Abstract
Climate change is driving poleward shifts in species distributions worldwide. In the Gulf of Mexico (GOM), warming temperatures foster black mangrove (Avicennia germinans L.) expansion into GOM wetlands replacing wetland plants including Spartina alterniflora Loisel, Salicornia depressa L., and Batis maritima L. We investigated insect community assemblages in wetlands with and without A. germinans to assess potential effects of A. germinans expansion on insect fauna. Insect abundance, biomass, richness, diversity, community structure, and feeding guild composition were measured in both the spring and the fall across three levels of A. germinans abundance. Insect abundance and biomass were larger in both the spring and the fall in wetlands where A. germinans abundance was low. Significant differences in community structure were associated with the presence of A. germinans. Feeding guild composition was also different in wetlands containing A. germinans, having less predator biomass. Shifting vegetation caused by climate change can alter insect communities in coastal wetlands, illustrating the need for a more comprehensive understanding of climate change effects on fauna in response to shifting foundation plant species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal ecosystems are experiencing significant effects of climate change (e.g., Loarie et al. 2009), which is of concern because two-thirds of the planet’s human population and some of its most productive ecosystems and biodiversity hot spots occur within these areas (Agardy et al. 2005). Challenges associated with climate change are and will continue to affect coastal ecosystems through sea level rise, ocean acidification as well as warming temperatures (e.g., Micheli et al. 2008; Craft et al. 2009; Koch et al. 2012; Armitage et al. 2015). Warming temperatures are associated with distribution changes in many taxa (Thomas et al. 2004), and they are driving a poleward expansion of species across the globe (Hickling et al. 2006). Tropical species are becoming more abundant in temperate areas, which has significant effects on biodiversity and ecosystem function (Hickling et al. 2006; Micheli et al. 2008; Vergés et al. 2014; Guo et al. 2017). For example, tropical herbivores may move into temperature regions and alter occurrence and distribution of aquatic vegetation (Vergés et al. 2014). Warming temperatures are also facilitating shifts in foundation species, which may fundamentally alter community structure and function as well as ecosystem processes (Ellison et al. 2005, Micheli et al. 2008; Armitage et al. 2015; Guo et al. 2013).
Plants are the primary source of food and habitat for consumers, and a shift in the abundances or distributions of existing plant fauna may alter the composition of and interactions between the organisms that inhabit them (Gratton and Denno 2005, 2006; Armitage et al. 2015). For example, invasion by the common reed Phragmites australis (Cav.) changed salt marsh faunal composition on the US Atlantic Coast (Osgood et al. 2003; Kimball et al. 2010). In estuaries, warming temperatures have facilitated seagrass species shifts in North Carolina and Texas, which were associated with changes in faunal assemblages and primary productivity (Micheli et al. 2008). In Gulf of Mexico (GOM) wetlands, milder winters without severe freezing events have allowed black mangroves Avicennia germinans L. to expand northward into salt marshes, displacing native wetland plants (Osland et al. 2013; Cavanaugh et al. 2014). This change is associated with significant changes in microclimates, soil organic content, sedimentation rates, and associated birds (Guo et al. 2017). Salt marshes provide numerous ecosystem services including storm surge and coastal erosion protection, carbon sequestration, primary production, and habitat for numerous aquatic and terrestrial species (Pennings and Bertness 2001). Alteration of this vital ecosystem through changes in vegetation make-up could dramatically alter coastal wetland food webs, change ecosystem properties, and create new niches for invasive species (Gedan et al. 2009).
The salt marsh–mangrove barrier exists at or near A. germinans temperature thresholds (Record et al. 2013), suggesting that even a minimal increase in mean annual temperature accompanied by a decrease in severe freezing events (colder than − 4 °C) could lead to extensive increases in A. germinans distribution, altering the structure and function of coastal wetlands (Cavanaugh et al. 2014). Cold temperatures have historically limited the northern range limit for A. germinans to around 30° N (Kangas and Lugo 1990). Over the last few decades, a decrease in the frequency of sustained severe freezing events has allowed the area of mangrove forests to double at the northern end of this range in the Gulf Coast of the United States (Cavanaugh et al. 2014). In marshes near Corpus Christi, TX, USA, mangrove cover has expanded from less than 100 acres to more than 27,000 acres since 1980 (Montagna et al. 2011.; Armitage et al. 2015). A. germinans encroachment alters below ground properties (e.g., soil characteristics, biomass, carbon storage), and these effects are more intense in drier wetlands like those in the Western GOM (Yando et al. 2016).
In some areas, A. germinans dominates the lower tidal elevations (Geldenhuys et al. 2016), but in the Western GOM, A. germinans tends to occupy the higher tidal elevations replacing upper elevations of Spartina alterniflora Loisel and other marsh plants in the process (Smee et al. 2017). S. alterniflora remains at the lowest tidal elevations in a small border surrounding a dwarf A. germinans forest, and S. alterniflora facilitates A. germinans seedlings by creating a buffer against cold temperatures (Guo et al. 2013) and by trapping the A. germinans propagules from becoming dispersed outside of optimal retention ranges (Peterson and Bell 2012). A. germinans are replacing the existing flora in the Southern GOM, and anticipated warming trends are likely to promote A. germinans expansion poleward, causing further displacement of marsh plants.
Insects are ubiquitous in coastal wetlands (Pennings and Bertness 2001). Insect families are generally restricted in diet to a small group of related plants due to coevolutionary defense strategies (Futuyma and Mitter 1996; Burrows 2003; Nagelkerken et al. 2008). For instance, tropical plants generally invest heavily in chemical defenses relative to temperate plants (Coley 1998). Evidence of coevolution has been seen in arthropods (Coley and Aide 1990), fish (Bertness et al. 1981), crustaceans (Heck and Wilson 1987), and various intertidal species (Menge and Lubchenco 1981). In general, A. germinans invests more in defense strategies to combat insect herbivory than do temperate marsh plants (He and Silliman 2015). Thus, replacement of a temperate plant species by a tropical one such as A. germinans might affect grazing rates and energy transfer. Insects play an important role in decomposition and the cycling of nutrients through ecosystems as well as being a major food source for fish, birds, amphibians, reptiles, and other invertebrates making them essential for habitat functional integrity (Angermeier and Karr 1994). Aquatic fauna were significantly different in wetlands dominated by A. germinans (Diskin and Smee 2017; Smee et al. 2017), but the effects of mangrove expansion on salt marsh insect communities remain largely unexplored in the GOM.
Predicting potential effects of A. germinans expansion on insects is difficult. By displacing other plants and developing a monoculture, A. germinans may lower insect diversity and abundance. Alternatively, by creating a new ecotone, A. germinans might increase insect diversity and enhance wetland food webs, particularly in wetlands transitioning from domination by grasses and succulents to dwarf A. germinans forests. The purpose of this study was to determine what changes, if any, are occurring in the GOM estuarine wetland insect communities as A. germinans replace salt marshes and attempt to identify patterns associated with this change.
Materials and methods
Study sites
Samples were collected from marshes in estuaries near Port Aransas and Rockport, TX, USA. A. germinans has become well established in many areas but is nearly completely absent in others. In some places, A. germinans has displaced all marsh plants common in higher intertidal elevations, such as Salicornia depressa L. and Batis maritima L., as well as the upper tidal elevations typically occupied by S. alterniflora (Smee et al. 2017). In these areas with established and abundant A. germinans, A. germinans has formed dense monocultures at higher intertidal elevations excluding all other plants and has restricted S. alterniflora to narrow bands at the lowest tidal elevations (Fig. 1). Thus, marshes change from a diverse plant assemblage containing grasses and succulent plants to one dominated by A. germinans. We categorized marshes by the abundance of A. germinans, which was inversely related to the abundance of S. depressa, B. maritima, and S. alterniflora. Abundance was based on measurements of vegetation width (distance from lowest to highest tidal elevations, Diskin 2016). For this study, marshes were grouped into three types based on the relative abundances of these plants: (1) rare A. germinans, and S. depressa, B. maritima, S. alterniflora abundant, (2) intermediate A. germinans co-occurring with similar abundance of S. alterniflora, S. depressa, and B. maritima, and (3) abundant A. germinans, in which other plants were in low abundance.
Sampling design
Insects were collected within each wetland type in June (late spring) and October (fall) of 2016. A. germinans were flowering during spring sampling and not during the fall with no other vegetation flowering while samples were taken. Eight samples were collected from each wetland type (24 from each season, 48 total). Insect specimens were collected using a suction sampler made from a converted leaf blower-vac (Buffington and Redak 1998). Samples were taken using a sweeping motion from side to side in a near 180° arc with 1 sweep made, then 1 step forward taken, and another sweep made, until 15 sweeps were completed and 15 constituted one sample (Buffington and Redak 1998). Catch per unit effort (CPUE) is therefore defined in this paper as the number of individuals per 15 sweeps of the suction sampler. Other techniques such as sweep nets and light traps were tested and determined to be less effective in capturing insects (Loveless 2017). We collected fewer insect species and overall many fewer individuals using these methods as compared to the suction sampler. Wetland plants were also searched by hand to ensure that insects had not been missed with the vacuum sampler. Insects such as lepidopterans that are not effectively sampled with a suction sampler were not found (Loveless 2017).
Once collected, insects were stored in 95% ethanol and brought to the lab for sorting, identification, and enumeration. The primary focus of this study was on insect taxa; however spiders were enumerated and simply identified as arachnid for both order and family. Feeding guilds were grouped using the classifications of Papp (2002) and Sinu and Sharma (2013): fungivore, herbivore, parasite, parasitoid, predator, and saprophage. Herbivores were further separated into chewers, sap feeders, and a third miscellaneous category (Pomeroy and Wiegert 1981) encompassing all remaining groups (e.g., gall formers, nectar feeders). Blood-sucking insect families (e.g., Culicidae, Tabanidae) were considered parasites, and detritivores were characterized as saprophagous.
Data analysis
Insect communities were compared among seasons (spring and fall) and wetland types (A. germinans rare, A. germinans intermediate, A. germinans abundant) using a 2-way PERMANOVA (PRIMER™). Many more insects were collected in spring than fall; therefore, insect community differences between wetland types were compared separately among seasons. Non-metric MDS plots were created for both the spring and the fall to visualize differences among insect communities by wetland type. An analysis of similarity (ANOSIM) in PRIMER™ was performed to examine the differences in communities among wetland types in spring and fall, and a similarity percentage (SIMPER) analysis in PRIMER™ was used to determine which insect families contributed most to insect community differences among wetland types.
Univariate analyses (insect abundance, biomass, richness), and Shannon–Weiner diversity, were compared in the spring and the fall using ANOVA in JMP Pro 13.1 with wetland type as a fixed factor. Tukey post hoc tests were performed to compare pairwise differences among wetland types.
Comparisons of feeding guilds were simplified by separating wetland types into two groups: with (intermediate + abundant) and without (rare) A. germinans. Many of the insects collected were small phorid flies (< 2 mm, < 2 mg) which contributed substantially to the number of individuals collected; however, they contributed very little to total biomass. Because of this, biomass contributions were used when comparing the feeding guilds between the wetland types.
Results
Community structure
Insect communities were significantly different among wetland types (Pseudo F = 13.731,42, p < 0.001) and seasons (Pseudo F = 13.731,42, p < 0.001). The interaction between wetland type and season was also significant (Pseudo F = 8.201,42, p < 0.001). MDS plots for the spring (Fig. 2a) and fall (Fig. 2b) showed distinct grouping patterns based on wetland type. Consistent with MDS, ANOSIM indicated a significant effect of wetland type in both spring (R = 0.92; p = 0.001) and fall (R = 0.42; p = 0.001).
SIMPER analysis revealed that regardless of season, insect communities were most dissimilar when wetlands with rare A. germinans were compared to those containing A. germinans, regardless of whether A. germinans abundance was intermediate or if A. germinans was established and had excluded most other plants (Tables 1, 2).
Abundance and biomass
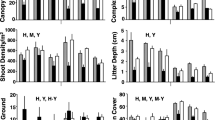
In spring, 2947 insects from 31 families were collected. Individuals in the family Phoridae were the most abundant group collected (1136). Insect abundances were significantly different among wetland types, (F2, 21 = 8.24, p < 0.001, Fig. 3); wetlands with rare A. germinans had more individuals than areas with intermediate or abundant A. germinans. Similarly, biomass was significantly different among wetland types (F2, 21 = 19.95, p < 0.001, Fig. 3) and was highest in areas with rare A. germinans. Biomass was not different between areas with intermediate or abundant A. germinans.
Fewer insects were collected overall in fall: 992 individuals, 25 families. Ephydridae was the most common family with 356 individuals. Like spring, insect abundance in the fall between wetland types was also significantly different (F2, 21 = 28.7, p < 0.001, Fig. 3), and areas without A. germinans had more insects than areas with intermediate and abundant levels of A. germinans. Biomass was also significantly different among wetland types (F2, 21 = 19.95, p < 0.001, Fig. 3), and as with abundance, wetlands without A. germinans had higher insect abundance than those containing A. germinans at intermediate and abundant levels. Arachnids contributed most of the fall biomass, comprising 3.38 g of the 10.06 g collected. Insect abundance was significantly higher in wetlands without A. germinans in both spring (F2, 21 = 13.8, p < 0.001) and fall (F2, 21 = 38.8, p < 0.001), as compared to areas with A. germinans (Fig. 3).
Richness and diversity
Species richness was significantly different among wetland types in the spring (F2, 21 = 18.8, p < 0.001) and fall (F2, 21 = 46.5, p < 0.001, Fig. 4). Richness was significantly higher in wetlands with abundant A. germinans. Shannon–Weiner diversity was significantly greater in wetlands containing A. germinans in spring (F2, 21 = 7.28, p < 0.01) and fall (F2, 21 = 6.2, p < 0.001, Fig. 4).
Feeding guild composition
All eight of the defined feeding guilds were represented in each wetland type. Herbivores (chewers, sapsuckers, and miscellaneous) were generally the most abundant feeding group followed by carnivorous guilds (predators, parasitoids, and parasites, Table 3). In the fall, sites with both intermediate and abundant levels of A. germinans had significantly more arachnids than other sites. Sites in which A. germinans were rare/absent contained an herbivore-to-carnivore ratio of 9:1 in the spring and 5:1 in the fall. In wetlands with A. germinans present, this ratio was 6:1 in the spring, but switched to a 1:1 herbivore-to-carnivore ratio in the fall.
Discussion
The presence of A. germinans was associated with reductions of other commonly occurring marsh plants in the Western GOM (Diskin and Smee 2017; Smee et al. 2017). As climate change trends continue, it is likely that the range of mangrove forest will move northward, displacing native marsh plants. The effects of this expansion of mangrove forests on salt marsh insect communities has largely been unexplored in the GOM. Coevolution of insects and marsh plants in coastal wetlands suggests that the disappearance of native marsh plants could significantly alter associated insect communities. Insects play an important role in nutrient cycling and energy transfer in their ecosystem; a change in insect communities could affect decomposition rates and food abundance for higher trophic levels such as fish, birds, and other invertebrates. Understanding if and how insect communities are changing as mangrove forests replace salt marsh plant species will help elucidate other potential cascading effects throughout salt marsh food webs and ecosystem functioning.
In this study, we collected and investigated insect community assemblages in wetland areas that had three levels of A. germinans abundance. Insects were less abundant and had lower biomass in areas with A. germinans. Insect communities were significantly different in areas where A. germinans was established. Areas in which vegetation diversity declines due to establishment of new plant species often have lower insect diversity (Bezemer et al. 2014), which is consistent with our findings. Because A. germinans creates monocultures, we anticipate significant changes of coastal insect communities and a lowering of insect diversity and biomass, which may have large effects on ecosystem processes.
Insect community structure in GOM wetlands containing A. germinans was different from those reported from tropical mangrove forests, which tend to be dominated by Lepidopteran species influencing pollination (Landry 2013) and herbivory (Menezes and Peixoto 2009), with Dipterans making minimal contributions (Simberloff and Wilson 1969; Murphy 1990; Veenakumari et al. 1997; Burrows 2003). In our study, Lepidopterans only constituted about 5% of the groups collected, while Dipterans were the most abundant (31%). However, our Dipteran total is lower than totals reported in other salt marsh communities (Luckett and Gray 1966; Cameron 1972). Similarly, Murphy (1990), and Burrows (2003), only found herbivorous insects from four orders in mangrove forests, compared to seven orders found in this study, and the 10–12 found in studies looking at salt marshes (Cameron 1972; Wu et al. 2009).
There are some similarities between the fauna collected in mangrove forests in the GOM and those collected from tropical climates. For instance, when comparing feeding guilds, we found that sap-sucking insects were nearly three times less abundant in sites with A. germinans. These results are consistent with tropical mangrove forests which also show a lower abundance of sap-sucking insects (Veenakumari et al. 1997; Burrows 2003). Similarly, the areas with A. germinans in this study and the mangrove forests sampled by Burrows (2003) and Murphy (1990) found very few Coleopterans, which are normally abundant in coastal marshes (Cameron 1972). Mangrove forests can show a high degree of insect specialization with little to no overlap of herbivorous species in adjacent habitats (Burrows 2003). In contrast, we did not see separate families in sites with and without A. germinans, possibly indicating that A. germinans in the GOM are in a transition between the communities found in tropical mangrove forest and those found in salt marshes. Our sites were within 15 km of one another and insects were likely able to travel between sites, which may also explain why we did not see distinct families associated with each wetland type.
Wetlands with rare A. germinans contained a larger diversity of plant species (e.g., S. virginica, B. maritima, S. alterniflora) than those dominated by A. germinans monocultures. It is not uncommon for encroaching plants to eliminate existing vegetation by competitive exclusion leading to decreased plant diversity (Wu et al. 2009; Harvey et al. 2010; Quan et al. 2016). For example, in China, the area occupied by S. alterniflora increased from 260 ha (Chung 1989) to 112,000 ha (An et al. 2007) in only 15 years with this expansion coming at the expense of existing vegetation (Wu et al. 2009). The diversity of lower trophic levels exerts significant controls on the abundances of the consumers that utilize them (Murdoch et al. 1972; Siemann et al. 1998; Knops et al. 1999). Encroaching plants can also alter habitat structure as they become established, influencing the existing arthropod communities (Gratton and Denno 2005). Our sites were exposed to similar abiotic factors (salinity, temperature, precipitation, etc.), and thus, the higher plant diversity, accompanied by the more labile plant materials associated with S. depressa, B. maritima, and the changes in the habitat structure are likely primary contributors to the higher insect abundances and biomass found in sites with rare A. germinans.
Changing plant compositions can alter food web structures in coastal wetlands (Gratton and Denno 2005, 2006; Levin et al. 2006; Harvey et al. 2010); however, patterns of community change have varied widely. In coastal marshes in southeastern Australia, native plants (Juncus kraussii) are being replaced by a congener (J. acutus). This change is associated with a lower diversity and abundance of predators (Harvey et al. 2010). In the northeastern United States, P. australis has displaced S. alterniflora in many areas. Gratton and Denno (2005) found feeding guilds in marshes containing abundant stands of P. australis to shift from a roughly 2:1:1 predator-to-herbivore-to-detritivore ratio to a 1:1 detritivore-to-predator ratio, with changes being driven by altered plant composition and physical structure. In our study, herbivores were the dominant guild in both the S. alterniflora and A. germinans habitat with the A. germinans dominated wetlands generally having fewer predators. The one exception to this is the inverted pattern found in fall mangrove sites. While it is possible that some environmental factor exists to explain this, for example, inverted biomass pyramids are common in aquatic systems (Wang et al. 2009), it is more likely that the dearth of fauna collected in fall mangrove sites has skewed the data. For example, only 18 arachnids were collected within these wetland types; however, this small number accounted for nearly 23% of individuals, and just under 52% of the total biomass.
Although mangrove encroachment may alter community and ecosystem traits, mangroves provide benefits inlcuding protecting coastlines from storm surges and rising sea levels (Comeaux et al. 2012), which salt marshes can do as well. Bianchi et al. (2013) found that A. germinans may reduce some effects of climate change by sequestering carbon. If the current trend of mangrove expansion continues, the increased area occupied by A. germinans would increase the overall levels of carbon sequestration in coastal wetlands (Bianchi et al. 2013), providing an invaluable carbon sink which could help reduce the levels of CO2 gas in the atmosphere. Based on this information, Bianchi et al. (2013) suggests implementing A. germinans planting programs to help increase the amount of carbon storage available in coastal wetlands. While the increased ability to sequester carbon is desirable, the possible consequences that may arise from a transition to mangrove forests from salt marsh habitat must be considered.
Avicennia germinans monocultures are replacing more diverse vegetation assemblages, and their presence has been associated with changes in insect community structure and changes in aquatic species composition (Diskin and Smee 2017; Smee et al. 2017). These changes may prove detrimental to coastal wetlands and the nearby ecosystems that rely on them. For instance, terrestrial arthropod species comprise a significant proportion of the diet of commercially important fish species in estuaries (Gray et al. 2002; Romanuk and Levings 2003) which further enforces the importance of wetland vegetation in estuarine food webs (Morley et al. 2012). This habitat loss is even more alarming when considered with other studies showing A. germinans presence adversely affects marsh nekton and benthic communities as well (Lunt et al. 2013; Diskin 2016; Smee et al. 2017).
This study provides insights into ongoing changes that are occurring in GOM estuarine wetland insect communities as A. germinans replaces native wetland plants. Future studies are needed to further elucidate the affect expanding mangrove forests could have on coastal wetland communities and food webs and the mechanisms by which these changes are occurring. Information gained would allow better action in the construction and implementation of future conservation policies and the management and restoration of coastal wetlands.
References
Agardy TR, Hassan RS, Ash N (2005) Coastal systems. The millennium ecosystem assessment: ecosystems and human well-being: current state and trends, vol 1. Island, Washington, DC, pp 515–549
An SQ, Guà BH, Zhou CF, Wang ZS, Deng ZF, Zhi YB, Li HL, Chen L, Yu DH, Liu YH (2007) Spartina invasion in China: implications for invasive species management and future research. Weed Res 47:183–191
Angermeier PL, Karr JR (1994) Biological integrity versus biological diversity as policy directives. Bioscience 44:690–697
Armitage AR, Highfield WE, Brody SD, Louchouarn P (2015) The contribution of mangrove expansion to salt marsh loss on the Texas Gulf Coast. PLoS ONE 10:1–17
Bertness MD, Garrity SD, Levings SC (1981) Predation pressure and gastropod foraging: a tropical-temperate comparison. Evolution 35:995–1007
Bezemer TM, Harvey JA, Cronin JT (2014) Response of native insect communities to invasive plants. Annu Rev Entomol 59:119–141
Bianchi TS, Allison MA, Zhao J, Li X, Comeaux RS, Feagin RA, Kulawardhana RW (2013) Historical reconstruction of mangrove expansion in the Gulf of Mexico: linking climate change with carbon sequestration in coastal wetlands. Estuar Coast Shelf Sci 119:7–16
Buffington ML, Redak RA (1998) A comparison of vacuum sampling versus sweep-netting for arthropod biodiversity measurements in California coastal sage scrub. J Insect Conserv 2:99–106
Burrows D (2003) The role of insect leaf herbivory on the mangroves Avicennia marina and Rhizophora stylosa. Ph.D. Thesis, James Cook University, Australia
Cameron GN (1972) Analysis of insect trophic diversity in two salt marsh communities. Ecology 53:58–73
Cavanaugh KC, Kellner JR, Forde AJ, Gruner DS, Parker JD, Rodriguez W, Feller IC (2014) Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc Natl Acad Sci USA 111:723–727. https://doi.org/10.1073/pnas.1315800111
Chung CH (1989) Ecological engineering of coastline with salt marsh plantations in China. Ecol Eng 27:49–57
Coley PD (1998) Possible effects of climate change on plant/herbivore interactions in moist tropical forests. Clim Change 39:455–472
Coley PD, Aide TM (1990) Comparison of herbivory and plant defenses in temperate and tropical broad-leaved forests. In: Price PW, Lewinsotin TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 25–49
Comeaux RS, Allison MA, Bianchi TS (2012) Mangrove expansion in the Gulf of Mexico with climate change: implications for wetland health and resistance to rising sea levels. Estuar Coast Shelf Sci 96:81–95
Craft C, Clough J, Ehman J, Joye S, Park R, Pennings S, Guo H, Machmuller M (2009) Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front Ecol Environ 7:73–78
Diskin MS (2016) Effects of black mangrove (Avicennia germinans) expansion on salt marsh fauna in south Texas before and after a major flooding event. Thesis, Texas A&M University Corpus Christi, Corpus Christi, Texas, USA
Diskin M, Smee DL (2017) Effects of black mangrove Avicennia germinans expansion on salt marsh nekton assemblages before and after a flood. Hydrobiologia. https://doi.org/10.1007/s10750-017-3179-2
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM, Mohan J (2005) Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486
Futuyma DJ, Mitter C (1996) Insect-plant interactions: the evolution of component communities. Philos Trans R Soc Lond Ser B 351:1361–1366
Gedan KB, Silliman BR, Bertness MD (2009) Centuries of human-driven change in salt marsh ecosystems. Annu Rev Mar Sci 1:117–141
Geldenhuys C, Cotiyane P, Rajkaran A (2016) Understanding the creek dynamics and environmental characteristics that determine the distribution of mangrove and salt marsh communities at Nahoon Estuary. S Afr J Bot 107:137–147
Gratton C, Denno RF (2005) Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis. Restor Ecol 13:358–372
Gratton C, Denno RF (2006) Arthropod food web restoration following removal of an invasive wetland plant. Ecol Appl 16:622–631
Gray A, Simenstad CA, Bottom DL, Cornwell TJ (2002) Contrasting functional performance of juvenile salmon in recovering wetlands of the Salmon River Estuary, Oregon, USA. Restor Ecol 10:514–526
Guo HY, Zhang YH, Lan ZJ, Pennings SC (2013) Biotic interactions mediate the expansion of black mangrove (Avicennia germinans) into salt marshes under climate change. Glob Chang Biol 19:2765–2774
Guo H, Zhang Y, Lan Z, Pennings SC (2017) Biotic interactions mediate the expansion of black mangrove (Avicennia germinans) into salt marshes under climate change. Glob Change Biol 19:2765–2774
Harvey KJ, Britton DR, Minchinton TE (2010) Insect diversity and trophic structure differ on native and non-indigenous congeneric rushes in coastal salt marshes. Austral Ecol 35:522–534
He Q, Silliman BR (2015) Biogeographic consequences of nutrient enrichment for plant-herbivore interactions in coastal wetlands. Ecol Lett 18:462–471
Heck KL Jr, Wilson KA (1987) Predation rates on decapod crustaceans in latitudinally separated seagrass communities: a study of spatial and temporal variation using tethering techniques. J Exp Mar Biol Ecol 107:87–100
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Change Biol 12:450–455
Kangas PC, Lugo AE (1990) The distribution of mangroves and saltmarsh in Florida. Trop Ecol 31:32–39
Kimball ME, Able KW, Grothues TM (2010) Evaluation of long-term response of intertidal creek nekton to Phragmites australis (common reed) removal in oligohaline Delaware Bay salt marshes. Restor Ecol 18:772–779
Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, Ritchie ME, Howe KM, Reich PB, Siemann E, Groth J (1999) Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol Lett 2:286–293
Koch M, Bowes G, Ross C, Zhang X (2012) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Change Biol 19:103–132
Landry CL (2013) Pollinator-mediated competition between two co-flowering neo-tropical mangrove species Avicennia germinans (Avicenniaceae) and Languncularia racemose (Combretaceae). Ann Bot 2:207–214
Levin LA, Neira C, Grosholz ED (2006) Invasive cordgrass modifies wetland trophic function. Ecology 87:419–432
Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD (2009) The velocity of climate change. Nature 462:1052–1055
Loveless JB (2017) Community structure shifts in response to Avicennia germinans expansion into Gulf of Mexico wetlands. M.S. Thesis, Texas A&M University, Corpus Christi
Luckett DV, Gray IE (1966) Zonal and seasonal distributions of insects in North Carolina salt marshes. Ecol Monogr 36:275–295
Lunt J, McGlaun K, Robinson EM (2013) Effects of black mangrove (Avicennia germinans) expansion on salt marsh (Spartina alterniflora) benthic communities of the South Texas coast. Gulf Caribb Res 25:125–129
Menezes LFTD, Peixoto AL (2009) Leaf damage in a mangrove swamp at Sepetiba Bay, Rio de Janeiro, Brazil. Rev Bras Bot 32:715–724
Menge BA, Lubchenco J (1981) Community organization in temperate and tropical rocky intertidal habitats: prey refuges in relation to consumer pressure gradients. Ecol Monogr 51:429–450
Micheli F, Bishop MJ, Peterson CH, Rivera J (2008) Alteration of seagrass species composition and function over two decades. Ecol Monogr 78:225–244
Montagna PA, Brenner J, Gibeaut J, Morehead S (2011) Coastal impacts. In: Schmandt J, North GR, Clarkson J (eds) The impact of global warming on Texas, 2nd edn. University of Texas Press, Austin, pp 96–123
Morley SA, Toft JD, Hanson KM (2012) Ecological effects of shoreline armoring on intertidal habitats of a Puget Sound urban estuary. Estuaries Coasts 35:774–784
Murdoch WW, Evans FC, Petersen CH (1972) Diversity and pattern in plants and insects. Ecology 53:819–829
Murphy DH (1990) The natural history of herbivory on mangrove trees in and near Singapore. Raffles Bull Zool 38:119–203
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185
Osgood DT, Yozzo DJ, Chambers RM, Jacobson D, Hoffman T, Wnek J (2003) Tidal hydrology and habitat utilization by resident nekton in Phragmites and non-Phragmites marshes. Estuaries 26:522–533
Osland MJ, Enwright N, Day RH, Doyle TW (2013) Winter climate change and coastal wetland foundation species: salt marshes vs. mangrove forests in the southeastern United States. Glob Change Biol 19:1482–1494
Papp L (2002) Dipterous guilds of small-sized feeding sources in forests of Hungary. Acta Zool Acad Sci Hung 48:197–213
Pennings SC, Bertness MD (2001) Salt marsh communities. In: Bertness MD, Gaines SD, Hay M (eds) Marine community ecology. pp 289–316
Peterson JM, Bell SS (2012) Tidal events and salt-marsh structure influence black mangrove (Avicennis germinans) recruitment across an ecotone. Ecology 93:1648–1658
Pomeroy LR, Wiegert RG (1981) Ecology of a salt marsh. Springer, New York
Quan W, Zhang H, Wu Z, Jin S, Tang F, Dong J (2016) Does invasion of Spartina alterniflora alter microhabitats and benthic communities of salt marshes in Yangtze River estuary? Ecol Eng 88:153–164
Record S, Charney ND, Zakaria RM, Ellison AM (2013) Projecting global mangrove species and community distributions under climate change. Ecosphere 4:1–23
Romanuk TN, Levings CD (2003) Associations between arthropods and the supralittoral ecotone: dependence of aquatic and terrestrial taxa on riparian vegetation. Environ Entomol 32:1343–1353
Siemann E, Tilman D, Haarstad J, Ritchie M (1998) Experimental tests of the dependence of arthropod diversity on plant diversity. Am Nat 152:738–750
Simberloff DS, Wilson EO (1969) Experimental zoogeography of islands: the colonization of empty islands. Ecology 50:278–296
Sinu P, Sharma M (2013) Insect functional guilds in the flowering canopy of Myristica fatua in a lowland swamp, central Western Ghats, India. Trop Conserv Sci 6:653–662
Smee DL, Sanchez JA, Diskin M, Trettin C (2017) Mangrove expansion into salt marshes alters associated faunal communities. Estuar Coast Shelf Sci 187:306–313
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BF, De Siqueira MF, Grainger A, Hannah L, Hughes L (2004) Extinction risk from climate change. Nature 427:145–148
Veenakumari K, Mohanraj P, Bandyopadhyay AK (1997) Insect herbivores and their natural enemies in the mangals of the Andaman and Nicobar islands. J Nat Hist 31:1105–1126
Vergés A, Steinberg PD, Hay ME et al (2014) The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc R Soc B 281:1–10
Wang H, Morrison W, Singh A, Weiss H (2009) Modeling inverted biomass pyramids and refuges in ecosystems. Ecol Model 220:1376–1382
Wu YT, Wang CH, Zhang XD, Zhao B, Jiang LF, Chen JK, Li B (2009) Effects of saltmarsh invasion by Spartina alterniflora on arthropod community structure and diets. Biol Invasions 11:635–649
Yando ES, Osland MJ, Willis JM, Day RH, Krauss KW, Hester MW (2016) Salt marsh-mangrove ecotones: using structural gradients to investigate the effects of woody plant encroachment on plant-soil interactions and ecosystem carbon pools. J Ecol 104:1020–1031
Acknowledgements
Funding was provided by the USDA Forest Service Southern Research Station agreements 12-DG-11330101-096 and 13-CA-11330140-116 to D.L. Smee. The NSF-MSP ETEAMS Grant #1321319 provided funding for boat time and their interns, E. Urban in particular, assisted in the field. Members of the Marine Ecology Lab, and C. Trettin, J. Arnold, and C. Stringer from USFS provided important assistance in the field. L. Patrick helped with mansucript formatting and proofreading. S. Bock was instrumental in writing and data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Miriama Malcicka.
Rights and permissions
About this article
Cite this article
Loveless, J.B., Smee, D.L. Changes in arthropod communities as black mangroves Avicennia germinans expand into Gulf of Mexico salt marshes. Arthropod-Plant Interactions 13, 465–475 (2019). https://doi.org/10.1007/s11829-018-9643-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-018-9643-8