot). About 20–25 days later, they reached around 20 cm and we removed the topmost buds of each plant; this allowed the production of more flowers by the start time of the experiments two weeks later.
Laboratory experiment: impact of marigold flowers on intra- and interspecific IGP
We assessed the impact of the presence of marigold flowering plants in intraspecific IGP in H. axyridis. Forty 4th instar larvae of H. axyridis were starved for 12 h and then placed in a cage with three tomato plants (plastic frame covered by 40-mesh, 50 cm3). In the treatment cages, one marigold plant was placed simultaneously in the cage, but not in the control cages. To estimate the impact of the number of open flowers of marigold, we varied the density of flowers per cage from one to five. We manipulated marigold plants accordingly using tinfoil paper to wrap up entire exceeding flowers. This led to a 5-level treatment (1–5 flowers) and a total of 180 cages (30 cages for control and 30 cages for each treatment level). No other food was provided to ladybirds in cages, and we recorded larvae survival after 24 h. All cages were maintained simultaneously in different rooms of the insectary (each room: 7 × 4 m) under controlled environmental conditions as above.
Dead larvae were further observed under a stereo microscope (Stereo V20, Zeiss, Germany), and those without external wounds were recorded as dead from starvation. We calculated the number of larvae victims of IGP as the total number of larvae introduced in each cage (40) minus the number of observed survivors minus the number of larvae dead from starvation.
Finally, to assess the impact of the abundance of marigold flowers on IGP between H. axyridis on P. japonica, we repeated the entire experiment above, except this time we placed 30 H. axyridis and 30 P. japonica 4th instar larvae in each cage. We used these extreme conditions in laboratory experiments—high ladybird densities, and no prey provided—to increase the likelihood and intensity of IGP, so as to properly evaluate intra- and interspecific IGP and to assess the impact of flower provision on the intensity of IGP. This choice of larvae density was made based on authors experience who observed the occurrence of IGP at such high ladybird densities in rearing experiments.
Greenhouse experiment: impact of marigold on ladybird population dynamics and biological pest control
To evaluate the practical effectiveness of marigold plants in enhancing ladybird populations and aphid pest control in systems with combined released of H. axyridis and P. japonica, we carried out a one-year survey in three greenhouses in Lanhu Organic Farmland (GPS: E116.75, N39.98), Tong’zhou county, Beijing, China. All three greenhouses were the same size (length: 80 m; width: 12 m; height: 6 m). We split each greenhouse into five isolated chambers using transparent plastic sheets preventing arthropod movement between chambers (each chamber: 10 × 8 m; Fig. 1). In every chamber, 80 tomato plants were grown in 10 rows. All tomato plants were transplanted at the stage 8–10 true leaves, from seedlings grown in climatic chambers (see Sect. 2.1). Forty-eight hour later, we infested 3rd instar nymphs of M. persicae (provided by IPEP, BAAFS) on 20 randomly selected tomato plants per chamber with 250 aphids each. Then we placed marigold potted plants in each chamber according to three treatments: (a) low flower density: five marigold plants with a total of 15 open flowers; (b) high flower density: 15 marigold plants with a total of 45 open flowers; and (c) control: no marigold plant. The position of each treatment in the five chambers of each greenhouse was fully randomized. Twenty-four hour later, 80 4th instar larvae of H. axyridis and P. japonica each were released evenly on 10 randomly selected tomato plants in each chamber. The aphid and ladybird densities, as well as the release strategy, were chosen based on authors’ experience and pilot observations. Such aphid densities simulate real greenhouse densities before aphid pest populations reach the outbreak peak. The plants chosen for ladybird larvae release were not necessarily the same as for aphid release due to random selection.
Layout of one greenhouse divided into five chambers (top), and spatial arrangement of tomato plants (green) and marigold plants (orange) in one chamber (bottom). One and five marigold plants were provided at each location (orange dots) at low and high flower densities, respectively, and each plant had exactly three open flowers. In control chambers, no marigold plant was provided
From the day we introduced ladybirds (May 6th 2019), we measured the densities of aphids and of the two species of ladybirds (including larvae from all instars and adults) by visual count on six randomly selected tomato plants per chamber (30 plants per treatment) every Monday until the last week of August 2019. Marigold plants were blooming throughout the duration of the experiment. In case one plant had stopped flowering it was immediately replaced, making sure no insect was present on this plant. Environmental conditions inside greenhouses followed seasonal trends and temperature reached 32–34 °C in average.
Statistical analysis
To test the impact of intraspecific IGP on larvae survival in our laboratory experiment, we performed a generalized linear mixed model (GLMM; function ‘glmer’, library ‘lme4’; Bates et al. 2015) with a binomial distribution and the response variable implemented as a matrix whose first and second columns corresponded to the number of IGP victims versus live larvae. We implemented the number of marigold flowers (0–5) as a factorial fixed effect, and the insectary room identifier as a random effect, followed by an ANOVA with a χ2 test. Model validity was verified a posteriori (functions ‘simulateResiduals’ and testDispersion’, library ‘DHARMa’; Hartig 2019). To assess whether means across treatments with a different number of flowers were significantly different, we performed a post hoc comparison of means across treatment levels (function ‘emmeans’, library ‘emmeans’; Lenth 2019). The impact of interspecific IGP on larvae survival was assessed with the same methodology, except that we used the factorial number of marigold flowers in interaction with the species (H. axyridis versus P. japonica) as fixed effects. The significance of the interaction and fixed effects was estimated through a type-II model comparison based on a χ2 test. A post hoc mean comparison across treatment levels was performed for each species independently as above (‘emmeans’: ‘specs = pairwise ~ Treatment | Species’).
To assess the impact of marigold flowers on ladybird abundances in the greenhouse experiment, we used a GLMM with a negative binomial distribution (to account for data dispersion; function ‘glmer.nb’, library ‘lme4’; Bates et al. 2015) on the number of live ladybirds per plant, with the treatment (control/low number of flowers/high number of flowers) in interaction with the species (H. axyridis versus P. japonica) as fixed effects. Random effects were the greenhouse chamber nested in the greenhouse to account for repeated measures through time and the week (implemented as a factor) to account for changes in population dynamics. The significance of the interaction between fixed effects was assessed through a type-II model comparison based on a χ2 test. A post hoc mean comparison across treatments and for each species was performed (function ‘emmeans’: ‘specs = pairwise ~ Treatment | Species’, library ‘emmeans’). We used the same method to test the impact of the number of flowers on aphid number per plant, except that only the treatment was used as fixed effect. All statistics were performed using R version 3.6.2 (R Core Team 2019).
Results
Laboratory experiment: impact of marigold flowers on intra- and interspecific IGP
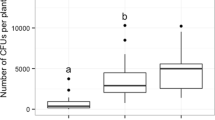
The number of marigold flowers significantly reduced the number of H. axyridis larvae affected by intraspecific IGP (χ2 = 225, df = 5, P < 0.001; Fig. 2). The number of victims gradually decreased in treatments from three to five flowers, but was not significantly different from control (no flowers) when less than three flowers were provided. The number of larvae which survived intraspecific IGP was more than twice as big when five flowers were present (mean ± SE: 31 ± 1) compared with control (no flower; 13 ± 1).
Similarly, the number of marigold flowers significantly reduced the number of larvae victims of IGP when both species were present (χ2 = 297, df = 5, P < 0.001; Fig. 3), with a 33% higher number of dead larvae in P. Japonica in average (χ2 = 290, df = 5, P < 0.001; mean number ± SE: H. axyridis 14.6 ± 0.2, P. japonica 19.4 ± 0.3), but the interaction between these effects was not significant (χ2 = 2.05, df = 5, P = 0.84). Similar to the intraspecific experiment, the number of victims gradually decreased in treatments from three to five flowers, but was not significantly different from control (no flowers) when less than three flowers were provided. The number of larvae which survived IGP was more than twice as big when five flowers were present (mean ± SE: H. axyridis 12.4 ± 0.3, P. japonica 7.6 ± 0.4) compared with control (no flower; H. axyridis 19.2 ± 0.5, P. japonica 14.9 ± 0.5).
Number of Harmonia axyridis (blue) and Propylea japonica (green) larvae victims of IGP out of 30 larvae each initially, as a function of the number of open flowers of the marigold companion plant (0 flowers = no plant; boxplot). Different letters indicate significant differences between the means of each group in the species of the corresponding color (P < 0.01)
The total proportion of larvae victims of IGP was not different in the two-species experiment compared with the one-species experiment in control (mean ± SE, no flowers: two species 67 ± 2%, one species 67 ± 2%), but it declined faster in the one-species experiment with increasing flower abundance (mean ± SE, five flowers: two species 43 ± 2%, one species 23 ± 2%). Also, the proportion of H. axyridis larvae victims of total IGP (cumulated intra- and interspecific IGP) in the two-species experiment was lower than in the one-species experiment at low flower density only (mean ± SE; no flowers: two species 58 ± 1%, one species 67 ± 2%; five flowers: two species 36 ± 2%, one species: 23 ± 2%).
Greenhouse experiment: impact of marigold on ladybird population dynamics and biological pest control
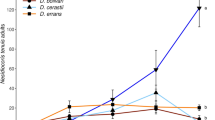
In our greenhouse experiment, we found that an increased number of marigold flowers per chamber resulted in a significant increase in the number of ladybirds of both species, and this was exacerbated for P. japonica (significant interaction between the number of flowers and the ladybird species: df = 2, χ2 = 60.7, P < 0.001; Fig. 4). The abundance of P. japonica was multiplied by almost three in average between the control (no flowers) and the high flower density, while the abundance of H. axyridis was multiplied by roughly two between these treatments (mean number of ± SE of ladybirds per plant: P. japonica control: 4.6 ± 0.1, high flower density: 12.5 ± 0.4; H. axyridis control 8.9 ± 0.2, high flower density 17.7 ± 0.5). All treatments were significantly different from each other for both species (Table 1). In absence of flowers, abundances of P. japonica increased before Week 1 (from 1 to ~ 2.5 ladybirds per plant) but were overall stable after that. Conversely, in all other treatments and in all treatments for H. axyridis, abundances increased from the date of release to Week 12. After that, only H. axyridis abundances kept increasing in the high flower density treatment, while P. japonica abundances remained stable in this treatment. After Week 12, abundances of both P. japonica and H. axyridis declined in the low flower density treatment and in absence of flowers for H. axyridis.
An increased number of flowers per chamber also induced a significant reduction in aphid populations (χ2 = 72.2, df = 2, P < 0.001; Fig. 5), and the three treatments were significantly different between each other (Table 1). Abundances were divided by almost two in average between control (no flower) and the high flower density treatment (mean number ± SE per plant: control 163 ± 2, high flower density 92 ± 1). In all three treatments, aphid abundances declined from Week 1 to Week 6. In absence of flowers, they increased from Week 6 to Week 11 and then decreased up to Week 18, while they remained more stable in the presence of flowers from Week 6 to 14 and then declined from Weeks 14 to 18.
Discussion
Intraguild predation—both intra- and interspecific—among biological control agents may negatively affect their population dynamics and consequently pest suppression. In the present study, we showed that the provision of marigold flowers reduced the negative impacts of intra- and interspecific IGP between predatory ladybirds in a laboratory setting. The provision of flowering marigold companion plants resulted in higher ladybird abundances and improved aphid pest control in a realistic greenhouse experiment similar to practical biological control application in tomato crops.
Marigold flowers decreased both intraspecific and interspecific IGP in H. axyridis and P. japonica, with less larvae victims of IGP in the laboratory experiment. We created a food shortage in this experiment via the starvation of ladybird larvae and the absence of prey; hence, conditions were ideal for a strong IGP (Polis and Myers 1989). Harmonia axyridis remained a dominant competitor in this system with higher survival no matter the number of marigold flowers. Total larvae mortality in the interspecific experiment was actually a combination of intraspecific and interspecific IGP. However, the lower total IGP rates in H. axyridis in the two-species experiment (Fig. 3) compared with the one-species experiment (Fig. 2) show that intraspecific IGP in H. axyridis was lower in the two-species experiment. This might be because it was compensated by high interspecific IGP by H. axyridis on P. japonica.
Interestingly, reduced IGP was found only when at least three flowers were provided in both the intraspecific and interspecific experiments. This points at a beneficial impact of marigold related to floral resources and likely the provision of food resources (Jaworski et al. 2019; Ma et al. 2019). Adding marigold floral resources in the diet of ladybirds has been shown to have a positive impact on their development (Wolf et al. 2018; El-Kareim et al. 2019). Preliminary video records in the laboratory showed that starving ladybird larvae tended to aggregate close to the flower pistil (source of pollen) where they showed reduced aggression to congeners (Chen and Wang, unpublished data). However, since the presence of a marigold plant with few flowers was not sufficient to reduce IGP, it is unlikely that marigold plants provided other services in our system, such as a refuge from IGP by plants (Gontijo 2018).
Many studies showed that companion plants may be beneficial via the provision of food resources to enhance the colonization, population growth and efficiency of pests’ natural enemies in conservation biological control (Jaworski et al. 2019; Landis et al. 2000; Li et al. 2021a). The present study is to our knowledge the first demonstration of reduced negative impacts of IGP by supplying companion plants that provide alternative floral resources to biological control agents. Similar to the present study with H. axyridis, Zhao et al. (2017) showed that marigold flowering plants effectively enhanced the development and population growth of the predatory flower bug Orius sauteri (Hemiptera: Anthocoridae) in both laboratory and greenhouse settings. Other companion plant species have been used to sustain the development and population growth of H. axyridis with the aim of enhancing pest biological control, including Perilla frutescens (Hatt and Osawa 2019), Fagopyrum esculentum and Centaurea cyanus (Wolf et al. 2018), and Hibiscus cannabinus (Xiu et al. 2017).
We also found a positive impact of flowering marigold companion plants on ladybird populations in our practical greenhouse biological control application. We observed that populations grew faster with increased flower density up to Week 12 and especially in P. japonica (Fig. 4). The flower provision may have benefit ladybird populations both via the provision of alternative resources improving fitness and reproduction and via reduced IGP. Hatt and Osawa (2019) found an increased fecundity of H. axyridis when fed with a mixed diet of Ephestia kuehniella eggs and Perilla frutescens flowers compared with prey only, while in a previous study we found a + 37% increase in female fecundity of P. japonica females when fed on a mixed diet of M. persicae aphids with C. officinalis flowers compared with aphids only (Jaworski et al. 2019). In our greenhouse experiment, the P. japonica abundances without flowers were roughly multiplied by three in Weeks 7–8 compared with Weeks 1–3 (Fig. 4), while during the same period P. japonica abundances at high flower density were roughly multiplied by seven, i.e., more than twice as fast. Hence, only higher mortality in absence of marigold flowers–very likely via IGP–could have caused such a slower population growth.
Evidence of IGP in mesocosms has been reported at even lower ladybird densities. Sato et al. (2003) found that 25% of H. axyridis and 14% of P. japonica larvae, out of nine larvae on a Hibiscus syriacus plant, died from IGP when aphid resource became rare. In mixed releases (three H. axyridis + three P. japonica + three Coccinella septempunctata larvae) (Coleoptera: Coccinellidae), about 20% only of H. axyridis larvae died, but up to 60% of P. japonica larvae died. In a previous study we observed IGP between H. axyridis, P. japonica and H. variegata for densities of around one ladybird per plant of horsebean in greenhouse conditions (Vicia faba; Wang et al. 2012). Finally, Hironori & Katsuhiro (1997) reported IGP by H. axyridis on C. septempunctata at densities of less than 40 ladybirds per 2 m-high Hibiscus syridis trees. Despite numerous reports of IGP by H. axyridis in agricultural systems, surprisingly few studies actually reported densities at which IGP occurred in field or mesocosm systems (Koch 2003; Pell et al. 2008). However based on the studies reported here, this seems very likely that IGP was at least partly responsible for the excess mortality observed in our greenhouse experiment.
Under the greenhouse temperatures (32–34 °C), we measured a time from ladybird releases to first larvae of three days for H. axyridis and two days for P. japonica, while the observed development time from egg to adult was 16–17 days for H. axyridis and 14–15 days for P. japonica. Therefore, larvae were observed in the greenhouses from the first week (Fig. 4). This confirms previous studies measuring a faster developmental rate for P. japonica than H. axyridis on M. persicae (first instar larvae to adult at 25 °C: 11.3 days for P. japonica, Zhang et al. 2012; 17.1 days for H. axyridis; Lanzoni et al. 2004). Similar to the laboratory interspecific experiment, H. axyridis remained the dominant competitor over P. japonica, with higher abundances.
After Week 12, population growth decreased in both control and low flower density treatments for both species (Fig. 4). This could be related to the reduction in available aphid prey (Fig. 5), potentially causing both reduced fecundity and increased IGP. Note that we could not directly assess the intensity of IGP in our greenhouse experiment because of logistic limitations in observing predation on eggs and larvae at the scale of the greenhouse chambers.
By promoting the diversity of natural enemies, conservation biological control generally enhances pest control, through a higher probability for very efficient natural enemies to be present but also through the niche complementarity of diverse natural enemies (Jonsson et al. 2017). However it may simultaneously result in negative impacts and poor pest control, notably via increased IGP (Straub et al. 2008). Our study yet demonstrated that the use of flowers helped reduce both intra- and interspecific IGP (shown in laboratory experiments) and instead enhanced pest control in a greenhouse crop. We found a better, long-term suppression of aphid populations at higher marigold flower density, related to higher ladybird densities and likely to reduced IGP. This was notably due to earlier suppression of aphid populations, otherwise increasing from Week 6 to Week 11 in absence of flowers.
Companion plants may act as a buffer against IGP in agroecosystems and improve natural enemy coexistence in simplified crop habitat (Zhang et al. 2016). They could improve the attraction of predators by emitting plant volatiles or provide separated micro-habitats or alternative food resources (Song et al. 2012; Li et al. 2014; Jaworski et al. 2019). Further increasing flower density may help further decrease IGP since we found that the effect of marigold flowers depended on flower density. Also, using other species as companion plants or a mixture of species may be beneficial to both ladybird species (Mathews et al. 2016; Xiu et al. 2017). A more diverse plant community has been shown to enhance biodiversity up to the upper trophic levels, increasing species richness and evenness of predation interactions in foodwebs (Barbosa 1998). Hence, this would be worth testing the use of multi-species companion plants in greenhouse systems relying on multiple biological control agents.
In commercial greenhouses, combined releases of multiple species of natural enemies targeting the same pest species are used to improve pest control (Tan et al. 2016). Theoretically, once the nutrition supply is sufficient and stable, the coexisting predators in the same ecological niche tend to segregate spatially (Rosenheim et al. 1995; Amarasekare 2008). However, in a greenhouse available space is strongly limited because of the simplified and isolated system. Thus, we observed no spatial segregation between ladybird species in our greenhouse experiment, and effective pest suppression was observed only with the ecological support of marigold companion plants.
Our study demonstrates the potential for companion plants to improve biocontrol. Such addition of floral resources could be used in systems with high risk of IGP, such as mass rearing of biological control agents, long-term storage and long-distance transportation, or inundative release of biological control agents prior to the exponential growth of pest populations. The provision of alternative floral food resources by easy to maintain companion plants would help reduce IGP and help omnivorous insect predators to overcome negative environmental conditions. Floral resources such as pollen are often integrated in the mass rearing of biological control agents such as predatory mites (Riahi et al. 2016).
We only considered one pest species, M. persicae, in our study. Yet, even a simplified greenhouse cropping system may be much more complex with multiple herbivorous pest and natural enemy species. The role of companion plants in reducing IGP in these more complex systems should be further investigated in the future. The use of molecular detection techniques of predator gut content (Eitzinger et al. 2019) could help quantify predation interactions and the use of companion plants as food supply by predators in complex systems. Finally, the companion plant species and diversity could be optimized to promote populations of the target natural enemies and with no benefits to pest populations.
6. References
Albajes R, Ghullino ML, van Lenteren JC, Elad Y (2000) Integrated pest and disease management in greenhouse crops. Luwer Academic Publishers, Dordrecht, Netherlands
Albrecht M, Kleijn D, Williams NM, Tschumi M, Blaauw BR, Bommarco R, Campbell AJ, Dainese M, Drummond FA, Entling MH, Ganser D, Arjen de Groot G, Goulson D, Grab H, Hamilton H, Herzog F, Isaacs R, Jacot K, Jeanneret P, Jonsson M, Knop E, Kremen C, Landis DA, Loeb GM, Marini L, McKerchar M, Morandin L, Pfister SC, Potts SG, Rundlöf M, Sardiñas H, Sciligo A, Thies C, Tscharntke T, Venturini E, Veromann E, Vollhardt IM, Wäckers F, Ward K, Wilby A, Woltz M, Wratten S, Sutter L (2020) The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecol Lett 23:1488–1498
Amarasekare P (2008) Coexistence of intraguild predators and prey in resource-rich environments. Ecology 89:2786–2797
Aparicio Y, Gabarra R, Arno J (2020) Interactions among Myzus persicae, predators and parasitoids may hamper biological control in Mediterranean peach orchards. Entomol Gen 40:217–228
Balzan MV, Bocci G, Moonen AC (2016) Utilization of plant functional diversity in wildflower stripes for the delivery of multiple agroecosystem service. Entomol Exp Appl 158:304–319
Barbosa PA (1998) Conservation biological control. Academic Press
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Stat Comput 67:1–48
Berkvens N, Landuyt C, Deforce K, Berkvens D, Tirry L, Clercq P (2010) Alternative foods for the multicoloured asian lady beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 107:189–195
Biondi A, Zappalà L, Di Mauro A, Garzia GT, Russo A, Desneux N, Siscaro G (2016) Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis. BioControl 61:79–90
Bommarco R, Ekborm B (2000) Landscape management and resident generalist predators in annual crop systems. In: Ekbom B, Irwin ME, Robert Y (eds) Interchanges of insects between agricultural and surrounding landscapes. Springer, Netherlands
Burgio G, Santi F, Maini S (2002) On intra-guild predation and cannibalism in Harmonia axyridis (Pallas) and Adalia bipunctata L. (Coleoptera: Coccinellidae). Biol Control 24:10–116
Cai ZP, Ouyang F, Su JW, Zhang XR, Liu CL, Xiao YL, Zhang JP, Ge F (2020) Attraction of adult Harmonia axyridis to volatiles of the insectary plant Cnidium monnieri. Biol Control 143:104189
Cai Z, Fang O, Chen J, Yang QF, Desneux N, Xiao YL, Zhang J, Ge F (2021) Biological control of Aphis spiraecola in apples using an insectary plant that attracts and sustains predators. Biol Control 155:104532
Chailleux A, Bearez P, Pizzol J, Amiens-Desneux E, Ramirez-Romero R, Desneux N (2013) Potential for combined use of parasitoids and generalist predators for biological control of the key invasive tomato pest Tuta absoluta. J Pest Sci 86:533–541
Chailleux A, Stirnemann A, Leyes J, Deletre E (2019) Manipulating natural enemy behavior to improve biological control: attractants and repellents of a weaver ant. Entomol Gen 38:191–210
Damien M, Le Lann C, Desneux N, Alford L, Al Hassan D, Georges R, Van Baaren J (2017) Flowering cover crops in winter increase pest control but not trophic link diversity. Agr Ecosyst Environ 247:418–425
Damien M, Llopis S, Desneux N, van Baaren J, Lann C (2020) How does floral nectar quality affect life history strategies in parasitic wasps? Entomol Gen 40:147–156
Desneux N, Decourtye A, Delpeuch J (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Eitzinger B, Abrego N, Gravel D, Huotari T, Vesterinen EJ, Roslin T (2019) Assessing changes in arthropod predator-prey interactions through DNA-based gut content analysis-variable environment, stable diet. Mol Ecol 28:266–280
El-Kareim A, Rashed AA, Marouf AE, Fouda SR (2019) Attractiveness and effects of some flowering plants on the longevity and foraging behavior of certain predatory insects. J Plant Protect Pathol 10:537–541
Fedriani JM, Fuller TK, Sauvajot RM, York EC (2000) Competition and intraguild predation among three sympatric carnivores. Oecologia 125:258–270
Foti MC, Peri E, Wajnberg E, Colazza S, Rostás M (2019) Contrasting olfactory responses of two egg parasitoids to buckwheat floral scent are reflected in field parasitism rates. J Pest Sci 92:747–756
Gagnon AÈ, Heimpel GE, Brodeur J (2015) The ubiquity of intraguild predation among predatory arthropods. PLoS ONE 6:e28061
Gao XY, Shi J, Qiao ZH, Lv DL, Wu HC (2016) Study on the synergistic predation effects of Harmonia axyridis and Propylea japonica on Aphis gossypii. Agric Technol 36:19–20
Gardarin A, Plantegenest M, Bischoff A, Valantin-Morison M (2018) Understanding plant-arthropod interactions in multitrophic communities to improve conservation biological control: useful traits and metrics. J Pest Sci 91:943–955
Gontijo LM (2018) Engineering natural enemy shelter to enhance conservation biological control in field crops. Biol Control 130:155–163
Gurr GM, Wratten SD, Landis DA, You M (2017) Habitat management to suppress pest populations: progress and prospects. Annu Rev Entomol 62:91–109
Hartig F (2019) DHARMa: Residual Diagnostics for Hierarchical (Multi–Level / Mixed) Regression Models. R package version 0.2.4. https://CRAN.R-project.org/package=DHARMa
Hatt S, Osawa N (2019) The role of Perilla frutescens flowers on fitness traits of the ladybird beetle Harmonia axyridis. BioControl 64:381–390
Hatt S, Xu Q, Francis F, Osawa N (2019) Aromatic plants of East Asia to enhance natural enemies towards biological control of insect pests. Entomol Gen 38:275–315
Hironori Y, Katsuhiro S (1997) Cannibalism and interspecific predation in two predatory ladybirds in relation to prey abundance in the field. Entomophaga 42:153–163
Hodek I, Michaud JP (2008) Why is Coccinella septempunctata so successful? (A point-of-view). Eur J Entomol 105:1–12
Hodek I, van Emden HF, Honěk A (2012) Ecology and behaviour of the ladybird beetles (Coccinellidae). Wiley
Jaworski CC, Xiao D, Xu QX, Ramirez-Romero R, Guo XJ, Wang S, Desneux N (2019) Varying the spatial arrangement of synthetic herbivore-induced plant volatiles and companion plants to improve conservation biological control. J Appl Ecol 56:1176–1188
Jonsson M, Kaartinen R, Straub CS (2017) Relationships between natural enemy diversity and biological control. Curr Opin Insect Sci 20:1–6
Koch RL (2003) The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. J Insect Sci. https://doi.org/10.1093/jis/3.1.32
Koch RL, Galvan TL (2008) Bad side of a good beetle: the north American experience with Harmonia axyridis. BioControl 53:23–25
Kuroda T, Miura K (2003) Comparison of the effectiveness of two methods for releasing Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) against Aphis gossypii Glover (Homoptera: Aphididae) on cucumbers in a greenhouse. Appl Entomol Zool 38:271–274
Lamichhane JR, Barzman M, Booij K, Boonekamp P, Desneux N, Huber L, Kudsk P, Langrell SRH, Ratnadass A, Ricci P, Sarah J-L, Messéan A (2015) Robust cropping systems to tackle pests under climate change. Agron Sustain Dev 35:443–459
Landis DA, Wratten SD, Gurr GM (2000) Habitat manipulation to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45:175–201
Lanzoni A, Accinelli G, Bazzocchi GG, Burgio G (2004) Biological traits and life table of the exotic Harmonia axyridis compared with Hippodamia variegata, and Adalia bipunctata (Col., Coccinellidae). J Appl Entomol 128:298–306
Lei ZL, Zong LB, Yang GJ, Xiao C (1988) The influence of temperature on development and predation to larvae of Propylea japonica (Thunberg). Zool Res 9:50
Lenth R (2019) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.3.5. https://CRAN.R-project.org/package=emmeans
Li MT (2013) Biological characteristics and control method of Myzus persicae (Sulzer). J Agric Catastrophol 3:1–4
Li S, Tan XL, Desneux N, Benelli G, Zhao J, Li XH, Zhang F, Gao XW, Wang S (2015) Innate positive chemotaxis to pollen from crops and banker plants in predaceous biological control agents: towards new field lures? Sci Rep 5:12729
Li S, Jaworski CC, Hatt S, Zhang F, Desneux N, Wang S (2021a) Flower strips adjacent to greenhouses help reduce pest populations and insecticide applications inside organic commercial greenhouses. J Pest Sci 94:679–689
Li H, Li B, Lövei GL, Kring TJ, Obrycki JJ (2021b) Interactions among native and non-native predatory Coccinellidae influence biological control and biodiversity. Ann Entomol Soc Am. https://doi.org/10.1093/aesa/saaa047
Liu YJ, Yu JX, Zhou G, Dai LX, Yang ZQ, Zhang LN (2012) Evaluation of biological control of Monochamus alternatus by releasing Scleroderma sichuanensis and Dastarus helophoroides. Hunan For Sci Technol 39:20–23
Lu YH, Wu KM, Jiang YY, Guo YY, Desneux N (2012) Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487:362–365
Lu ZX, Zhu PY, Gurr GM, Zheng XS, Read DME, Heong KL, Yang YJ, Xu HX (2014) Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: prospects for enhanced use in agriculture. Insect Sci 21:1–12
Ma YY, Zhang F, Wang S, Di N (2019) Synergistic effect of functional plant Calendula officinalis (Asterales: Asteraceae) to the colonization of Coccinella septempunctata (Coleoptera: Coccinellidae) in greenhouse. J Environ Entomol 41:276–282
Mathews CR, Brown MW, Wäckers FL (2016) Comparison of peach cultivars for provision of extrafloral nectar resources to Harmonia axyridis (Coleoptera: Coccinellidae). Environ Entomol 45:649–658
Michaud JP (2010) A comparative study of larval cannibalism in three species of ladybird. Ecol Entomol 28:92–101
Mirande L, Desneux N, Haramboure M, Schneider MI (2015) Intraguild predation between an exotic and a native coccinellid in Argentina: the role of prey density. J Pest Sci 88:155–162
Mohammadpour M, Hosseini M, Michaud JP, Karimi J, Hosseininaveh V (2020) The life history of Nabis pseudoferus feeding on Tuta absoluta eggs is mediated by egg age and parasitism status. Biol Control 151:104401. https://doi.org/10.1016/j.biocontrol.2020.104401
Ortiz-Martínez S, Staudacher K, Baumgartner V, Traugott M, Lavandero B (2020) Intraguild predation is independent of landscape context and does not affect the temporal dynamics of aphids in cereal fields. J Pest Sci 93:235–249
Osawa N (2015) Sex-dependent effects of sibling cannibalism on life history traits of the ladybird beetle Harmonia axyridis. Biol J Lin Soc 76:349–360
Ovchinnikov AN, Belyakova NA, Ovchinnikova AA, Reznik SY (2019) Factors determining larval cannibalistic behavior in invasive and native populations of the multicolored Asian ladybird, Harmonia axyridis. Entomol Gen 38:243–254
Parolin P, Bresch C, Poncet C, Desneux N (2012) Functional characteristics of secondary plants for increased pest management. Int J Pest Manag 58:369–377
Pell JK, Baverstock J, Roy HE, Ware RL, Majerus MEN (2008) Intraguild predation involving Harmonia axyridis: a review of current knowledge and future perspectives. BioControl 53:147–168
Pervez A, Gupta AK (2010) Role of surface chemicals in egg cannibalism and intraguild predation by neonates of two aphidophagous ladybirds, Propylea dissecta and Coccinella transversalis. J Appl Entomol 128:691–695
Perovic DJ, Gámez-Virués S, Landis DA et al (2018) Managing biological control services through multi-trophic trait interactions: review and guidelines for implementation at local and landscape scales. Biol Rev 93:306–321
Polis GA, Myers CA (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Polis GA, Holt RD (1992) Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol Evol 7:151–154
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/.
Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N (2011) Ecology and management of the soybean aphid in North America. Ann Rev Entomol 56:375–399
Riahi E, Fathipour Y, Talebi AA, Mehrabadi M (2016) Pollen quality and predator viability: life table of Typhlodromus bagdasarjani on seven different plant pollens and two-spotted spider mite. Syst Appl Acarol 21:1399–1412
Rosenheim JA, Wilhoit LR, Armer CA (1993) Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 96:439–449
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological-control agents: theory and evidence. Biol Control 5:303–335
Sanchez-Hernandez CV, Desneux N, Bao-Fundora L, Ramirez-Romero R (2021) Alternative extraguild prey modifies focal extraguild prey consumption and parasitism but not intraguild predation intensity. Biol Control 153:104475
Sato S, Dixon AFG, Yasuda H (2003) Effect of emigration on cannibalism and intraguild predation in aphidophagous ladybirds. Ecol Entomol 28:628–633
Snyder WE, Evans EW (2006) Ecological effects of invasive arthropod generalist predators. Annu Rev Ecol Evol Syst 37:95–122
Snyder WE (2019) Give predators a complement: Conserving natural enemy biodiversity to improve biocontrol. Biol Control 135:73–82
Song BZ, Zhang J, Wiggins NL, Yao YC, Tan GB, Sang XS (2012) Intercropping with aromatic plants decrease herbivore abundance species richness, and shifts arthropod community trophic structure. Environ Entomol 4:872–879
Straub CS, Finke DL, Snyder WE (2008) Are the conservation of natural enemy biodiversity and biological control compatible goals? Biol Control 45:225–237
Sun H, Song Y (2019) Establishment of a wheat banker plant system for the parasitoid Aphidius gifuensis against Myzus persicae in greenhouse chili pepper. Appl Entomol Zool 54:1–9
Tan XL, Zhao J, Wang S, Zhang F (2015) Optimization and evaluation of microencapsulated artificial diet for mass rearing the predatory ladybird Propylea japonica (Coleoptera: Coccinellidae). Insect Sci 22:111–120
Tan XL, Hu NN, Zhang F, Ramirez-Romero R, Desneux N, Wang S, Ge F (2016) Mixed release of two parasitoids and a polyphagous ladybird as a potential strategy to control the tobacco whitefly Bemisia tabaci. Sci Rep 6:28245
Thomine E, Rusch A, Supplisson C, Monticelli LS, Amiens-Desneux E, Lavoir AV, Desneux N (2020) Highly diversified crop systems can promote the dispersal and foraging activity of the generalist predator Harmonia axyridis. Entomol Gen 40:133–145
van Veen FJF, Morris RJ, Godfray HCJ (2006) Apparent competition, quantitative food webs, and structure of phytophagous insect communities. Annu Rev Entomol 51:187–208
Vuong PT, Kim J, Song Y (2001) The seasonal occurrence of the two aphid species, Myzus persicae and Aphis gossypii, and their natural enemies on vegetable crops in Chinju, Korea. J Asia-Pacific Entomol 4:41–44
Wäckers FL, van Rijn PCJ (2012) Pick and mix: selecting flowering plants to meet the requirements of target biological control insects. In: Gurr G, Wratten S, Snyder W, Read D (eds) Biodiversity and insect pests. Wiley
Wang S, Tan XL, Xu HX, Zhang F (2012) Interspecific competition among three predacious ladybirds (Coleoptera: Coccinellidae). Scientia Agricultura Sinica 45:3980–3987
Wang YS, Yao FL, Soares MA, Basiri SE, Amiens-Desneux E, Campos MR, Lavoir AV, Desneux N (2020) Effects of four non-crop plants on life history traits of the lady beetle Harmonia axyridis. Entomol Gen 40:243–252
Ware RL, Majerus MEN (2008) Intraguild predation of immature stages of British and Japanese coccinellids by the invasive ladybird Harmonia axyridis. BioControl 53:169–188
Wolf S, Romeis J, Collatz J (2018) Utilization of plant-derived food sources from annual flower strips by the invasive harlequin ladybird Harmonia axyridis. Biol Control 122:118–126
Xiu CL, Pan HS, Ali A, Lu YH (2017) Extrafloral nectar of Hibiscus cannabinus promotes adult populations of Harmonia axyridis. Biocontrol Sci Tech 27:1009–1013
Xu Q, Wang S, Li S, Hatt S (2020) Conservation Biological Control in Organic Greenhouse Vegetables. In: Gao Y, Hokkanen H, Menzler-Hokkanen I (eds) Integrative Biological Control. Progress in Biological Control, vol 20. Springer, Cham
Yang NW, Zang LS, Wang S, Guo JY, Xu HX, Zhang F, Wan FH (2014) Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol Control 68:92–102
Zhang SZ, Li JJ, Shan HW, Zhang F, Liu TX (2012) Influence of five aphid species on development and reproduction of Propylaea japonica (Coleoptera: Coccinellidae). Biol Control 62:135–139
Zhang ZQ, Zhou C, Xu YY, Huang XQ, Zhang LX, Mu W (2016) Effects of intercropping tea with aromatic plants on population dynamics of arthropods in Chinese tea plantations. J Pest Sci 90:227–237
Zhao J, Guo XJ, Tan XL, Desneux N, Zappala L, Zhang F, Wang S (2017) Using Calendula officinalis as a floral resource to enhance aphid and thrips suppression by the flower bug Orius sauteri (Hemiptera: Anthocoridae). Pest Manag Sci 73:515–520
Acknowledgements
The study was funded by the project 32072479 supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China (2017YFD0201000; 2017YFD0200400), the Beijing Key Laboratory of Environment Friendly Management on Fruit Diseases and Pests in North China (BZ0432) and the Key Research and Development Program of Jiangxi Province (20202BBF62006).
Author information
Authors and Affiliations
Contributions
SW, CCJ, YL and XG designed the study; CX and JW performed the experiments; CX, HDJ and CCJ analyzed the data; YL, CX, CCJ and SW wrote the manuscript. All authors read and approved the manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare they have no competing interests. SW is a Subject Editor of Journal of Pest Science and was not involved in the journal’s review of, or decision related to, this manuscript.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, Y., Chen, X., Dai, H. et al. Flower provision reduces intraguild predation between predators and increases aphid biocontrol in tomato. J Pest Sci 95, 461–472 (2022). https://doi.org/10.1007/s10340-021-01396-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01396-x