Abstract
Our objective was to gain an understanding of the influences of habitat context and seasonal and interannual factors on arthropod assemblage structure in a wetland environment. We hypothesized that river and pond riparian habitats in the wetland would have greater diversity and abundance than core wetland habitat, and that these differences would be driven by aquatic subsidy via emerging aquatic insects. We also hypothesized that diversity and abundance of terrestrial fauna would decline through the dry summer. We sampled the study wetland, in Yosemite National Park, California, USA, through the growing seasons of 2013 and 2014; a large wildfire (> 100,000 ha) burned the entire study site during late summer of 2013. Assemblage structure was strongly influenced by habitat context, season, and year. Diversity and abundance were high at the river riparian sites, but these results were driven by a diverse and abundant terrestrial fauna, rather than by large numbers of emerging aquatic insects. Faunal assemblages became increasingly depauperate through the summer, likely due to drying of wetland habitat in this hot Mediterranean-type climate. Fire probably had a strong influence on faunal assemblages and vegetation structure, but we cannot rule out interannual variability independent of the fire.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Faunal assemblage structure in wetlands can be influenced by a number of factors, including landscape configuration and context (Armitage et al. 2013; Holmquist et al. 2014). There is high faunal richness and abundance at habitat edges in many environments (Forman 1995), often because faunal components from two adjoining habitat elements are present (Polis and Hurd 1996; Puth and Wilson 2001). Arthropod richness and abundance in forested riparian habitat can be directly increased by immigration of emerging aquatic insects (Murakami and Nakano 2002; Kato et al. 2004; Baxter et al. 2005; Jackson et al. 2015) which, in turn, can attract invertebrate predators, further increasing complexity of assemblage structure (Henschel et al. 2001; Jackson and Sullivan 2018). Although wetlands are periodically saturated or inundated, nearby lotic and lentic habitats have the potential to be important influences on the structure of wetland faunal assemblages via such direct and indirect influences.
Faunal assemblage structure in low-canopy, vegetated habitats can vary across months in a variety of tropical and temperate environments (e.g., Denlinger 1980; Holmquist et al. 2013a), and infusion of emerging aquatic insects from streams can vary seasonally in terrestrial habitats bordering streams (Puth and Wilson 2001; Kato et al. 2004; Baxter et al. 2005; Jackson and Sullivan 2018). The assemblage structure of seasonal ponds also changes throughout the year (Bischof et al. 2013) and may also drive assemblage changes in adjoining wetland habitats via aquatic insect emergence. Montane wetland fauna in drier Mediterranean climates might be expected to be influenced by both climate-driven changes in vegetation structure and temporal patterns in emergence of aquatic fauna through the short growing season. Differences in faunal assemblage structure between wetland edge and core habitats could thus shift through the growing season and across years, i.e., habitat-time interactions may be present.
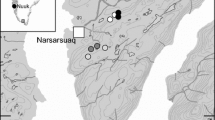
We investigated spatial and temporal influences on arthropod assemblages in a montane wetland complex (Yosemite National Park, California, USA) with portions that border lotic or lentic habitat. Poopenaut Valley represents the largest montane wetland along the Tuolumne River, which has been designated as a U.S. Wild and Scenic River and is important both ecologically and as a major source of water for the San Francisco Bay Area. This wetland complex is spatially isolated from other wetland habitats (see Study Area and Design, below).
Although response of wetland fauna to fire was not part of the study design, the wetland was completely burned by the 2013 Rim Fire, which was the largest fire (104,131 ha) recorded in the extensive mountain range of the Sierra Nevada (Lydersen et al. 2014). The fire occurred during late summer, after the first season of sampling. Such late-season fires have the potential to cause additional mortality, because some species are already in less motile and thus more vulnerable states, such as eggs, pupae, or other overwintering stages (Swengel 2001). We sampled sites immediately before the fire and during the growing season subsequent to the fire (9 months post-fire), but there was no unburned habitat in the wetland, or nearby, that could be used as a post-fire reference (see also Bess et al. 2002). The mid-study occurrence of the Rim Fire has the potential to provide some coarse insights into response of wetland fauna to fire (see also Panzer 2002), but conclusions regarding apparent effects must be limited, particularly given the nuanced responses to fire that have been observed for arthropods (Panzer and Schwartz 2000; Andersen et al. 2014; Moranz et al. 2014; Jackson and Sullivan 2015; Rose and Goebel 2015).
We addressed several central questions in this study. 1) How does assemblage structure vary as a function of wetland context? We compared a) core wetland versus edge wetland near b) river or c) pond. Based on previous work at stream-upland interfaces (Henschel et al. 2001; Murakami and Nakano 2002; Kato et al. 2004; Baxter et al. 2005), we anticipated that emerging aquatic insects would drive higher richness and abundance at river and pond edges, relative to core habitat. Wetlands are, however, productive habitats, and thus such allochthonous subsidy might be proportionally less influential than in uplands. 2) Are there strong seasonal trends for the wetland faunal assemblage, and are there interactions with habitat context? Emerging aquatic insects have been shown to decrease in abundance in near-stream forest through the growing season, whereas terrestrial arthropods can increase during the same period (Kato et al. 2003). Given the dry Mediterranean summers in this montane wetland, with vegetation senescence by July, we hypothesized that abundances of terrestrial, as well as emerging aquatic, insects would decrease, rather than increase, through the growing season. We anticipated that changes in assemblage structure through the growing season would be strongest at wetland-aquatic edges, because of the potential influence of neighboring river and pond habitat and associated fauna (Baxter et al. 2005). Our overall aim was to gain an understanding of several factors that might influence wetland faunal assemblages; we found that all study factors did have strong influences, though not necessarily as anticipated.
Materials and Methods
Study Area and Design
Poopenaut Valley is isolated by the steep granitic walls that line much of the Tuolumne River along the mid-elevation reaches, and no wetlands of the same size (26 ha) are found within 50 river km up- or downstream of the study area. The Valley is rarely visited by people, despite being only 1.75 km by trail from a road in heavily-visited Yosemite National Park, probably because the trail loses 400 m rapidly before reaching the Valley at 1017 m. Poopenaut Valley has been little-studied until recently (Russo et al. 2012). The area receives 89 cm/y of precipitation, three-quarters of which falls between November and March, primarily as snow (Russo et al. 2012), with an ensuing three-month growing season.
The studied wetland habitat is wet meadow that is seasonally-saturated but generally not inundated. Dominant vegetation in sampled areas included beardless wildrye Leymus triticoides (Buckley) Pilger, Mugwort Artemisia douglasiana Besser, grass-leaved goldenrod Euthamia occidentalis Nutt., inflated sedge Carex vesicaria L., and Kentucky bluegrass Poa pratensis L. Although found in the other habitats, Poa was most common in core habitat, Carex was most common near the pond, and tule Scirpus acutus (S. Watson) Beetle was found exclusively near the pond. The Valley wetland is bisected by the Tuolumne River, which, at this elevation, is a fourth-order, perennial stream with a 1% gradient that is characterized by riffle-pool habitat. A three-hectare, seasonal pond lies 150 m from the north bank of the river and varies in depth and length of inundation. The pond sediment was saturated, but not flooded, during 2013 but was inundated to a depth of ~0.6 m between February and April of 2014. When only saturated, the wetted pond habitat continues to support semi-terrestrial taxa and midge and mosquito larvae not found in wet meadow habitat. The Rim Fire burned the wetland at low to moderate intensity (0–50% basal area; CalFire 2013) during August of 2013. The Valley had burned previously during the 1996 Ackerson Fire.
We sampled three wetland habitats during 2013 and 2014: 1) core wetland habitat that was at least 70 m from the closest upland or aquatic habitat, 2) wetland habitat directly adjoining the river, and 3) wetland habitat directly adjoining the pond. We sampled fauna and associated vegetation structure through the growing season, i.e., starting after snow was completely melted (May) and ending just before high temperatures (mean during sampling hours = 34.3 °C, maximum >40 °C) drove complete senescence of wetland vegetation (late July; see also Fukui et al. 2006). Most major emergences of aquatic insects also occur during these months on the west slope of the Sierra Nevada (Schalla 2015). We thus used a 3 × 3 × 2 design: Habitat (Core, River, Pond) x Month (May, June, July) x Year (2013, 2014). There were four randomly-located samples for each of the Habitat x Month x Year combinations, yielding a total of 72 faunal samples. There were two randomly-selected subsample locations within each sampling location for fauna, and there were two additional randomly-selected vegetation subsamples nested within each of the first pair of subsamples. There were thus two subsampling locations for fauna and four subsampling locations for vegetation at each sampling site. A Scientific Research and Collecting permit was obtained from the US National Park Service for work in Yosemite National Park for each year of the study. No protected species were sampled.
Faunal and Vegetation Methodology
Each sample represented 50 standard sweep net sweeps (New 1998; Henderson and Southwood 2016), evenly divided between each pair of subsampling locations and covering a total of 400 m2. The sweep net had a mesh size of 0.5 × 0.75 mm and a 30.5 cm aperture. Sweeping was done before vegetation data collection at each sampling location so as to minimize disturbance (see Holmquist et al. 2010, 2011, 2013a for additional faunal sampling details). The same individual collected all faunal samples and vegetation data for consistency. All samples were collected between 0800 and 1800 in full sun and when wind speed was less than 12 km/h; a Kestrel 3000 m was used to record air and ground temperature and wind speed. All arthropod fauna were identified in the laboratory to species or morphospecies (particularly for immature individuals, Kremen et al. 1993; Oliver and Beattie 1996; Gerlach et al. 2013). Arthropods from all taxa were identified, rather than only those from a single order or other taxonomic group. Analysis across all arthropod groups facilitates detection of responses to habitat characteristics and other drivers that structure ecosystems (Fahrig and Jonsen 1998; Koricheva et al. 2000; Pocock et al. 2012).
We measured percent bare ground, percent green vegetation cover, percent standing brown (senescent) vegetation cover, and percent litter cover using a 10 m point-intercept transect (20 points) centered and randomly-oriented at each subsample location. We measured stem density, canopy height, litter depth, and structural complexity (pole-touch method, Bestelmeyer and Wiens 2001) at two random locations along each of the two transects for each faunal collection. We estimated plant species richness by counting taxa that were contacted anywhere along the full length of the transect.
Analysis
Univariate analyses were primarily 3 × 3 × 2 ANOVAs (Habitat × Month × Year), which were followed by Tukey’s multiple comparison tests, both using SYSTAT 12. Vegetation and physical response variables were as outlined above. Faunal response variables included total arthropod abundance, family and species richness, Margalef’s index (Magurran and McGill 2011), dominance (percent of total sample abundance represented by the most abundant species in each sample), number and percentage of aquatic and terrestrial arthropods, percentage of herbivores and predators, and individual order, family, and species abundances. Proportional variables were square-root transformed, and all other variables were log-transformed. We adjusted multiple comparisons to per-family error rate with the sequential Bonferroni correction (Holm 1979; Jaccard and Guilamo-Ramos 2002) with MacBonferroni 1.6.
Multivariate analyses included multi-response permutation procedures (MRPP) and nonmetric multidimensional scaling (NMS, McCune and Grace 2002; Peck 2010) using PC-ORD 6, as well as analyses of dispersion using PERMDISP2 (Anderson 2004). Data from all factors and samples were included in the response matrices. There were two explanatory matrices; both included habitat variables and a coding variable for Year, but one matrix included a coding variable for Habitat, and the other included a coding variable for Month. The response matrices of faunal species included only taxa that were collected in at least three sites so as to reduce sparsity (Peck 2010) but not discard excessive information (Poos and Jackson 2012). Response matrices were relativized by maximum abundance for each species. The final response matrix contained 162 species/morphospecies, with a moderate (McCune and Grace 2002) coefficient of variation of 63%. The Sørensen distance measure was used for all analyses.
We assessed dimensionality of data via stress tests and construction of scree plots as part of the NMS analyses. After assessing multiple levels of dimensionality, the best balance of stress level and dimensionality was achieved at three dimensions. We then used three dimensions as an initial configuration for 250 runs with real data. Final stress was moderately high at 18, but was less than expected by chance (p = 0.0040; Monte Carlo test, 249 runs). There were 82 runs for the final solution, and stress stabilized at 51 iterations in stress versus iteration plots. Eight complete additional NMS analyses confirmed consistency of results. The permutational analyses of dispersion were based on 9999 permutations, used the same datasets and distance measure used for MRPP, and results were derived from deviations from spatial medians and ANOVA tables. We supplemented these analyses with sign tests and rank abundance plots to provide additional perspectives on diversity, richness, and evenness (Underwood and Fisher 2006; Magurran and McGill 2011; Savage et al. 2011). The datasets generated and/or analyzed during the current study are freely available from the corresponding author upon request.
Results
Main effects differences for the twelve vegetation and physical variables were common, and arthropod habitat quality was generally highest in May (early-season), in Pond and Core, and in 2013 (Fig. 1, Online Resource 1). Ten variables differed by Habitat, eight by Year, and six by Month, although three-quarters of the variables also indicated one or more interactions. Canopy height was lowest in May and highest in Pond habitat during 2014 (Habitat × Year). Structural complexity was halved from 2013 to 2014, and was highest in Pond habitat during July (Habitat × Month). Shoot density was lowest in River habitat, during July, and in 2014 (all main effects). Litter depth was similarly lowest for River and was reduced by a factor of two in 2014 (main effects only). There was essentially no bare ground for Pond and Core, and only ~1% for River, in 2013, but bare ground increased to ~10% for all habitats in 2014 (Fig. 1, Online Resource 1). Green cover was lowest in River, during July, and in 2013 (main effects); a Month × Year interaction was apparent (stronger monthly trends in 2014). Thus both percent bare ground and green cover were higher during 2014, and standing senescent vegetation and litter were reduced during 2014 (Fig. 1, Online Resource 1). Plant species richness was highest at River and did not differ by month or year. Air temperature was lowest in River habitat; temperatures exceeded 30 °C by July at all sites (Online Resource 1). Soil surface temperature was also lowest at the River sites. Wind speed was higher at River and Core than at Pond sites (Online Resource 1).
Vegetation means (SE) as a function of Habitat (H), Month (M), and Year (Y). Letters indicate ANOVA contrasts for main effects and interactions that were significant at p < 0.01; see Online Resource 1 for additional parameters and detailed test results
Vegetation metrics suggested poorer habitat structure in River, but faunal abundance, richness, diversity, and % aquatic taxa were all higher in this habitat zone (Fig. 2, Online Resource 2). Faunal assemblage variables also generally had higher values earlier in the summer and in 2013. Richness and Margalef’s diversity both followed these trends for main effects, particularly for Habitat (River was two-fold higher), and interactions were absent (Fig. 2, Online Resource 2). Abundance results were similar, but there was also a Month x Year interaction. In accord with the trends for richness and diversity, dominance was low at River. The percent of adult taxa that had aquatic juvenile stages (% aquatic) was low for all months and habitats in 2013; aquatics represented only 0.46–2.71% of the fauna at River, but these animals were absent or essentially absent at Pond and Core. In 2014, these values increased slightly for Pond and Core, and % aquatic at River increased to a range of 3.68 to 9.71% (Fig. 2, Online Resource 2). Trends were similar for number of aquatics collected in the wetlands. Abundance of terrestrials was also greatest in River, but numbers were higher in 2013 than in 2014. The percent of the assemblage represented by predators was greatest in late summer, as was the predator:herbivore ratio; % herbivores was conversely highest in early summer (Fig. 2, Online Resource 2). Predator:herbivore ratio was greatest in 2014; this ratio was never greater than one during 2013 at any sites. Month × Year interactions were present for a number of variables, particularly for variables relating to the relative abundance of aquatics and terrestrials and for predators and herbivores. There was only a single, relatively weak, Habitat x Month interaction (species dominance).
Faunal assemblage means (SE) as a function of Habitat (H), Month (M), and Year (Y). All metrics were based on 50-sweep samples. Capital letters indicate ANOVA contrasts for main effects and interactions that were significant at p < 0.01, and lower case letters indicate significance at p < 0.05; see Online Resource 2 for additional parameters and detailed test results
The 7372 individuals collected during the study yielded representatives of seventeen orders, 127 families, and 310 species/morphospecies. Hemiptera was the most abundant order overall (60.1 individuals/50 sweeps, SE = 6.0, Fig. 3, Online Resource 3), followed by Coleoptera (\( \overline{\mathrm{x}}=10.8 \), SE = 1.6), Araneae (\( \overline{\mathrm{x}}=9.6 \), SE = 0.80), Diptera (\( \overline{\mathrm{x}}=9.1 \), SE = 1.1), and Hymenoptera (\( \overline{\mathrm{x}}=5.9 \), SE = 0.82). The most abundant species were all hemipterans (Fig. 4, Online Resource 3): the aphid Sitobion avenae (Fabricius) (overall \( \overline{\mathrm{x}}=6.9 \), SE = 2.0), the mirid plant bug Europiella artemisiae (Becker) (\( \overline{\mathrm{x}}=4.7 \), SE = 2.2) the delphacid leafhopper Nothodelphax consimilis (Van Duzee) (\( \overline{\mathrm{x}}=4.7 \), SE = 1.2), and the cicadellid leafhoppers Hebecephalus discessus (Van Duzee) (\( \overline{\mathrm{x}}=6.0 \), SE = 1.1), Mesamia sp. (\( \overline{\mathrm{x}}=3.9 \), SE = 1.7), and Dikraneura carneola (Stål) (\( \overline{\mathrm{x}}=3.3 \), SE = 0.7). Overall family richness was highest for Diptera (32), Hymenoptera (26), and Coleoptera (20); species/morphospecies richness was greatest for Diptera and Hemiptera (both 71) and Hymenoptera (65). The most speciose families were cicadellid leafhoppers (25 species/morphospecies), braconid and pteromalid wasps (13 and 12, respectively), and aphids (12).
Mean (SE) abundances of most abundant faunal orders as a function of Habitat (H), Month (M), and Year (Y). All metrics were based on 50-sweep samples. Note differing y-axes. Capital letters indicate ANOVA contrasts for main effects and interactions that were significant at p < 0.01, and lower case letters indicate significance at p < 0.05; see Online Resource 3 for additional orders and detailed test results
Mean (SE) abundances of abundant species as a function of Habitat (H), Month (M), and Year (Y). All metrics were based on 50-sweep samples. Note differing y-axes. Capital letters indicate ANOVA contrasts for main effects and interactions that were significant at p < 0.01, and lower case letters indicate significance at p < 0.05; see Online Resource 3 for additional species and detailed test results
Abundances of dominant orders reflected many of the patterns observed at the assemblage level, but there was also variability by order (Fig. 3, Online Resource 3). Hemiptera and Coleoptera were most abundant early in the season, near the river, and in 2013. Diptera were most abundant along the river but did not have lower abundances in 2014. Diptera decreased in abundance through the growing season in 2014 but not in 2013 (Fig. 3, Online Resource 3). Hymenoptera (wasps and ants) were most abundant near the river and in 2013, but monthly patterns were absent. Araneae (spiders) did not demonstrate differences as a function of habitat, and temporal differences were the opposite of those more generally observed: numbers were lowest in early season and rose thereafter. Spider abundances were much lower in 2014 than in 2013. Lepidoptera (moths and butterflies) were most abundant near the river, but abundances were low after the fire (Fig. 3, Online Resource 3). Peak lepidopteran abundances occurred in June in 2013, but there were no monthly patterns in 2014. There were no Habitat x Month interactions among the abundant orders.
Dominant species showed strong trends as a function of study factors, particularly Year. (Fig. 4, Online Resource 3). The aphid Sitobion avenae had low abundances throughout the study—except in May of 2014, when there was a 40-fold increase in abundance. There was also a two-fold increase in the cicadellid leafhopper Dikraneura carneola at this time. Conversely, a number of dominant species demonstrated the common pattern of higher abundances in River habitat, low abundances in 2014, and variable seasonal patterns: the delphacid leafhopper Nothodelphax consimilis, the cicadellid leafhopper Mesamia sp., and the plant bug Europiella artemisiae (Fig. 4, Online Resource 3). Yet another cicadellid, Hebecephalus discessus, was also virtually absent in 2014, but lacked clear patterns as a function of habitat or month. There were significant overall trends of higher abundances in River habitat (p = 0.0015, sign test across taxa in Online Resource 3) and in 2013 (p = 0.019) but not for a given month (p > 0.063 for all). Habitat x Month interactions were uncommon.
Rank-abundance relationships and multivariate analyses were consistent with the univariate trends of overall higher diversity near the river, in early season, and in 2013. Rank-abundance slopes were low for River, and high for Pond and July (Fig. 5). Multiple response permutation procedure results as a function of Month and Year were highly significant (p < 0.000001; A > 0.53), and all multiple comparisons were significant (all p < 0.0064). There were similar levels of significance for MRPP on Habitat and Year (p < 0.000001, A > 0.41; all multiple comparisons p < 0.035). Permutational analyses of dispersion were non-significant for the factor combinations in both MRPP analyses, indicating that the differences observed via MRPP were due to differences in assemblage structure rather than being attributable to dispersion. The overall PERMDISP result for Month x Year was p = 0.61, and pairwise contrasts ranged from 0.70 to 0.98. The Habitat x Year result was p = 0.54; pairwise comparisons ranged from 0.83 to 0.91.
Nonmetric multidimensional scaling showed lack of overlap between years in ordinal space (Figs. 6 and 7). Months were also somewhat disjunct (Fig. 6), but there was more overlap among habitats (Fig. 7). Cumulative R2 was 0.66 for both ordinations. Important explanatory variables in the Month-Year ordination included complexity (R2 = 0.34), litter depth (0.22), green cover (0.22), and litter cover (0.21), which were most strongly associated with Axis 2 (Fig. 6). Results were similar for the Habitat-Year ordination, but percent cover by senescent vegetation (R2 = 0.20) also met the threshold for variable-axis correlation for inclusion in the joint plot (Fig. 7). Explanatory variables were again most closely associated with Axis 2 (Fig. 7).
Ordination of faunal assemblages by Month and Year across samples using nonmetric multidimensional scaling. Distance between site icons increases with dissimilarity among samples; convex hulls surround all samples of a given Month-Year combination. White and black symbols indicate 2013 and 2014 samples, respectively. Squares indicate May, triangles June, and diamonds July. Plots were scaled by proportion of maximum; orthogonality was 100% for each axis pair. Axis labels note R2 values estimating post-hoc percent of variation within the distance matrix that is explained by each axis. Cumulative R2 was 0.66. Explanatory variables in joint plot: Co = Complexity, LC = Litter Cover, GC = Green Cover, LD = Litter Depth. Minimum explanatory variable-axis correlation for inclusion in the joint plot was R2 = 0.20
Ordination of faunal assemblages by Habitat and Year across samples using nonmetric multidimensional scaling. Distance between site icons increases with dissimilarity among samples; convex hulls surround all samples of a given Habitat-Year combination. White and black symbols indicate 2013 and 2014 samples, respectively. Squares indicate Pond, triangles River, and diamonds Core. Plots were scaled by proportion of maximum; orthogonality was 100% for each axis pair. Axis labels note R2 values estimating post-hoc percent of variation within the distance matrix that is explained by each axis. Cumulative R2 was 0.66. Explanatory variables in joint plot: Co = Complexity, LC = Litter Cover, GC = Green Cover, BC = Brown (standing senescent) Cover, LD = Litter Depth. Minimum explanatory variable-axis correlation for inclusion in the joint plot was R2 = 0.20
Discussion
We found a high diversity and abundance of fauna in riparian edge habitat, relative to core wetland, as we had hypothesized, but this relationship only held for the river riparian sites. Contrary to expectations, the pond riparian fauna was similar to that of core habitat that was distant from water. Further, the trends observed at River sites were driven by terrestrial fauna, rather than by emerging aquatic insects as had been anticipated on the basis of previous work (Murakami and Nakano 2002; Kato et al. 2004; Baxter et al. 2005; Fukui et al. 2006). It seems unlikely that the dearth of aquatic taxa near the river was the result of low lotic abundance. Limited sampling of the river near the wetland, coincident with each wetland sample (Holmquist and Schmidt unpublished data; Online Resource 4) yielded a faunal assemblage that was analogous to that of other montane river habitat (Holmquist and Waddle 2013) and should have provided a source pool of emerging lotic fauna.
If there were few aquatic fauna sampled in river riparian habitat, and habitat structure was relatively poor near the river, why were wetland fauna so diverse and abundant at the River sites? There are several non-mutually exclusive possibilities. a) Summer microclimate may have been more favorable for arthropods near the river. River sites had lower air and ground temperatures than were recorded from the other sites. Wind speeds were higher at River than Pond, which may have also contributed to the cooling effect. Humidity was not recorded but may have been higher near the river as well, particularly after the wetlands dried later in the season. b) Vegetation structure can have important influences on wetland arthropods, particularly in mountain environments with short growing seasons (Holmquist et al. 2013b, 2014). Structure was unlikely to have been responsible for the rich faunal assemblage of the river riparian wetland, given that structure metrics indicated poorer habitat quality near the river than in Core and Pond habitat. It is possible that unknown factors associated with Scirpus acutus and Carex near the pond and Poa in core habitat were unfavorable for arthropods, but taller plants, such as Carex vesicaria and Scirpus acutus, are known to provide good habitat for wetland arthropods (Holmquist et al. 2011, 2013b; Cunha et al. 2012). The River habitat did have higher plant species richness, which should have a positive influence on fauna (Schaffers et al. 2008), though plant species richness can be less important than vegetation structure in driving wetland arthropod richness (Cunha et al. 2012; Holmquist et al. 2013b). c) Many terrestrial insects undertake long, active flights or are carried passively by winds, and rivers are flyways (Forman 1995; Puth and Wilson 2001). Many of the taxa found in the study wetland are strong fliers or are small enough to be transported passively by wind. There may be a settlement shadow (Gaines and Roughgarden 1985; Lewin 1986) that increases diversity and abundance near the river. There is little wetland habitat along the montane portion of the river, which is largely bordered by steep canyon walls, and insects flying along the river corridor may settle in the first portion of acceptable habitat that is encountered after a long flight, i.e., river riparian habitat. d) Many of the terrestrial taxa may be “multi-habitat” species (Forman 1995) that, though lacking an aquatic life stage, make use of the river bank for puddling (drinking), cooling, or egg laying in sand. e) We may have largely missed the emergences of aquatic insects in either time or space, if the emergences of the variety of aquatic taxa had been devoured or otherwise perished before these animals could be sampled or if the emerging individuals largely avoid wetland vegetation. Some combination of these phenomena, or others, apparently yields substantial edge effects resulting in high diversity and abundance (Polis and Hurd 1996; Fukui et al. 2006) at the river-wetland ecotone. In contrast, the pond riparian fauna may have been as depauperate as core wetland because of distance from the river flyway and because of low water levels during the study.
We had hypothesized that both terrestrial fauna and aquatic adults would decrease in abundance through the growing season in this Mediterranean climate, and this pattern was indeed evident. The congruent directionality for terrestrials and aquatics contrasted with previously observed opposing trends through the growing season in other locations: decreasing aquatic abundance but increasing terrestrial abundance through the growing season (Nakano and Murakami 2001; Kato et al. 2003). Summer in the montane Sierra Nevada is a stressful period after early season, in contrast with wetter environments. In these Sierrran wetlands, soils dry and plant productivity slows or ceases before temperatures cool, (Online Resource 1, % senescent vegetation; Holmquist et al. 2013a), and faunal diversity and abundance appear to also decline well before the end of summer. Terrestrial arthropods are generally in diapause-- variously as eggs, larvae, nymphs, pupae, or adults— during times of the year in which photoperiod, temperature, and food resources are not optimal (Wolda 1988; Cardoso et al. 2007). In the Sierra Nevada, the optimal period between the wet winter and dry summer is short indeed. These seasonal faunal declines at our montane study sites were more precipitous than previously observed in subalpine wetlands (Holmquist et al. 2013a), likely because of less snow accumulation, less soil saturation, and warmer summer temperatures at these lower elevations. Neither the terrestrial or aquatic seasonal decreases are likely to be supply-side in nature, as a function of decreasing aquatic subsidy; lotic densities tend to be highest in mid- to late season (Online Resource 4; Holmquist et al. 2015). There were few Habitat x Month interactions, indicating that differences among habitats were, contrary to our hypothesis, consistent through the growing season.
Predators, particularly spiders, were an exception to the trend of decreasing arthropod abundances through the growing season. The high early-season abundances of herbivores, particularly leafhoppers and beetles, may have fueled spider abundances that remained high after seasonal reductions in herbivore densities (Henschel et al. 2001; but see Denlinger 1980), although seasonal drying and senescence are likely to have caused at least as much of the observed herbivore decrease as predation (Holmquist et al. 2013a).
Interannual effects for fauna were common and strong and indicated an overall negative trend from 2013 to 2014. We cannot unequivocally claim that these trends were caused by fire, due to lack of available reference habitat (see also Rose and Goebel 2015), but trends for both vegetation and fauna were consistent with frequently-reported fire effects. Fire in grass and sedge-dominated habitats burns away litter and standing senescent vegetation, increases the proportion of bare ground, and increases green cover within a year (Kato et al. 2003; Vogel et al. 2010; Little et al. 2013, Masunga et al. 2013; see also Hosoishi et al. 2014). We observed identical directionality for these metrics at our sites following the Rim Fire. Faunal assemblages can be strongly influenced by indirect fire effects, via these shifts in vegetation structure, and by direct effects (Vogel et al. 2010; Little et al. 2013), though responses can vary among environments and taxa (Warren et al. 1987; Siemann et al. 1997; Swengel 2001; Panzer 2002; Hanula and Wade 2003; Doamba et al. 2014). Affected fauna may be killed directly by wildfire (Bock and Bock 1991; Swengel 2001) or may emigrate during or after the fire (Swengel 2001; Doamba et al. 2014). Direct mortality is most likely for species that are in immobile stages just prior to the coming fall and winter (Swengel 2001; Malmström et al. 2009). Many leafhoppers and Lepidoptera are univoltine, and eggs and dormant juveniles are likely to be sequestered in litter in late season (Panzer and Schwartz 2000). These groups may be particularly susceptible to fire and other disturbances (Armitage et al. 2013), and leafhoppers and Lepidoptera had much lower abundances on our sites in 2014 than in 2013. There were also major 2014 decreases in Coleoptera, Hymenoptera, and Araneae, as well as decreases in overall abundance, species richness, and diversity. Similar trends were common at the species level, but the aphid Sitobion avenae and the leafhopper Dikraneura carneola were exceptions. Both taxa can produce outbreaks under certain conditions, and may have been able to respond rapidly to the additional food resources present during greenup in 2014. In contrast, fire-sensitive taxa may be slow to recover (Vogel et al. 2010), particularly if source habitat is limited and/or distant (Anderson et al. 1989; Swengel 2001; Panzer 2002). There were no unburned portions of the study wetland, and source wetlands were distant and at higher elevation; this level of isolation may have contributed to the low diversity and abundance present in the study wetlands in 2014. The decreases that we observed in 2014 may or may not have been due to fire effects, but were unlikely to have been a proximate result of reduced aquatic subsidy, though fire and stream productivity can demonstrate complex interactions (Malison and Baxter 2010; Jackson et al. 2012; Jackson and Sullivan 2018). Abundance and richness of emergent lotic fauna were nominally greater in 2014 than in 2013, and pond inundation occurred in 2014 and likely increased the supply of emerging lentic fauna. Emerging aquatics nonetheless represented a small proportion of the wetland fauna in either year.
Conclusions
Wetland arthropods were strongly influenced by habitat context and seasonal and interannual factors, but emerging aquatic insects had little proximate influence on these patterns, which was an unexpected result, and powerful aquatic subsidies to riparian habitats should not be assumed to be a universal phenomenon. Faunal diversity and abundance were markedly reduced through the summer, likely due to drying of wetland habitat. Differences among habitats were consistent through the growing season and did not shift as a function of changes in aquatic subsidy or increasing wetland senescence. Fire probably had a strong influence on faunal assemblages and vegetation, though we cannot rule out stochastic change between 2013 and 2014.
References
Andersen AN, Ribbons RR, Pettit M, Parr CL (2014) Burning for biodiversity: highly resilient ant communities respond only to strongly contrasting fire regimes in Australia's seasonal tropics. Journal of Applied Ecology 51:1406–1413
Anderson MJ (2004) PERMDISP: a FORTRAN computer program for permutational analysis of multivariate dispersions (for any two-factor ANOVA design) using permutation tests. Department of Statistics, University of Auckland, New Zealand
Anderson RC, Leahy T, Dhillion SS (1989) Numbers and biomass of selected insect groups on burned and unburned sand prairie. American Midland Naturalist 122:151–162
Armitage AR, Ho C-K, Quigg A (2013) The interactive effects of pulsed grazing disturbance and patch size vary among wetland arthropod guilds. PLoS ONE 8:e76672
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biology 50:201–220
Bess EC, Parmenter RR, McCoy S, Molles MC Jr (2002) Responses of a riparian forest-floor arthropod community to wildfire in the middle Rio Grande Valley, New Mexico. Environmental Entomology 31:774–784
Bestelmeyer BT, Wiens JA (2001) Ant biodiversity in semiarid landscape mosaics: the consequences of grazing vs. natural heterogeneity. Ecological Applications 11:1123–1140
Bischof MM, Hanson MA, Fulton MR, Kolka RK, Sebestyen SD, Butler MG (2013) Invertebrate community patterns in seasonal ponds in Minnesota, USA: response to hydrologic and environmental variability. Wetlands 33:245–256
Bock CE, Bock JH (1991) Response of grasshoppers (Orthoptera: Acrididae) to wildfire in a southeastern Arizona grassland. American Midland Naturalist 125:162–167
CalFire (2013) Vegetative burn severity – Rim Fire. In: Forest Practice Geographical Information System. Available via https://www.calfire.ca.gov/resource_mgt/resource_mgt_forestpractice_gis. Accessed Feb 2015
Cardoso P, Borges PAV, Gaspar C (2007) Biotic integrity of the arthropod communities in the natural forests of Azores. Biodiversity and Conservation 16:2883–2901
Cunha E, Thomaz S, Mormul R, Cafofo E, Bonaldo A (2012) Macrophyte structural complexity influences spider assemblage attributes in wetlands. Wetlands 32:369–377
Denlinger DL (1980) Seasonal and annual variation of insect abundance in the Nairobi National Park, Kenya. Biotropica 12:100–106
Doamba SWMF, Savadogo P, Nacro HB (2014) Effects of burning on soil macrofauna in a savanna-woodland under different experimental fuel load treatments. Applied Soil Ecology 81:37–44
Fahrig L, Jonsen I (1998) Effect of habitat patch characteristics on abundance and diversity of insects in an agricultural landscape. Ecosystems 1:197–205
Forman RTT (1995) Land mosaics: the ecology of landscapes and regions. Cambridge University Press, Cambridge
Fukui D, Murakami M, Nakano S, Aoi T (2006) Effect of emergent aquatic insects on bat foraging in a riparian forest. Journal of Animal Ecology 75:1252–1258
Gaines S, Roughgarden J (1985) Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proceedings of the National Academy of Sciences 82:3707–3711
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. Journal of Insect Conservation 17:831–850
Hanula JL, Wade DD (2003) Influence of long-term dormant-season burning and fire exclusion on ground-dwelling arthropod populations in longleaf pine flatwoods ecosystems. Forest Ecology and Management 175:163–184
Henderson PA, Southwood TRE (2016) Ecological methods, 4th edn. Wiley, Chichester
Henschel JR, Mahsberg D, Stumpf H (2001) Allochthonous aquatic insects increase predation and decrease herbivory in river shore food webs. Oikos 93:429–438
Holm S (1979) A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, Theory, and Applications 6:65–70
Holmquist JG, Waddle TJ (2013) Predicted macroinvertebrate response to water diversion from a montane stream using two-dimensional hydrodynamic models and zero flow approximation. Ecological Indicators 28:115–124
Holmquist JG, Schmidt-Gengenbach J, Haultain SA (2010) Does long-term grazing by pack stock in subalpine wet meadows result in lasting effects on arthropod assemblages? Wetlands 30:252–262
Holmquist JG, Jones JR, Schmidt-Gengenbach J, Pierotti LF, Love JP (2011) Terrestrial and aquatic macroinvertebrate assemblages as a function of wetland type across a mountain landscape. Arctic, Antarctic, and Alpine Research 43:568–584
Holmquist JG, Schmidt-Gengenbach J, Haultain SA (2013a) Effects of a long-term disturbance on arthropods and vegetation in subalpine wetlands: manifestations of pack stock grazing in early versus mid-season. PLoS ONE 8:e54109
Holmquist JG, Schmidt-Gengenbach J, Haultain SA (2013b) Equine grazing in managed subalpine wetlands: effects on arthropods and plant structure as a function of habitat. Environmental Management 52:1474–1486
Holmquist JG, Schmidt-Gengenbach J, Demetry A (2014) Efficacy of low and high complexity vegetation treatments for reestablishing terrestrial arthropod assemblages during montane wetland restoration. Restoration Ecology 22:649–656
Holmquist JG, Schmidt-Gengenbach J, Roche JW (2015) Stream macroinvertebrates and habitat below and above two wilderness fords used by mules, horses, and hikers in Yosemite National Park. Western North American Naturalist 75:311–324
Hosoishi S, Tasen W, Park S-H, Le Ngoc A, Kuboki Y, Ogata K (2014) Annual fire resilience of ground-dwelling ant communities in Hiraodai Karst Plateau grassland in Japan. Entomological Science 18:254–261. https://doi.org/10.1111/ens.12117
Jaccard J, Guilamo-Ramos V (2002) Analysis of variance frameworks in clinical child and adolescent psychology: advanced issues and recommendations. Journal of Clinical Child Psychology 31:278–294
Jackson BK, Sullivan SMP (2015) Responses of riparian tetragnathid spiders to wildfire in forested ecosystems of the California Mediterranean climate region, USA. Freshwater Science 34:1542–1557
Jackson BK, Sullivan SMP (2018) Ecosystem size and flooding drive trophic dynamics of riparian spiders in a fire-prone Sierra Nevada river system. Canadian Journal of Fisheries and Aquatic Sciences 75:308–318
Jackson BK, Sullivan SMP, Malison RL (2012) Wildfire severity mediates fluxes of plant material and terrestrial invertebrates to mountain streams. Forest Ecology and Management 278:27–34
Jackson BK, Sullivan SMP, Baxter CV, Malison RL (2015) Stream-riparian ecosystems and mixed- and high-severity fire. In: DellaSala DA, Hanson CT (eds) The ecological importance of mixed-severity fires: nature’s phoenix. Elsevier, Amsterdam, pp 1–31
Kato C, Iwata T, Nakano S, Kishi D (2003) Dynamics of aquatic insect flux affects distribution of riparian web-building spiders. Oikos 103:113–120
Kato C, Iwata T, Wada E (2004) Prey use by web-building spiders: stable isotope analyses of trophic flow at a forest-stream ecotone. Ecological Research 19:633–643
Koricheva J, Mulder CPH, Schmid B, Joshi J, Huss-Danell K (2000) Numerical responses of different trophic groups of invertebrates to manipulations of plant diversity in grasslands. Oecologia 125:271–282
Kremen C, Colwell RK, Erwin TL, Murphy DD, Noss RF, Sanjayan MA (1993) Terrestrial arthropod assemblages: their use in conservation planning. Conservation Biology 7:796–808
Lewin R (1986) Supply-side ecology. Science 234:25–27
Little IT, Hockey PAR, Jansen R (2013) A burning issue: fire overrides grazing as a disturbance driver for South African grassland bird and arthropod assemblage structure and diversity. Biological Conservation 158:258–270
Lydersen JM, North MP, Collins BM (2014) Severity of an uncharacteristically large wildfire, the Rim Fire, in forests with relatively restored frequent fire regimes. Forest Ecology and Management 328:1–9
Magurran AE, McGill BJ (2011) Biological diversity: frontiers in measurement and assessment. Oxford University Press, Oxford
Malison RL, Baxter CV (2010) The fire pulse: wildfire stimulates flux of aquatic prey to terrestrial habitats driving increases in riparian consumers. Canadian Journal of Fisheries and Aquatic Sciences 67:570–579
Malmström A, Persson T, Ahlström K, Gongalsky KB, Bengtsson J (2009) Dynamics of soil meso- and macrofauna during a 5-year period after clear-cut burning in a boreal forest. Applied Soil Ecology 43:61–74
Masunga GS, Moe SR, Pelekekae B (2013) Fire and grazing change herbaceous species composition and reduce beta diversity in the Kalahari sand system. Ecosystems 16:252–268
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Moranz RA, Fuhlendorf SD, Engle DM (2014) Making sense of a prairie butterfly paradox: the effects of grazing, time since fire, and sampling period on regal fritillary abundance. Biological Conservation 173:32–41
Murakami M, Nakano S (2002) Indirect effect of aquatic insect emergence on a terrestrial insect population through by birds predation. Ecology Letters 5:333–337
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences 98:166–170
New TR (1998) Invertebrate surveys for conservation. Oxford University Press, New York
Oliver I, Beattie AJ (1996) Invertebrate morphospecies as surrogates for species: a case study. Conservation Biology 10:99–109
Panzer R (2002) Compatibility of prescribed burning with the conservation of insects in small, isolated prairie reserves. Conservation Biology 16:1296–1307
Panzer R, Schwartz M (2000) Effects of management burning on prairie insect species richness within a system of small, highly fragmented reserves. Biological Conservation 96:363–369
Peck JE (2010) Multivariate analysis for community ecologists: step-by-step using PC-ORD. MjM Software Design, Gleneden Beach
Pocock MJO, Evans DM, Memmott J (2012) The robustness and restoration of a network of ecological networks. Science 335:973–977
Polis GA, Hurd SD (1996) Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. American Naturalist 147:396–423
Poos MS, Jackson DA (2012) Addressing the removal of rare species in multivariate bioassessments: the impact of methodological choices. Ecological Indicators 18:82–90
Puth LM, Wilson KA (2001) Boundaries and corridors as a continuum of ecological flow control: lessons from rivers and streams. Conservation Biology 15:21–30
Rose SJ, Goebel PC (2015) Short-term impacts of prescribed burning on the spider community (order: Araneae) in a small Ohio grassland. Ohio Journal of Science 115:79–89
Russo TA, Fisher AT, Roche JW (2012) Improving riparian wetland conditions based on infiltration and drainage behavior during and after controlled flooding. Journal of Hydrology 432:98–111
Savage J, Wheeler TA, Moores AMA, Taillefer AG (2011) Effects of habitat size, vegetation cover, and surrounding land use on Diptera diversity in temperate Nearctic bogs. Wetlands 31:125–134
Schaffers AP, Raemakers IP, Sýkora KV, ter Braak CJF (2008) Arthropod assemblages are best predicted by plant species composition. Ecology 89:782–794
Schalla S (2015) Hatches- West Side Sierra. In: Fly fishing the Sierra Available via https://stevenojai.tripod.com/hatchWest.htm. Accessed Feb 2015
Siemann E, Haarstad J, Tilman D (1997) Short-term and long-term effects of burning on oak savanna arthropods. American Midland Naturalist 137:349–361
Swengel AB (2001) A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodiversity and Conservation 10:1141–1169
Underwood EC, Fisher BL (2006) The role of ants in conservation monitoring: if, when, and how. Biological Conservation 132:166–182
Vogel JA, Koford RR, Debinski DM (2010) Direct and indirect responses of tallgrass prairie butterflies to prescribed burning. Journal of Insect Conservation 14:663–677
Warren SD, Scifres CJ, Teel PD (1987) Response of grassland arthropods to burning: a review. Agriculture, Ecosystems and Environment 19:105–130
Wolda H (1988) Insect seasonality: why? Annual Review of Ecology and Systematics 19:1–18
Acknowledgements
This project was supported by the U.S. National Park Service (P12AC10902), and the current study built upon earlier work funded by the NPS (J8R07070007, J8C07110016, and P12AC10902). We very much appreciate project support from, and discussion with, Greg Stock, Monica Buhler, Breeanne Jackson, Laura Jones, Linda Mazzu, Joe Meyer, Jim Roche, Sarah Stock, and Steve Thompson (all National Park Service), Tom Francis, Mike Horvath, Elizabeth Lilley, Adam Mazurkiewicz, Bruce McGurk, Tim Ramirez, Bill Sears, and Jen Vick (all San Francisco Public Utilities Commission), and Jeremiah Eanes, Joan Koyama, Antony Orme, Elizabeth Sally, Shahara Vasquez, and staff at White Mountain Research Center (all UCLA). We thank Marie Pavlovsky, Steve Case, Val Case, Kristen Klinefelter, and Jamey Wilcher for field and/or lab assistance. Ray Gill and Alessandra Rung kindly provided hemipteran identifications. This project was facilitated by the Californian and Great Basin Cooperative Ecosystems Studies Units, with guidance from Angela Evenden. We dedicate this paper to the memory of Steve Thompson, inspirational wildlife biologist, who gave us our first tour of Poopenaut Valley many years ago.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online Resource 1
Vegetation and physical parameters. Means (standard errors) for vegetation and physical parameters and ANOVA results for main effects and two-way interactions. (pdf) (PDF 81 kb)
Online Resource 2
Faunal assemblage parameters. Means (standard errors) for faunal assemblage parameters (all based upon 50 sweeps) and ANOVA results for main effects and two-way interactions. (pdf) (PDF 81 kb)
Online Resource 3
Faunal orders and most abundant families and species. Mean number of individuals (standard errors) for faunal orders and ten most abundant families and species (all based upon 50 sweeps) and ANOVA results for main effects and two-way interactions. (pdf) (PDF 1144 kb)
Online Resource 4
Lotic fauna near wetland. Raw data, means, and standard errors for Tuolumne River lotic fauna near wetland sites. Results are from 1 m2 kick net samples from cobble habitat. (xlsx) (XLSX 37 kb)
Rights and permissions
About this article
Cite this article
Holmquist, J.G., Schmidt-Gengenbach, J. Arthropod Assemblages in a Montane Wetland Complex: Influences of Adjoining Lotic and Lentic Habitat and Temporal Variability. Wetlands 40, 259–271 (2020). https://doi.org/10.1007/s13157-019-01175-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01175-6