Abstract
Predaceous ladybird beetles are known to consume alternative foods from flowers, especially when prey is scarce. Flower-rich semi-natural habitats in agroecosystems generally host a diversity of natural enemies, including predaceous ladybird beetles, suggesting that the availability of flowers may have a positive role in their fitness traits. In this study, we test whether feeding on flowers of Perilla frutescens (Lamiaceae) increases longevity and fecundity in Harmonia axyridis Pallas (Coleoptera: Coccinellidae). The longevity of H. axyridis females and males fed with five flowers was significantly greater than those fed with one flower and in the control group (no food), although the provision of flowers had no positive effect on the increase of body weight in males and females. The number of eggs and oviposition frequency in H. axyridis fed with flowers plus prey, as well as with prey only, were significantly larger than those fed with only flowers, whereas no significant difference was observed between individuals fed with the mixed diet and those with only prey. However, on the first day of the diet assignment, the number of eggs from individuals with the mixed diet was significantly higher than of those with only prey and those with only flowers. The results show that flowers of P. frutescens, mixed with prey, have a positive effect on H. axyridis survival and early reproduction, suggesting that flowers may play an important role in increasing fitness in H. axyridis. The possibility of P. frutescens sown in fields to support populations of the predator toward conservation biological control is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in predator–prey interactions has risen in a move to enhance the natural regulation of pests in agroecosystems toward relying less on chemical inputs, especially insecticides (e.g., Brewer and Eliott 2004; Eubanks and Finke 2014; Jonsson et al. 2017). Diversifying agroecosystems can favor populations of pest predators by increasing the number of shelters and habitats that host prey and non-prey food (Gurr et al. 2017; Hatt et al. 2018). Notably, flower-rich semi-natural habitats in agricultural landscapes host a diversity of natural enemies, among them predaceous ladybird beetles (Coleoptera: Coccinellidae) (Hatt et al. 2017, 2019a). Predaceous ladybird beetles, which feed on aphids (Hemiptera: Aphididae) (Dixon 2000), weevils (Coleoptera: Curculionoidea) (Evans et al. 1999), scales (Hemiptera: Coccoidea), whiteflies (Hemiptera: Aleyrodidae), and psyllids (Hemiptera: Psylloidea) (Hodek and Honek 2009), can also feed on floral pollen and nectar as well as extrafloral nectar of flowers (Lundgren 2009). However, different types of food may play different roles in their development, reproduction, and survival because these foods are not equally nutritive. Hodek and Honek (1996) distinguished essential food from alternative food: essential food allows adults to oviposit and larvae to develop, while alternative food allows them to survive only. Mixed feeding (i.e., of both essential and alternative food) also happens and can improve ladybird beetle reproduction, especially when essential food is limited (Evans et al. 1999).
The timing of food availability can determine ladybird beetle survival and reproduction schedule. Therefore, resource tracking (i.e., functional and numerical responses to temporal and spatial variation of resources) determines population levels and the related behavioral variations (e.g., Osawa 2000). Notably, oviposition of aphidophagous ladybird beetles normally occurs before the aphid density peaks to optimize the availability of food resources for their offspring (Hemptinne et al. 1992). Still, the larvae may lack such essential food when the aphid population collapses before the end of their development (Osawa 1992), pushing them to cannibalism, intraguild predation, or non-prey food consumption (e.g., Takahashi 1989; Agarwala and Dixon 1992). Moreover, when adult beetles emerge from pupae, it is very likely that aphids are no longer available (Osawa 1992) and here again, their survival would depend on the availability of alternative food. Later, their nutritional status will also determine their ability to oviposit. Notably, feeding on alternative flower food instead of starving before finding essential prey food reduces their pre-oviposition period (Wolf et al. 2018).
Flower pollen and nectar are generally alternative foods for predaceous ladybird beetles (Lundgren 2009). Understanding the role of flowers on ladybird longevity and fecundity is essential to design agroecosystems sustaining populations of predators toward pest control (Obrycki et al. 2009). Harmonia axyridis Pallas (Coleoptera: Coccinellidae), a native ladybird beetle in East Asia (Sasaji 1971), is such a predaceous species that feeds on a diversity of prey (among which potential pests for agricultural plants) (Koch 2003), as well as nectar and pollen of some wild flowers (Wolf et al. 2018) and extrafloral nectar of some fruit trees (Mathews et al. 2016). Past experiments evaluated the effect of sugar-only or pollen-only diet, as well as a mixed diet of prey with pollen, on H. axyridis longevity and fecundity (Evans and Gunther 2005; Berkvens et al. 2008a). However, further research is needed to evaluate the effect of flower food on H. axyridis longevity and fecundity, by using fresh flowers that may more clearly reflect natural conditions, instead of solely pollen or sugar water. Indeed, nectar is not composed of sugar only but also of a diversity of additional nutrients such as amino acids, proteins, lipids, mineral ions, alkaloids, ethanol, eugenol, and methyl eugenol (Lu et al. 2014) that should not be ignored. Moreover, ladybird beetles will probably simultaneously consume pollen and nectar when foraging on flowers and there is no reason to separate them in experiments.
By using Perilla frutescens (L.) Britton (Lamiaceae), an aromatic flower plant that has been cultivated for centuries in East Asia, this study aims at evaluating the effect of flowers on fitness traits of H. axyridis. First, the benefit of flower availability to the survival of ladybird beetles just emerging is assessed, and it is more in particular explored whether the number of available flowers is of significant importance. Second, the benefit of flower availability on adult fecundity is evaluated, by comparing the effect of flower only, prey only, and a mixed diet on adult oviposition. Finally, the role of flowers on the longevity and fecundity of H. axyridis is discussed in regard to potential applications for conservation biological control.
Materials and methods
Flower plant
Perilla frutescens is an aromatic plant that has been cultivated for centuries in East Asia because both leaves and flowers can be used in food cuisine as well as in medicine (e.g., Nitta et al. 2003; Seo and Baek 2009). It can have red leaves with purple flowers (the type considered in the present study) but also green leaves with white flowers. In agricultural landscapes, it is sown in rows, but it is also observed spreading through fields because it can naturally regrow from seeds kept in soil and it does not need any particular care. Its wide spread in agricultural landscapes makes it a potential source of floral food for insects such as ladybird beetles. Nevertheless, interactions between P. frutescens and insect predators have not been studied so far (Hatt et al. 2019b). Because of the use of the flowers in local cuisine, fresh cut flowers can be obtained every day in the daily wholesale market in Kyoto (Japan). Such fresh flowers were purchased on a regular basis during the experiments, always from the same provider (Syuyo, commercial supplier of P. frutescens flowers at Kyoto City central wholesale market, Kyoto, Japan), and they were kept at 5 °C in the laboratory to always provide them fresh to the ladybird beetles.
Longevity experiment
Harmonia axyridis ladybird beetles used in the longevity experiment were purchased (Agrisect®, Tsukuba, Japan) and delivered at the pupal stage. One pupa was placed per plastic Petri dish (7 cm wide, 2 cm high) on a filter paper and kept in an incubator (25 °C, 8:16 L:D photoperiod). Once adults emerged, they were assigned to one of three treatments: (1) five P. frutescens flowers provided daily, (2) one P. frutescens flower provided daily, and (3) no food (control). Every day, newly cut fresh flowers were provided to ladybird beetles and the remains of those of the previous day were removed. Water was provided to all treatments with a piece of cotton that was humidified every day in each Petri dish. Alive or dead individuals were monitored on a daily basis. Adults were weighed (A&D Electronic Balance® FA-200, 0.001 g) at emergence (day 1) and at death. Sex was determined at death. The whole experiment was conducted over three runs to manage a reasonable number of individuals at the same time. Moreover, it happened that some pupae did not turn into adults and new ones were purchased. The objective was to obtain at least 30 replications per treatment per sex, and in the end, between 29 and 46 individuals were considered per treatment and sex (Table 1).
Fecundity experiment
Harmonia axyridis ladybird beetles used in the fecundity experiment were the second generation offspring of 13 individuals (seven males and six females) collected at the botanical garden of Kyoto University (35° 03′N, 135° 79′E) in June 2018. The 13 collected individuals reared in the laboratory were fed with eggs of Ephestia kuehniella (Lepidoptera: Pyralidae) (Beneficial Insectary®, Ontario, Canada) to allow them to reproduce. Several hundred offspring adults were obtained as the second generation. All the adults used in the experiment were the offspring of the second generation (i.e., the third generation). They were reared as same-sex groups of unmated individuals in plastic cages (13 cm wide, 10 cm high) to prevent their mating before the experiment began and they were fed with lyophilized drone pupae powder (Agrisect®, Tsukuba, Japan), the nutritional quality of which allowed them to stay alive but limited the development of their sexual maturity. At the start of the experiment, one male and one female were placed together in a plastic Petri dish (7 cm wide, 2 cm high) with a filter paper and were fed with E. kuehniella eggs. The quantity of E. kuehniella eggs provided daily corresponded to half of a small laboratory spoon (0.027 g ± 0.004, measured by weighing half a spoon of eggs 20 times). This same quantity was provided daily all along the experiment to the ladybird beetles whose diet treatment included prey food (see hereafter). Couples were randomly divided into three groups (i.e., the three future treatments). Once the female in a pair oviposited a first egg batch (i.e., indicating that the female had the ability for oviposition), the diet of the pair was changed to its previously assigned diet (i.e., treatment): (1) E. kuehniella eggs provided daily (i.e., prey only), (2) five P. frutescens flowers provided daily (i.e., flower only), and (3) both E. kuehniella eggs and five P. frutescens flowers provided daily (i.e., prey + flower). Frozen E. kuehniella eggs were used as a substitute for natural prey food instead of aphids. Indeed, despite nutritional variations between E. kuehniella eggs and aphids (e.g., pea aphid Acyrthosiphon pisum (Harris)) (Specty et al. 2003), H. axyridis can reproduce on a diet of E. kuehniella eggs with equivalent egg batch size as when feeding on A. pisum (Berkvens et al. 2008b) and E. kuehniella eggs are intermediate suitable food for H. axyridis development (Noriyuki and Osawa 2012). During the experiment, we observed signs of flowers chewed by the ladybird beetles in the treatments with flowers only and prey plus flowers. The ladybird beetles were offered their respective diet in a clean Petri dish with filter paper every day. The number of eggs laid was counted on a daily basis during ten days from the first day after diet change (i.e., the very first egg batch laid before diet change was excluded). When eggs were cannibalized by ladybird beetles, the remainders of eaten eggs sticking to the filter paper or to the Petri dish were used to estimate the total number of oviposited eggs. It happened that the male or the female in one pair died, and, in this case, the whole pair was excluded. Because of the number of ladybird beetles available from the laboratory culture and because of some deaths, the experiment was conducted over four runs. In the end, 30 pairs were considered for each treatment.
Statistical analysis
Longevity
The Kaplan–Meier survival curve was used to assess the effect of diet (three treatments: five flowers daily, one flower daily, control) on ladybird beetle longevity (package “survival,” Therneau and Grambsch 2000). The effect of diet was tested by using a Log-rank test (package “survival”) followed by a multiple comparisons of survival curves test with a Bonferroni p-value adjustment (package “survminer,” Kassambara and Kosinski 2018) (p < 0.05). Males and females were considered separately. Similar analyses were also conducted to compare the longevity of males and females within each diet. The effect of diet on percentage of weight gain between emergence from pupae and adult death was compared, for males and females separately, using an analysis of variance (ANOVA, p < 0.05). The normality of the data and the homogeneity of variances across the samples were verified by using the Shapiro–Wilk test (p < 0.05) and Bartlett’s test (p < 0.05), respectively. The means of percentage of weight gain were compared between diets using a pairwise Tukey test (package “multcomp,” Hothorn et al. 2008) (p < 0.05).
Fecundity
The effect of diet (three treatments: prey only, flower only, prey + flower) on the number of eggs laid over ten days was assessed by fitting a Generalized Linear Mixed effect Model (GLMM, package “lme4,” Bates et al. 2015) with the Poisson error distribution (log link function). Diet was included as a fixed factor and ladybird beetle couple (identified by a single number) nested within their respective run was included as a random factor because eggs laid by the same couples were counted over ten successive days. The effect of diet was tested using a likelihood ratio test (p < 0.05) and the means of oviposited eggs were compared between diets using a pairwise Tukey test (p < 0.05). A focus on egg numbers oviposited on day 1 was realized by fitting a GLMM with Poisson error distribution (log link function) considering diet as a fixed factor, runs as a random factor and tested with a likelihood ratio test (p < 0.05) followed by a pairwise Tukey test (p < 0.05).
The effect of diet on oviposition frequency (i.e., the number of laying-egg days over the ten-day observation) was also assessed by fitting a GLMM with Poisson error distribution (log link function) considering diet as a fixed factor and runs as a random factor, tested with a likelihood ratio test (p < 0.05) followed by a pairwise Tukey test (p < 0.05). All statistical analyses were conducted with R software (R Core Team 2017).
Results
Longevity
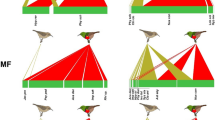
Diet had a significant effect on longevity of H. axyridis adults for females (df = 2; χ2 = 97.3; p < 0.001) and males (df = 2; χ2 = 84.9; p < 0.001) (Fig. 1). Females and males lived significantly longer when they were fed with five flowers daily compared with the other diets, and significantly longer when they were fed with one flower daily compared with water only (i.e., control) (Fig. 1, Table 1). When considering each diet separately, it appears that males lived significantly longer than females when fed with five flowers daily (df = 1; χ2 = 7.1; p = 0.008), but no significant differences were found between male and female longevity when fed with one flower daily (df = 1; χ2 = 0.7; p = 0.401), or water only (df = 1; χ2 = 0.1; p = 0.706).
Kaplan–Meier survival curves of female (a) and male (b) Harmonia axyridis fed with daily provided diets: five Perilla frutescens flowers and water (five flowers), one P. frutescens flower with water (one flower), and water only (control). Details on replication numbers are given in Table 1
Diet did not have a significant effect on weight gain differences between emergence from pupae and death for females (F2,122 = 1.79; p = 0.171) and males (F2,95 = 2.83; p = 0.064). During their lifetime, females and males lost from about 19% to 24% of their initial weight on average (Table 1).
Fecundity
Diet had a significant effect on the number of eggs laid (df = 2; χ2 = 73.0; p < 0.001) and oviposition frequency (df = 2; χ2 = 150.48; p < 0.001) over ten days (Fig. 2). Harmonia axyridis females feeding only on flowers laid significantly fewer eggs and significantly less often than those assigned to the other diets, and no significant differences were found between ladybird beetles fed with prey only and those fed with a mix of prey and flowers (Fig. 2). Looking at the number of eggs laid on each day reveals that diet affected the number of eggs laid on the first day (df = 2; χ2 = 375.11; p < 0.001): ladybird beetles fed with flowers only still laid significantly fewer eggs than those assigned to other diets, but ladybird beetles fed with the mixed diet laid significantly more eggs than those fed with prey only (Fig. 3).
Effect of daily provided diets (Prey + Flower: Ephestia kuehniella frozen eggs and five Perilla frutescens flowers, Prey: E. kuehniella frozen eggs, Flower: five P. frutescens flowers), on mean (± SE) number of eggs laid per day per female over ten days (a) and mean (± SE) oviposition frequency per female over ten days (b). Letters above bars represent pairwise significant differences (p < 0.05) between treatments using a post-hoc Tukey test on GLMM
Change along ten days of the mean (± SE) number of eggs laid per female according to daily provided diets (Prey + Flower: Ephestia kuehniella frozen eggs and five Perilla frutescens flowers, Prey: E. kuehniella frozen eggs, Flower: five P. frutescens flowers) with respective linear regressions and coefficients of determination (R2) (a). A focus on the effect of diet on day 1 is given with different letters above bars representing pairwise significant differences (p < 0.05) between treatments using a post-hoc Tukey test on GLMM (b)
Discussion
Longevity
The present results indicate that the predaceous ladybird beetle H. axyridis can feed on resources provided by P. frutescens flowers, which allows it to survive longer than when starving (Fig. 1; Table 1). Moreover, increasing the number of available flowers enhances its survival ability in males and females (Fig. 1; Table 1). Thus, P. frutescens flowers provide key nutrients allowing adult ladybird beetles to survive in the absence of prey, although these specific nutrients were not identified in the present study. This is consistent with previous studies exploring the effect of other non-insect alternative food on H. axyridis longevity, such as fruits of apple tree (Malus domestica L., Rosaceae), pear tree (Pyrus communis L., Rosaceae), or raspberry tree (Rubus idaeus L., Rosaceae) (Berkvens et al. 2010) as well as on extrafloral nectar of fruit trees like peach (Prunus persica L., Rosaceae) (Mathews et al. 2016).
In this study, individuals fed with five flowers daily lived on average from 12 to 14 days only (in comparison, prey-fed H. axyridis can live for several months, Soares et al. 2001) (Table 1). Moreover, their diet did not lead to an increase in their body weight, which even decreased (Table 1). This loss of weight was equivalent across diets, regardless of whether ladybird beetles were starving, fed with one or five flowers daily. Hagen (1962) highlighted that some non-insect food may not be of value for fat synthesis by predaceous ladybird beetles. Our results suggest that floral food from P. frutescens is a resource that is not converted to fat, which explains why this flower cannot sustain H. axyridis in the long term. Instead it can be hypothesized that the energy obtained from P. frutescens has been involved in the short-term survival and searching behavior for potential better resources. Adults of H. axyridis are known to be highly mobile when intensively searching for favorable habitats (Osawa 2000). However, they face a trade-off between saving energy to sustain themselves or consuming it to find better resources (Evans 2003). Harmonia axyridis females have been observed staying 4.70 ± 0.60 (mean ± SE, n = 6) days in a given habitat through six-generation analysis (Osawa 2000), a shorter time than the 12–14 days of average survival observed when feeding on several P. frutescens flowers daily (Table 1). Thus, this circumstantial evidence strongly suggests that the presence of P. frutescens in the environment could allow H. axyridis males and females to survive when individuals emerging from pupae are prey-limited, until migrating toward higher-quality habitats for mating and ovipositing.
Fecundity
The present results also indicate that feeding only on P. frutescens does not allow H. axyridis to reproduce, and that a supplement of P. frutescens to prey food does not greatly increase its reproduction ability in the long term, compared with prey alone (Figs. 2, 3). Still, ladybird beetles laid significantly more eggs on the first day when they were fed with both flower and prey food, compared with prey food alone (Fig. 3). In past studies exploring alternative food for ladybird beetles, pollen collected from honeybees or sugar water to simulate nectar were provided, and it was shown that ladybird beetles cannot reproduce on such diets alone (Evans et al. 1999; Evans and Gunther 2005; Berkvens et al. 2008a). Regarding a mixed diet of prey with flower food, Berkvens et al. (2008a) did not observe an increase in egg batch sizes and oviposition frequency when H. axyridis was fed with E. kuehniella eggs and pollen compared with E. kuehniella eggs only. Our own observations with fresh P. frutescens flowers are generally consistent with these previous studies and in accordance with the fact that non-prey food is not nutritionally and metabolically satisfactory for ovigenesis of most predaceous ladybird beetles (Hagen 1962).
Nonetheless the present results reveal a significant increase of oviposited eggs per female on the first day when flowers are mixed with prey compared with prey alone (Fig. 3). It suggests that floral resources, combined with prey food, accelerate oviposition in ladybird beetles. Previously, Evans and Dixon (1986) observed that ladybird beetles laid eggs in the presence of aphid honeydew, which is also a sugar-based non-prey food, like nectar (although they did not confirm the effect of aphid honeydew on fitness traits of ladybird beetles).
This result is of significant importance because it is predicted from the Ruler–Lotka equation that early reproduction plays an important role in increasing fitness (Stearns 1992), especially for organisms with a short life-span (Varpe et al. 2007). To lay their eggs, ladybird beetles females generally search for patches of aphids that will be used as nursery prey for their offspring (Dixon 2000). It is acknowledged that their offspring’s chance to survive will be high when females oviposit during a limited time window, that is above a minimum aphid density but below their population peak (Hemptinne et al. 1992; Kindlmann and Dixon 1993; Osawa 2000). In addition, it was proposed that few eggs should be laid during this “egg window” to guarantee the survival of all the larvae before the aphid population collapses (Dixon 2000). However, high larval mortality of H. axyridis is sometimes observed, especially at the fourth instar in fields because of food shortage (Osawa 1993), suggesting that it is difficult for females to determine a suitable timing for oviposition. In this context, females will probably take a bet hedging strategy for oviposition (e.g., Hooper 1999): to lay as many eggs as possible in many habitats to guarantee the survival of some offspring. Environments rich in flowers and prey (i.e., comprising both essential and alternative food) could be seen as high-quality habitats for ladybird beetle females and their future offspring. For instance, Wolf et al. (2018) reported that larvae of H. axyridis fed with both flowers (Fagopyrum esculentum Moench, Polygonaceae) and prey (Spodoptera littoralis Boisduval caterpillars, Lepidoptera: Noctuidae) developed better than larvae fed with each diet separately. The acceleration of oviposition induced by flower nutrients could be an adaptive behavior pushing females to lay most of their eggs as soon as possible. A resultant effect being an enhanced predation pressure of the larvae on the available prey. Flowers may have here an important role regarding the sustainable use of natural enemies in agroecosystems.
Perspectives: implications for conservation biological control
In East Asia, P. frutescens blooms in September, when temperature and air humidity decrease after a hot and wet summer. Hence, P. frutescens blooming occurs when H. axyridis beetles start to be active after summer aestivation (summer aestivation is seen as an adaptation to high temperature, e.g., Osawa 2011). According to the present results, available flowers might be especially useful during this transition to secure to ladybird beetles a source of energy to survive and to allow them to search for prey. Perilla frutescens flowers would also enhance the oviposition of H. axyridis females when the prey is already available (i.e., mixed diet), which would secure the ladybird beetle’s reproductive cycle in addition to increasing the number of predatory larvae. Indeed, potential prey like aphids can become abundant in autumn and be an important threat to autumn crops (e.g., foxglove aphid Aulacorthum solani (Kaltenbach) on soybean [Glycine max L. Merr., Fabaceae] (Inoue 1981); cabbage aphid Brevicoryne brassicae (Linnaeus) on cabbage [Brassica oleracea L., Brassicaceae] (pers. obs.)). Thus, planting P. frutescens at field margins, as an intercrop, potentially within a mixture of other flowering plant species, or just letting them regrow naturally, is a promising ecological practice to enhance conservation biological control by predaceous ladybird beetles like H. axyridis (Obrycki et al. 2009). In addition, other natural enemies than ladybird beetles like aphidophagous hoverflies (Diptera: Syrphidae) have been observed visiting P. frutescens flowers (pers. obs.). Future research could assess the diversity of natural enemies that visit the flowers of P. frutescens and evaluate the effect of P. frutescens plantings on pest suppression in a diversity of adjacent crops.
References
Agarwala BK, Dixon AFG (1992) Laboratory study of cannibalism and interspecific predation in ladybirds. Ecol Entomol 17:303–309
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Berkvens N, Bonte J, Berkvens D, Deforce K, Tirry L, De Clercq P (2008a) Pollen as an alternative food for Harmonia axyridis. BioControl 53:201–210
Berkvens N, Bonte J, Berkvens D, Tirry L, De Clercq P (2008b) Influence of diet and photoperiod on development and reproduction of european populations of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). BioControl 53:211–221
Berkvens N, Landyuit C, Deforce K, Berkvens D, Tirry L, De Clercq P (2010) Alternative foods for the multicoloured asian lady beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 107:189–195
Brewer MJ, Eliott NC (2004) Biological control of cereal aphids in north America and mediating effects of host plant and habitat manipulations. Annu Rev Entomol 49:219–242
Dixon AFG (2000) Insect predator–prey dynamics: ladybird beetles and biological control. Cambridge University Press, Cambridge
Eubanks MD, Finke DL (2014) Interaction webs in agroecosystems: beyond who eats whom. Curr Opin Insect Sci 2:1–6
Evans EW (2003) Searching and reproductive behaviour of female aphidophagous ladybirds (Coleoptera: Coccinellidae): a review. Eur J Entomol 100:1–10
Evans EW, Dixon AFG (1986) Cues for oviposition by ladybird beetles (Coccinellidae): response to aphids. J Anim Ecol 55:1027–1034
Evans EW, Gunther DI (2005) The link between food and reproduction in aphidophagous predators: a case study with Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 102:423–430
Evans EW, Stevenson AT, Richards DR (1999) Essential versus alternative foods of insect predators: benefits of a mixed diet. Oecologia 121:107–112
Gurr GM, Wratten SD, Landis DA, You M-S (2017) Habitat management to suppress pest populations: progress and prospects. Annu Rev Entomol 62:91–109
Hagen KS (1962) Biology and ecology of predaceous Coccinellidae. Annu Rev Entomol 7:289–326
Hatt S, Uyttenbroeck R, Lopes T, Mouchon P, Chen J, Piqueray J, Monty A, Francis F (2017) Do flower mixtures with high functional diversity enhance aphid predators in wildflower strips? Eur J Entomol 114:66–76
Hatt S, Boeraeve F, Artru S, Dufrêne M, Francis F (2018) Spatial diversification of agroecosystems to enhance biological control and other regulating services: an agroecological perspective. Sci Total Environ 621:600–611
Hatt S, Uyttenbroeck R, Lopes T, Mouchon P, Osawa N, Piqueray J, Monty A, Francis F (2019a) Identification of flower functional traits affecting abundance of generalist predators in perennial multiple species wildflower strips. Arthropod-Plant Interact 13:127–137
Hatt S, Xu Q, Francis F, Osawa N (2019b) Aromatic plants of east Asia to enhance natural enemies towards biological control of insect pests. A review. Entomol Gen 38:275–315
Hemptinne J-L, Dixon AFG, Coffin J (1992) Attack strategy of ladybird beetles (Coccinellidae): factors shaping their numerical response. Oecologia 90:238–245
Hodek I, Honek A (1996) Ecology of Coccinellidae. Kluwer, Dordrecht
Hodek I, Honek A (2009) Scale insects, mealybugs, whiteflies and psyllids (Hemiptera, Sternorrhyncha) as prey of ladybirds. Biol Control 51:232–243
Hooper KR (1999) Risk-spreading and bet-hedging in insect population biology. Annu Rev Entomol 44:535–560
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Inoue H (1981) Major species of aphids and their seasonal occurrence on soybean in Chikugo. Proc Assoc Plant Prot Kyushu 27:109–111
Jonsson M, Kaartinen R, Straub CS (2017) Relationships between natural enemy diversity and biological control. Curr Opin Insect Sci 20:1–6
Kassambara A, Kosinski M (2018) survminer: drawing survival curves using “ggplot2”. R package version 0.4.3. https://cran.r-project.org/package=survminer. Accessed 22 Nov 2018
Kindlmann P, Dixon AFG (1993) Optimal foraging in ladybird beetles (Coleoptera: Coccinellidae) and its consequences for their use in biological control. Eur J Entomol 90:443–450
Koch RL (2003) The multicolored asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J Insect Sci 3:32
Lu Z-X, Zhu P-Y, Gurr GM, Zheng X-S, Read DMY, Heong K-L, Yang Y-J, Xu H-X (2014) Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: prospects for enhanced use in agriculture. Insect Sci 21:1–12
Lundgren JG (2009) Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol Control 51:294–305
Mathews CR, Brown MW, Wäckers FL (2016) Comparison of peach cultivars for provision of extrafloral nectar resources to Harmonia axyridis (Coleoptera: Coccinellidae). Environ Entomol 45:649–657
Nitta M, Lee JK, Ohnishi O (2003) Asian Perilla crops and their weedy forms: their cultivation, utilization and genetic relations. Econ Bot 57:245–253
Noriyuki S, Osawa N (2012) Intrinsic prey suitability in specialist and generalist Harmonia ladybirds: a test of the trade-off hypothesis for food specialization. Entomol Exp Appl 144:279–285
Obrycki JJ, Harwood JD, Kring TJ, O’Neil RJ (2009) Aphidophagy by Coccinellidae: application of biological control in agroecosystems. Biol Control 51:244–254
Osawa N (1992) A life table of ladybird beetle Harmonia axyridis Pallas (Coleoptera, Coccinellidae) in relation to the aphid abundance. Jpn J Entomol 60:575–579
Osawa N (1993) Population field studies of the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): life tables and key factor analysis. Res Popul Ecol 35:335–348
Osawa N (2000) Population field studies on the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): resource tracking and population characteristics. Popul Ecol 42:115–127
Osawa N (2011) Ecology of Harmonia axyridis in natural habitats within its native range. BioControl 56:613–621
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org. Accessed 1 Apr 2019
Sasaji H (1971) Fauna japonica, Coccinellidae (Insecta: Coleoptera). Academic Press of Japan, Tokyo
Seo WH, Baek HH (2009) Characteristic aroma-active compounds of Korean Perilla (Perilla frutescens Britton) leaf. J Agric Food Chem 57:11537–11542
Soares AO, Coderre D, Schanderl H (2001) Fitness of two phenotypes of Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 98:287–293
Specty O, Febvay G, Grenier S, Delobel B, Piotte C, Pageaux J-F, Ferran A, Guillaud J (2003) Nutritional plasticity of the predatory ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae): comparison between natural and substitution prey. Arch Insect Biochem Physiol 52:81–91
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Takahashi K (1989) Intra- and inter-specific predation of lady beetles in spring alfalfa fields. Jpn J Entomol 57:199–203
Therneau TM, Grambsch PM (2000) Modeling survival data: extending the Cox model. Springer, New York
Varpe Ø, Jørgensen C, Tarling GA, Fiksen Ø (2007) Early is better: seasonal egg fitness and timing of reproduction in a zooplankton life-history model. Oikos 116:1331–1342
Wolf S, Romeis J, Collatz J (2018) Utilization of plant-derived food sources from annual flower strips by the invasive harlequin ladybird Harmonia axyridis. Biol Control 122:118–126
Acknowledgements
This research has been co-funded by the University of Liège, Belgium and the European Union (Marie-Curie Belgium International PostDoc-COFUND), Wallonie Bruxelles International (WBI.World), Postdoctoral Fellowship for Research in Japan to S. Hatt (No. P18396), and a Grant-in-Aid for Scientific Research to N. Osawa (No. 18F18396) from the Japan Society for the Promotion of Science. Thanks are also due to Agrisect, Tsukuba, Japan for obtaining pupae of Harmonia axyridis and the JA Federation of Economic Organizations of Aichi, Japan for supplying flowers of Perilla frutescens used in preliminary experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Patrick De Clercq
Rights and permissions
About this article
Cite this article
Hatt, S., Osawa, N. The role of Perilla frutescens flowers on fitness traits of the ladybird beetle Harmonia axyridis. BioControl 64, 381–390 (2019). https://doi.org/10.1007/s10526-019-09937-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-019-09937-1