Abstract
Rice sheath blight disease caused by the necrotrophic fungus Rhizoctonia solani, is an economically detrimental fungal disease which significantly affects rice productivity worldwide. Potato snakin-1, a cysteine-rich basic antimicrobial peptide (AMP), is a member of the novel Snakin AMP family. In order to assess the ability of the snakin-1 peptide in protecting rice against the sheath blight disease, we have developed transgenic rice constitutively expressing the snakin-1 peptide. The antimicrobial activity of snakin-1 was evaluated against the sheath blight pathogen Rhizoctonia solani both in vitro and in planta. Crude protein from transgenic rice leaves showed in vitro antifungal activity against Rhizoctonia solani. Moreover, in planta bioassay results also confirmed the same, wherein snakin-1 expressing rice plants showed significantly enhanced protection against the sheath blight disease. This report demonstrates how a member of the Snakin family of antimicrobial peptides has been successfully used to generate sheath blight resistance in rice, without compromising on its agronomic characteristics and at no phenotypic cost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is one of the most extensively grown and consumed cereal crops in the world, especially India and China being the leading producers. The worldwide rice production has been afflicted by various biotic stress factors among which are the major diseases like fungal blast, bacterial blight and sheath blight.
The causal agent of sheath blight is the basidiomycetous necrotrophic soil-borne fungus Rhizoctonia solani Kühn [teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk]. This cosmopolitan fungal pathogen affects a wide range of host plants belonging to more than 32 plant families (Molla et al. 2020). Sheath blight is one of the most destructive and economically damaging diseases affecting rice productivity worldwide. The disease proliferation is favored by a wide array of environmental conditions such as high temperature (28–32°C) and high relative humidity of 96–100% with frequent rainfall. Additionally due to various agricultural malpractices like, high levels of nitrogen in the soil due to excess usage of N-fertilizers, use of high yielding varieties, close spacing of plants and dense canopy, presence of sclerotia in the soil also are responsible for sheath blight development (Pin et al. 2012). It is a major production constraint of rice in most states of India. Yield losses have been reported in the range of 4–50%, depending on the cultivar, environmental conditions and stages at which the plants are infected (Singh et al. 2016). Due to the broad host range and flexibility of the pathogen, and lack of robust resistance in the existing germplasm (from both cultivated and wild varieties of rice), traditional breeding approaches have failed to yield durable sheath blight resistance in rice. Pesticides have been traditionally used worldwide to protect plants against this pathogen, but their rampant unregulated use is responsible for significant environmental and health-related issues worldwide (Molla et al. 2020). Therefore, the generation of transgenic rice by the integration of defense-related genes may well be the effective approach to cut productivity losses caused by sheath blight. Most of the transgenic strategies taken till date to generate sheath blight-resistant rice have involved the constitutive expression of pathogenesis-related (PR) proteins, such as PR-3 (chitinases) (Lin et al. 1995; Datta et al. 2000, 2001), PR-5 (thaumatin-like proteins) (Datta et al. 1999) and the tissue-specific expression of oxalate oxidase (Molla et al. 2013). There are also reports on co-expression of some PR proteins, like rice chitinase (chi11) and thaumatin-like protein (Kalpana et al. 2006), chi11 and β-1,3-glucanase (Sridevi et al. 2008) and chitinase and oxalate oxidase (Karmakar et al. 2016). Plants have acquired a two-tiered defense response against pathogen attacks over the course of evolution. The first innate layer is exemplified by readymade structures and molecules present during the regular life cycle of a plant, whereas the second adaptive layer is elicited during pathogen infection. In spite of the well-developed multilayered defense network, pathogens have managed to slip through and infect the host plants, thereby causing losses in productivity. Antimicrobial peptides (AMPs) are important members of a group of evolutionarily conserved low molecular weight defense peptides, which are a vital component of the innate and the adaptive immune responses in plants and help deliver broad spectrum resistance against a wide variety of phytopathogens. These AMPs directly act against the microbes via varying modes of action such as disrupting their membranes, inhibiting their growth and causing cell death (Barbosa Pelegrini et al. 2011). AMPs are mostly positively charged, rich in cysteine residues and possess a disulfide bridge-stabilized structure. The deadly combination of small size and antimicrobial efficacy in the nM to µM range makes AMPs an excellent choice for generating disease resistance against a wide spectrum of phytopathogens affecting diverse families of crops (Das et al. 2019). Transgenic approaches have been successfully used previously to express different AMPs such as puroindolines (Krishnamurthy et al. 2001), nonspecific lipid transfer proteins (ns-LTPs) (Patkar and Chattoo 2006) and defensins (Jha et al. 2009; Jha and Chattoo 2010) to generate sheath blight-resistant rice.
Snakin peptides are a novel family of antimicrobial peptides which share structural similarity with members of the Snakin/Gibberellic Acid-Stimulated Arabidopsis (GASA) protein family (Segura et al. 1999; Berrocal-Lobo et al. 2002). They share common features such as N-terminal putative signal sequence, a highly divergent intermediate region and a conserved C-terminal domain with 12 conserved Cys residues. In spite of the common structural features, they are engaged in diverse biological processes like cell division, cell elongation, cell growth, transition to flowering, signaling pathways and defense (Segura et al. 1999; Roxrud et al. 2007; Nahirñak et al. 2012). The Snakin/GASA family is proposed to have an important role is redox homeostasis as it contains putative redox active sites, i.e., cysteine residue pairs present in the GASA domain, and this has been validated in different Snakin/GASA family members in various plants (Wigoda et al. 2006; Rubinovich and Weiss 2010; Lee et al. 2015; Rubinovich et al. 2014; Nahirñak et al. 2019).
The Snakin-1 (StSN1) peptide, a cysteine-rich antimicrobial peptide isolated from potato (Solanum tuberosum), has 63 amino acid residues, 12 of which are highly conserved cysteines. The StSN1 peptide was shown to inhibit the following fungal pathogens such as Fusarium solani, Fusarium culmorum, Aspergillus flavus, Bipolaris maydis, Colletotrichum lagenarium, Colletotrichum graminicola, Plectosphaerella cucumerina and Botrytis cinerea in vitro (Segura et al. 1999; Berrocal-Lobo et al. 2002). Transgenic wheat overexpressing SN1 displayed significantly heightened resistance to the take-all disease as well as the powdery mildew disease (Rong et al. 2013; Faccio et al. 2011). Alfalfa modified to overexpress its endogenous SN1 was protected against two different fungal diseases, anthracnose and spring leaf spot (García et al. 2014). Overexpression of the snakin-1 gene was shown to confer enhanced resistance in transgenic potato plants to Rhizoctonia solani (Almasia et al. 2008). Lettuce plants expressing it also showed resistance against the bottom rot and drop disease causing pathogen, Rhizoctonia solani (Darqui et al. 2018).
In the present study, we aim to investigate whether the constitutive expression of snakin-1 in transgenic rice protects it against the sheath blight fungus, Rhizoctonia solani.
Materials and methods
Vector construction
The 1.8-kb fragment of the ubiquitin1 promoter (Ubi1) from rice, 785-bp fragment of snakin-1 gene from potato cv. Kufri Chipsona 3 and 320-bp fragment of nos terminator from pCAMBIA1301 were amplified by PCR using HotStar HiFidelity Polymerase from Qiagen. These were then cloned into the pBluescript SK(+) vector and sequenced. For restriction digestion-mediated verification, the rice ubiquitin1 promoter fragment was digested with SacI and KpnI, the snakin-1 gene with KpnI and SmaI and the nos terminator with SmaI and BamHI from the pBluescript SK(+) vector, and the construct was cloned into the pUC19 vector to make the final construct pUC-OSN. The 2.8-kb fragment of the final construct was subsequently cloned into the SacI-BamHI digested pCAMBIA1301 to form the pCAM-OSN.
Rice transformation
The genetic transformation of rice (Oryza sativa L. subspecies indica cv. Jaldi-13) was carried out by following the Agrobacterium-mediated method (Lin et al. 2009) with some minor modifications, using 50 mg/L hygromycin B as the selection agent. The putative transformed plants were moved to soilrite for hardening. The hardened plants were subsequently transplanted in pots containing soil in the greenhouse and grown to maturity.
PCR screening
Genomic DNA was extracted from the leaves of putative T0-positive rice plants as previously described (Huang et al. 1997). With 100 ng of genomic DNA template, PCR screening was performed using snakin-1-nos-specific primers (F = 5′-AAGGCAGGACTTGCAGACAG-3′ and R = 5′-GATCTAGTAACATAGATGACACCGC-3′).
Southern blot analysis
Genomic DNA was extracted from the leaves of non-transformed wild-type control (WT) and PCR-positive T1 transgenic plants according to the modified CTAB method (Stein et al. 2001) and quantified using a Nanodrop spectrophotometer (Thermo Fischer, USA). Fifteen micrograms of purified genomic DNA was digested with KpnI and BamHI restriction enzymes (Fermentas, USA). The digested DNA samples were electrophoretically separated by 1% agarose gel and blotted onto a nylon membrane (Hybond N+, Amersham, GE Healthcare, USA) via capillary transfer and UV crosslinked. Restriction-digested 1062-bp snakin1-nos fragment labeled with DIG-11-dUTP (as per the manufacturer’s protocol) was used as the probe for hybridization. The hybridization, washing and detection steps were performed in accordance with the manufacturer’s protocol (DIG DNA Labelling and Detection Kit, Roche, Germany).
RNA extraction, cDNA synthesis and quantitative real-time PCR
Rice leaf tissues were snap-frozen in liquid N2, and total RNA was extracted from 100 mg of frozen leaf samples using TRIzol reagent (Invitrogen, USA). The purified RNA was treated with DNase (Fermentas, USA), and first-strand cDNA was synthesized from 1 µg of purified total RNA using iScript™ cDNA synthesis kit (BioRad, USA). qRT-PCR was performed in the CFX-96 real-time PCR system (Bio-Rad) using a SYBR Green Kit (Fermentas, Canada) with snakin-1 gene-specific primers (F = 5′- AAGGCAGGACTTGCAGACAG-3′ and R = 5′-AGGGCATTTAGACTTGCCCTTA-3′). Rice β-tubulin gene was used as an internal control for qRT-PCR using tubulin-specific primers (F = 5′-GGAGTCACATGCTGCCTAAGGTT-3′ and R = 5′- TCACTGCCAGCTTACGGAGG-3′). Each experiment was performed in triplicate, and relative expression levels were determined using 2−ΔΔCTmethod (Livak and Schmittgen 2001).
Enzymatic antioxidant assays
The activity assays for various antioxidant enzymes were performed from leaf samples (WT and transgenic) that were collected at different time intervals post-infection following Paranidharan et al. (2003). The leaf samples were homogenized, and total protein concentration was determined by the Bradford method (1976) using BSA as the standard. The activity assays for peroxidase (POD), superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT) were performed as described (Chen and Zhang 2016; Das et al. 2015).
Estimation of lipid peroxidation
The level of lipid peroxidation was estimated by measuring the malondialdehyde (MDA) content, a by-product of lipid peroxidation (Chen and Zhang 2016).
Antifungal test
The crude protein was isolated from leaves of T0 transgenic and non-transformed WT plants by homogenizing with PBS (pH 7.0). A 5-day-old PDA plate of Rhizoctonia solani AG1-1A (Hyderabad isolates) was taken, and active young mycelia were incubated in 100 µg total protein solution for 4 h at 25 ± 2°C. The treated mycelia were then stained using lactophenol cotton blue (Hi-media), acridine orange-ethidium bromide (AO-EB) (Kasibhatla et al. 2006), Nile Red (Thrane et al. 1999) and MTT separately (Swain et al. 2017). They were subsequently photographed using a compound fluorescence microscope (Carl Zeiss, Switzerland).
Bioassay
Mycelial agar disc bioassay
The detached leaf bioassay using a mycelial agar disc was performed as previously described (Kumar et al. 2003). A spherical mycelial agar block (5 mm diameter) from a 5-day-old Rhizoctonia solani AG1-1A (Hyderabad isolates) on PDA media was placed on the middle of each leaf and incubated in the dark at 25 ± 2°C. After incubation for 72 h, leaf was stained using lactophenol cotton blue (Hi-media) and the number of infection cushions was determined under a microscope.
Microchamber bioassay
A mycelial disc of 1 cm diameter was used as inoculum carrier, from a 3-day-old PDA culture of Rhizoctonia solani AG1-1A (Hyderabad isolates). A single mycelial agar disc was placed at the base of each 21-day-old rice plant. Transgenic and WT plants were subjected to the microchamber infection bioassay according to Jia et al. (2007) with some modifications. The infected sheath 10 days post-infection (DPI) was stained using lactophenol cotton blue (Hi-media) and examined under a compound microscope (Carl Zeiss, Switzerland).
Whole plant bioassay
The whole plant bioassay was performed as described previously (Datta et al. 1999). PDA plate was inoculated with a single sclerotium of Rhizoctonia solani and incubated for 3 days in the dark at 30 °C. Subsequently it was again subcultured for another 3 days. The contents of the PDA plate were moved into a bottle containing a rice hull-rice grain mixture and incubated in the dark at 28°C for 10 days. Five grams of inoculated rice-hull mixture was carefully placed in the midst of the tillers of each plant at its maximum tillering stage. Then the tillers were tied together with rubber bands. Sterile water was sprayed every morning to maintain the humidity of the environment. The disease severity and progression were evaluated at 7, 14 and 21 DPI using a scale ranging from 0 to 9, which is based on the relative lesion height on the whole plant, in accordance with the Standard Evaluation System for Rice (IRRI 2002).
Agronomic evaluation of transgenic plants
The agronomic performance of both WT and snakin-1 transgenic rice plants were evaluated under the same growing conditions. There are different agronomic characters such as plant height (in cm), number of tillers, number of panicles per plant, panicle length (cm) and 1000 seeds weight (DW). All data were evaluated according to the Standard Evaluation System for Rice (IRRI 2002).
Statistical analysis
All statistical analysis was performed using the GraphPad Prism5 software (GraphPad software, La Jolla, CA, USA). One-way and two-way analyses of variance (ANOVAs) were used to compare the differences between the WT and the transgenic plants. The statistical significance at p < 0.05 was calculated.
Results
Molecular analysis of snakin-1 expressing transgenic rice
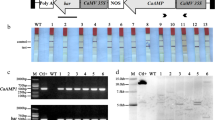
Agrobacterium strain LBA4404 harboring the gene construct pCAM-OSN (Fig. 1a) was used to transform dehusked mature rice seeds to generate transgenic plants. A total of 16 putative hygromycin-resistant transgenic plants (T0) were obtained through Agrobacterium-mediated transformation. PCR was used as a primary screening method for positive transformants. All the sixteen plants showed the amplification of the 437-bp PCR product, whereas WT plants did not show any amplified bands (Fig. 1b). Gene expression of the snakin-1 gene showed that two T0 plants viz. OSN-4 and OSN-10 showed comparatively higher expression than the others as seen in Fig. 1c. These were selected for further analysis. Southern hybridization analysis of T1 transgenic rice plants revealed integration of the transgene cassette into the rice genome (Fig. 2a) with no signal in the WT plant. Quantitative real-time PCR showed that three T1 plants from OSN-4 line, i.e., 4–21, 4–25 and 4–36, showed 3.47-, 1- and 2.11-fold higher expression of snakin-1 gene than the level of rice β-tubulin expression (Fig. 2b).
a Diagrammatic representation of the snakin-1 gene construct b PCR screening of putative transgenic plants using partial gene-specific primers (snakin-1-nos) to amplify the 437-bp snakin-nos fragment.(M)—GeneRuler 1 kb gene ladder used as marker; (N)—negative control or WT plant; (P)— positive control. c Expression analysis of snakin-1 gene using gene-specific primers by RT-PCR to generate 147-bp fragment. (M)—GeneRuler Low Range DNA Ladder used as marker; c WT control
a Southern blot analysis of KpnI-BamHI-digested genomic DNA of T1 transgenic plants with KpnI-BamHI-digested snakin1-nos probe of 1062 bp. (PC)- positive control, (NC)- negative control or WT plant. b Expression analysis of snakin-1 gene using quantitative real-time PCR, using the rice β-tubulin gene for normalization. Each bar represents the mean ± SE of three independent experiments.
Antioxidant enzyme activity and oxidative stress tolerance in progeny transgenic plants after infection
The activities of the major antioxidant enzymes were found to be greatly reduced in the transgenic plants as compared to the WT plants after infection (Fig. 3a–d). APX activity was 1.37- to 1.41-fold lower (p < 0.0001) in the transgenic plants than in the WT plants (Fig. 3a). Transgenic plant 4–21 showed a marked 3.69-fold reduction of CAT activity followed by 3.04- and 2.23-fold in plants 4–36 and 4–25 respectively (Fig. 3b), when compared to WT plants at 5DPI (p < 0.0001). The activity of POD was significantly reduced (3.09- to 3.66-fold) in transgenic plants (p < 0.0001) than the WT plants (Fig. 3c). SOD activity exhibited similar activity pattern as CAT with plant 4–21 (1.87-fold) showing the lowest activity followed by 4–36 (1.56-fold) and 4–25 (1.41-fold) (p < 0.0001), in comparison with the WT plants (Fig. 3d). The oxidative stress tolerance due to fungal infection was measured by the degree of lipid peroxidation and represented by the malondialdehyde (MDA) levels and was also found to be reduced by 1.95- to 3.56-fold in transgenic plants in relation to the WT plants (Fig. 3e).
The activities of the different antioxidant enzymes and evaluation of oxidative damage in WT and T1 transgenic rice plants at different days post-infection (DPI) with R. solani. a APX activity, b CAT activity, c POD activity, d SOD activity, e MDA content. Each bar represents the mean ± SE of three independent experiments
Antifungal activity of crude protein from transgenic rice plants
Lactophenol cotton blue is used to selectively stain fungi. The Rhizoctonia solani mycelia treated with the WT crude protein were mostly healthy, smooth and uniformly stained (Fig. 4a). In comparison, those treated with the transgenic crude protein showed a wide variety of abnormalities (Fig. 4b–f). The different abnormalities observed were (1) vacuolation (Fig. 4b), (2) aberrated hyphal membranes (Fig. 4c), (3) cytoplasmic granulation (Fig. 4d), (4) dead, empty and lightly stained hyphal fragments (Fig. 4e) and (5) swelling and bursting of the hyphal tips (Fig. 4f). These observations suggest that the snakin-1 had affected the mycelia.
Lactophenol cotton blue (LPCB)-stained Rhizoctonia solani. a Mycelia treated with WT crude protein showing mostly healthy uniformly stained hyphae. b Mycelia treated with transgenic crude protein showing vacuolation (indicated by red arrows). c Hyphae with aberrated membranes. d Hyphae showing granulated cytoplasm (indicated by the red arrow and in the magnified inset). e Lightly stained dead hyphae. f Hyphal tip swelling and tip bursting in the magnified inset indicated by red arrows (left arrow- burst tip; right arrow- swollen tip)
The acridine orange-ethidium bromide stain (AO-EB stain) is a viability stain, which detects and differentiates dead cells from live ones (Ribble et al. 2005). The mycelia treated with the WT crude protein have a majority of uniformly fluorescing healthy green hyphae with some dead orange hyphae (Fig. 5a, b). In contrast, the transgenic crude protein-treated fungi have a majority of the hyphae fluorescing orange indicating dead hyphae (Fig. 5c, d).
Acridine Orange-Ethidium Bromide (AO-EB)-stained Rhizoctonia solani: Mycelia treated with control WT crude protein showed a majority of live hyphae uniformly green fluorescing with few uniformly yellow-orange fluorescing necrotic hyphae (a, b). Mycelia treated with transgenic crude protein showed mostly necrotic hyphae (uniformly yellow-orange fluorescing) with some hyphae showing c early apoptosis (bright green dots) and d late apoptosis (bright orange dots) (both are marked by white arrows). Nile Red stained Rhizoctonia solani: Mycelia treated with WT control crude protein showed e mostly uniformly bright yellow fluorescing healthy hyphae with intact membranes. Mycelia treated with transgenic crude protein showed f mostly lightly fluorescing dead hyphae with damaged membranes and presence of lipid droplets (bright orange dots) (indicated by cyan arrows) indicating apoptosis. MTT staining of Rhizoctonia solani: g WT control crude protein-treated mycelia showing mostly purple-stained metabolically active live cells with few unstained dead cells. h Transgenic crude protein-treated mycelia showing mostly dead unstained cells with small purple-stained patches of live cells
Nile Red is hydrophobic dye which stains membranes and other lipid-containing structures in the hyphae (Yuan and Heath 1991). The WT crude protein-treated mycelia have a majority of bright uniformly fluorescing healthy yellow hyphae (Fig. 5e). Transgenic crude protein-treated hyphae showed reduced fluorescence as compared to the WT treated, indicating lower number of live hyphae (Fig. 5f).
Metabolically active cells can reduce MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), a yellow tetrazolium salt to purple formazan crystals. Mycelia treated by WT crude protein had a larger proportion of live purple-stained hyphae (Fig. 5g), while transgenic crude protein-treated mycelia showed mostly unstained dead hyphae with small patches of live purple cells (Fig. 5h).
Enhanced resistance of transgenic plants against sheath blight fungus
The microchamber method showed that transgenic plants OSN-4 (DI = 2.30) and OSN-10 (DI = 4.06) were significantly (p < 0.0001) better protected than the WT (DI = 5.89) at 10 DPI (Fig. 6a, b). Post-LPCB staining, we observed that the WT sheath was heavily infected with the fungus (Fig. 6ci), whereas the transgenic sheath was able to prevent the colonization of the plant cells by the fungi significantly (Fig. 6cii, iii).
Microchamber bioassay of young rice plants using Rhizoctonia solani. a Disease severity was measured as the disease index (DI) of transgenic and WT plants at 10 DPI. The values are represented as the mean ± SE (n = 6). b Representative images of infected sheath of (i) WT, (ii) OSN-10 and (iii) OSN-4 (lesion indicated by red arrows). c Lactophenol cotton blue-stained infected sheath of both WT and transgenic plants as observed under the microscope. (i) WT infected sheath showing blue-stained fungi inside the plant cells. (ii) Transgenic infected sheath showing mostly unaffected cells and a blue hyphae originating from an infection cushion. It is surrounding the plant cell but unable to penetrate it [indicated by cyan arrow]
The detached leaf inoculation test carried out using the mycelial agar disc demonstrated that the leaves from transgenic plants showed a significant drop in disease symptoms in contrast to that of WT plant. The number of infection cushions in transgenic plant 4–21 was significantly low (6.0) as compared to its WT counterpart (59.33) after 72 h (p < 0.0001) (Fig. 7a). The WT leaves displayed large areas of necrotic spots and surface browning, whereas the transgenic leaves showed minimal symptoms and were mostly green with small patches of necrosis at 72 h (Fig. 7b). The detached leaves post-staining confirmed that WT leaf (Fig. 7ci) was crowded by large number of infection cushions, whereas the transgenic leaf had a sparse population of infection cushions and mycelia (Fig. 7cii).
a Detached leaf bioassay showing reduced number of infection cushions being formed in the T1 transgenic plants than in the wild type (WT). The values are represented as the mean ± SE (n = 6). Experiments were replicated three times. b Representative images of reduced symptom formation in transgenic leaves as compared to WT leaf in the mycelial agar disc bioassay. c Lactophenol cotton blue-stained leaves of both WT and transgenic plants observed under the microscope. WT leaves are severely infected by large number of infection cushions (i), while transgenic leaves showed sparse hyphal network with few infection cushions (ii) (infection cushion is indicated by white arrows)
The whole plant bioassay using Rhizoctonia solani showed substantial restriction of the vertical advancement of the fungus in transgenic plants over the course of 7, 14 and 21 DPI as compared to WT plants. At 21 DPI, a significant decrease (16.98–20.59%) in the disease index was observed in the transgenic plants, whereas it was much higher in case of WT plants (59.82%) (p < 0.0001) (Fig. 8a, b).
Whole plant bioassay of transgenic and WT plants using Rhizoctonia solani. a Disease severity was evaluated as percentage disease index (PDI) in T1 transgenic and WT plants at 7, 14 and 21 DPI. The values are represented as the mean ± SE (n = 7). b Representative images showing disease symptoms in the rice tillers of WT and T1 transgenic plants. Pictures were taken at 30 DPI
Expression of snakin-1 does not affect agronomic traits
There were no significant differences observed between the WT and the transgenic plants (Fig. 9a). There were no observable phenotypic or morphological differences in the germinated seedlings of both WT and transgenic plants (Fig. 9b).
a Agronomical evaluation of WT and transgenic plant lines: The parameters were measured for both WT and transgenic plants grown in similar conditions in the green house. Values are presented as mean ± standard error (SE) (n = 10). b (i) Germination of wild-type (WT) and transgenic (T) seeds. (ii) Morphology of WT and transgenic snakin-1 rice seedlings
Discussion
Snakin-1 has been found to inhibit a variety of phytopathogens in vitro (Berrocal-Lobo et al. 2002; Segura et al. 1999). But the mechanism of its antifungal action has not been deciphered yet. Therefore, we explored its antifungal mode of action against the sheath blight fungus, Rhizoctonia solani in vitro, using the crude protein extract. We have used different types of staining methods to highlight different aspects of its antifungal activity.
Lactophenol cotton blue helps in studying the fungal morphology, as it selectively stains the fungal chitin and cytoplasm. The light or lack of staining of the transgenic-treated hyphae, suggests that the hyphal damage was due to both the loss of cellular contents and the chemical alterations of the fungal cytoplasmic components (Ikediugwu and Webster 1970). Granular cytoplasm was reported earlier due to increased hyphal membrane permeability caused by the cell-penetrative action of the AMP NaD1 (van Der Weerden et al. 2008). Increased vacuolation caused by stress-induced autophagy leads to hyphal compartments having large vacuoles, which arrests the cell cycle (du Pré et al. 2020). Defensins PsD1 and ApDef1 were previously reported to inhibit the fungal cell cycle progression (Lobo et al. 2007; Soares et al. 2017). Inhibition of phospholipid methyltransferases causes a lack of phosphatidylcholine (PC) in the fungal membrane leading to hyphal morphological abnormalities such as swollen tips (Tao et al. 2010; Akhberdi et al. 2018), which make membranes hypersensitive to stress conditions and eventually cause rupture of the tips.
Cell viability was studied using the dual AO-EB staining technique. Acridine orange is a cell-permeable vital dye which stains both live and dead cells, while ethidium bromide is non-permanent and exclusively stains dead cells. Live, apoptotic and necrotic cells display different fluorescence characteristics (Kasibhatla et al. 2006). The WT crude protein-treated fungal mycelia displayed mostly uniform green fluorescence (live) interspersed with some uniformly orange fluorescing hyphae (necrotic) (Fig. 5a, b). In contrast, the crude protein isolated from the transgenic plant-treated mycelia has a mixed population of cells displaying different stages of cell death. Hyphae were found in the early apoptotic stage (bright green dots) (Fig. 5c), some in the late apoptotic stage (bright yellow dots) and most of them in necrosis (uniform orange fluorescence) along with very few green live hyphae (Fig. 5d). These observations suggest that snakin-1 possibly triggered apoptosis as well as necrosis in Rhizoctonia solani.
Nile Red stains lipid-rich cellular structures like membrane and lipid droplets which helps evaluate membrane damage and cell death. The WT crude protein-treated mycelia had uniformly bright fluorescence suggesting healthy hyphae with intact membranes (Fig. 5e). The transgenic crude protein-treated mycelia mostly showed reduced fluorescence, indicating dead and damaged cells (Fig. 5f). The accumulation of lipid droplets (bright yellow dots) (Fig. 5f), which is a marker of apoptosis (Boren and Brindle 2012) was also observed. The reduced fluorescence indicates a marked reduction in the cell membrane hydrophobicity (Thrane et al. 1999), suggesting that snakin-1 probably altered the fungal membrane lipid composition by targeting its lipid biosynthesis pathways via unknown molecular mechanisms. This reduction in cell surface hydrophobicity (CSH) affects its adhesion capabilities, which is an important factor for the successful host infection capability of Rhizoctonia solani (Keijer 1996).
Living metabolically active cells can reduce the tetrazolium dye MTT to purple insoluble formazan crystals, via mitochondrial dehydrogenases. Transgenic-treated mycelia showed a greater live cell population than the WT-treated hyphae, suggesting that snakin-1 can affect fungal mitochondrial respiration, like other antifungal peptides (Aerts et al. 2011).
Infection-induced stress conditions cause a buildup of reactive oxygen species (ROS), and given the previously reported redox-modulating capabilities of snakin-1, we analyzed the rice antioxidant enzyme activity levels post-infection. Transgenic rice plants showed significantly reduced antioxidant enzyme activity post-infection than their WT counterparts. Snakin-1 reportedly regulates important non-enzymatic ROS scavengers like ascorbate, raffinose and galactinol (Nahirñak et al. 2012). Therefore, we hypothesize that membrane-bound snakin-1 in transgenic rice not only directly inhibits the incoming pathogen resulting in reduced pathogen-induced ROS generation, but also helps detoxify the ROS generated, by modulating the non-enzymatic ROS scavenging antioxidant levels. Therefore, the synergistic effect of the antimicrobial action and the redox modulation of snakin-1 significantly reduced ROS generation. This caused lower expression and resulted in reduced antioxidant enzymes activity levels in the transgenic rice plants. The reduced MDA levels in transgenic plants can also be explained by both the lowered ROS generation and reduced membrane penetration by the fungus.
Snakin-1 has successfully protected both potato and lettuce (Almasia et al. 2008; Darqui et al. 2018) against the necrotrophic fungus, Rhizoctonia solani. Therefore, we evaluated its in planta activity against the sheath blight pathogen in rice in three different ways.
Infection cushions are fungal structures which breach the plant’s primary defense barriers and help colonize the underlying plant tissue. Microscopic observations of the detached leaves after staining showed that the transgenic leaf had sparse hyphal filaments with significantly lesser number of infection cushions than its WT counterpart. Since the snakin-1 is membrane localized (Nahirñak et al. 2012), it directly encounters the entry of the fungus and inhibits the fungal growth before penetration, thus preventing its entry into plant cell. This explains the significantly fewer infection cushions in the transgenic plant.
The microchamber assay not only helped study the effect of the fungus on young rice sheaths, but also the protective effect of snakin-1 against Rhizoctonia solani in young rice plants. Rhizoctonia solani being a necrotrophic fungus also attacks young rice plants (Dath 1990). WT plants were rapidly colonized by the fungus (Fig. 6ci), whereas the transgenic plants had significant protection. This is evident from the microscopic evaluation of the stained infected sheaths, where the fungus was unable to penetrate the cell wall and enter the cell in the transgenic sheath. Instead, the fungal hypha is restricted to the apoplastic space (Fig. 6cii). Snakin-1 being membrane bound, had successfully prevented the fungus from rapidly penetrating and invading the young transgenic rice sheath.
The vertical disease progression was rapidly halted from the beginning of the bioassay till the end in the mature transgenic rice plants. The sheath blight disease symptoms were notably reduced as evident from the lower PDI value (on average, 65.6–71.6% resistance) at 21 DPI. The WT sheath underwent browning and chlorosis, whereas the transgenic sheath was healthy with few lesions and minimal browning. Snakin-1 thus effectively protected mature transgenic rice against this deadly fungus.
The antifungal mode of action of snakin-1 has not been deciphered yet. It has been proposed that snakin-1 can probably form pores in the pathogen membrane via self-aggregation, thereby causing membrane permeability. The presence of a helix-turn-helix (HTH) motif, a common structure found in all known gene-regulating DNA-binding proteins, together with its cationic nature, possibly allows snakin-1 to affect their vital gene expression by binding to the pathogen’s DNA/RNA (Almasia et al. 2020). Based on this context and our experimental observations, we have proposed a hypothetical model of the antifungal mode of action of snakin-1 (Fig. 10). Through the course of this work, we have observed that snakin-1 affects the fungus by triggering apoptosis via multiple pathways (Fig. 10g–j). It also probably alters the cell membrane permeability and its cell surface hydrophobicity, therefore affecting its adhesion capabilities (Fig. 10e, f, k). The combination of all these factors accounts for its antifungal action against Rhizoctonia solani. Future work can focus on the detailed aspects of the different pathways proposed here and further help in deciphering the exact mode of antifungal action of snakin-1. Therefore, we can summarize that snakin-1 was indeed effective against the rice sheath blight fungus both in vitro and in planta without affecting the normal growth and development of the transgenic rice. The strategy followed in this work can be extrapolated to other staple crops affected by this cosmopolitan and economically devastating phytopathogen.
Diagrammatic representation of the hypothetical model of the antifungal mode of action of snakin-1 against Rhizoctonia solani. a The transgenic rice plant expressing snakin-1 on being infected by Rhizoctonia solani shows a magnified view of an b infection cushion attacking the plant cellular structure by invading the apoplastic space via means of the mycelial network. A magnified view of the interaction between the c plant cell expressing the snakin-1 peptide (red structures) and the d fungal cell has been presented to propose the antifungal mode of action of snakin-1 against Rhizoctonia solani. The membrane-bound Snakin-1 being a redox regulator, modulates the levels of important non-enzymatic ROS scavenging antioxidant molecules such as ascorbate (Asc), galactinol (Gal) and raffinose (Raf). These help in neutralizing the ROS generated as a result of infection, thereby reducing its severity. Snakin-1 affects the fungal cell via its possible gene-regulating capability, in a wide variety of ways: e the snakin-1 affects the fungal membrane phosphatidylcholine biosynthesis, which leads to membrane deformation, ultimately creating hypha with swollen and/or ruptured tips. f Snakin-1 also affects the cell surface hydrophobicity which reduces the adhesion capabilities of the fungus, which is vital for a successful infection, thereby leading to reduced infection of the rice plant. g Vacuolation observed in the hyphal cell due to the action of snakin-1 occurs via unknown pathways, suggesting that the formation of enlarged vacuoles may be due to its possible fusion with stress-induced autophagosomes. This leads to arrest of the cell cycle, which in turn causes cellular death. h The snakin-1 peptide also inhibits mitochondrial respiration which consequently can trigger fungal cell death. i The formation and accumulation of lipid bodies triggered by snakin-1 in the fungal cell is a known marker for apoptosis. j Snakin-1 via unidentified pathways also triggers chromatin condensation and nuclear fragmentation, which precedes cell death. k Increased hyphal membrane permeability occurs as a result of the possible self-aggregation induced pore forming action of snakin-1, causing loss of cellular contents, which ultimately leads to fungal cell death
References
Aerts AM, Bammens L, Govaert G, Carmona-Gutierrez D, Madeo F, Cammue B, Thevissen K (2011) The antifungal plant defensin HsAFP1 from Heucher asanguinea induces apoptosis in Candida albicans. Front Microbiol 2:47
Akhberdi O, Zhang Q, Wang H, Li Y, Chen L, Wang D, Yu X, Wei D, Zhu X (2018) Roles of phospholipid methyltransferases in pycnidia development, stress tolerance and secondary metabolism in the taxol-producing fungus Pestalotiopsis microspore. Microbiol Res 210:33–42
Almasia NI, Bazzini AA, Hopp HE, Vazquez-Rovere CE (2008) Overexpression of snakin-1 gene enhances resistance to Rhizoctonia solani and Erwinia carotovora in transgenic potato plants. Mol Plant Pathol 9:329–338
Almasia NI, Nahirñak V, Hopp HE, Vazquez-Rovere CE (2020) Potato Snakin-1: an antimicrobial player of the trade-off between host defense and development. Plant Cell Rep 11:1–11
Barbosa Pelegrini P, del Sarto RP, Silva ON, Franco OL, Grossi-de-Sa MF (2011) Antibacterial peptides from plants: what they are and how they probably work. Biochem Res Int 5:250349
Berrocal-Lobo M, Segura A, Moreno M, López G, Garcıa-Olmedo F, Molina A (2002) Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. J Plant Physiol 128:951–961
Boren J, Brindle KM (2012) Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation. Cell Death Differ 19:1561–1570
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen T, Zhang B (2016) Measurements of proline and malondialdehyde content and antioxidant enzyme activities in leaves of drought stressed cotton. Bio Protoc 6:e1913
Darqui FS, Radonic LM, Trotz PM, López N, Rovere CV, Hopp HE, Bilbao ML (2018) Potato snakin-1 gene enhances tolerance to Rhizoctonia solani and Sclerotinia sclerotiorum in transgenic lettuce plants. J Biotechnol 283:62–69
Das P, Lakra N, Nutan KK, Singla-Pareek SL, Pareek A (2015) Pot level drought stress tolerance assay in tobacco through plant phenotyping and antioxidant assay. Bio Protoc 5:e1605
Das K, Datta K, Karmakar S, Datta SK (2019) Antimicrobial peptides—small but mighty weapons for plants to fight phytopathogens. Protein Pept Lett 26:720–742
Dath AP (1990) Sheath blight disease of rice and its management. Associated Pub. Co., New Delhi
Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK (1999) Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet 98:1138–1145
Datta K, Koukolikova-Nicola Z, Baisakh N, Oliva N, Datta SK (2000) Agrobacterium-mediated engineering for sheath blight resistance of indica rice cultivars from different ecosystems. Theor Appl Genet 100:832–839
Datta K, Tu J, Oliva N, Ona I, Velazhahan R, Mew TW, Muthukrishnan S, Datta SK (2001) Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci J 160:405–414
du Pré S, Birch M, Law D, Beckmann N, Sibley GE, Bromley MJ, Read ND, Oliver JD (2020) The dynamic influence of olorofim (F901318) on the cell morphology and organization of living cells of Aspergillus fumigatus. J Fungi 6:47
Faccio P, Vazquez-Rovere C, Hopp E, Gonzalez G, Decima-Oneto C, Favret E, Paleo AD, Franzone P (2011) Increased tolerance to wheat powdery mildew by heterologous constitutive expression of the Solanum chacoense snakin-1 gene. Czech J Genet Plant Breed 47:S135–S141
García AN, Ayub ND, Fox AR, Gómez MC, Diéguez MJ, Pagano EM, Berini CA, Muschietti JP, Soto G (2014) Alfalfa snakin-1 prevents fungal colonization and probably coevolved with rhizobia. BMC Plant Biol 14:248
Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS (1997) Pyramiding of bacterial blight resistance genes in rice: marker-assisted selection using RFLP and PCR. Theor Appl Genet 95:313–320
Ikediugwu FE, Webster J (1970) Antagonism between Coprinus heptemerus and other coprophilous fungi. Trans Br Mycol Soc 54:181–184
Jha S, Chattoo BB (2010) Expression of a plant defensin in rice confers resistance to fungal phytopathogens. Transgenic Res 19:373–384
Jha S, Tank HG, Prasad BD, Chattoo BB (2009) Expression of Dm-AMP1 in rice confers resistance to Magnaporthe oryzae and Rhizoctonia solani. Transgenic Res 18:59–69
Jia Y, Correa-Victoria F, Mcclung A, Zhu L, Liu G, Wamishe Y, Xie J, Marchetti MA, Pinson SR, Rutger JN, Correll JC (2007) Rapid determination of rice cultivar responses to the sheath blight pathogen Rhizoctonia solani using a micro-chamber screening method. Plant Dis 91:485–489
Kalpana K, Maruthasalam S, Rajesh T, Poovannan K, Kumar KK, Kokiladevi E, Raja JA, Sudhakar D, Velazhahan R, Samiyappan R, Balasubramanian P (2006) Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Sci J 170:203–215
Karmakar S, Molla KA, Chanda PK, Sarkar SN, Datta SK, Datta K (2016) Green tissue-specific co-expression of chitinase and oxalate oxidase 4 genes in rice for enhanced resistance against sheath blight. Planta 243:115–130
Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR (2006) Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb Protoc 21:pdb.prot4493
Keijer J (1996) The initial steps of the infection process in Rhizoctonia solani. In: Sneh B, Jabaji-Hare S, Neate S, Dijst G (eds) Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Springer, Dordrecht, Netherlands, pp 149–162
Krishnamurthy K, Balconi C, Sherwood JE, Giroux MJ (2001) Wheat puroindolines enhance fungal disease resistance in transgenic rice. Mol Plant Microbe Interact 14:1255–1260
Kumar KK, Poovannan K, Nandakumar R, Thamilarasi K, Geetha C, Jayashree N (2003) A high throughput functional expression assay system for a defense gene conferring transgenic resistance on rice against the sheath blight pathogen, Rhizoctonia solani. Plant Sci 165:969–976
Lee S-C, Han S-K, Kim SR (2015) Salt-and ABA-inducible OsGASR1 is involved in salt tolerance. J Plant Biol 58:96–101
Lin W, Anuratha C, Datta K, Potrykus I, Muthukrishnan S, Datta SK (1995) Genetic engineering of rice for resistance to sheath blight. Nat Biotechnol 13:686–691
Lin J, Zhou B, Yang Y, Mei J, Zhao X, Guo X, Huang X, Tang D, Liu X (2009) Piercing and vacuum infiltration of the mature embryo: a simplified method for Agrobacterium-mediated transformation of indica rice. Plant Cell Rep 28:1065–1074
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lobo DS, Pereira IB, Fragel-Madeira L, Medeiros LN, Cabral LM, Faria J, Bellio M, Campos RC, Linden R, Kurtenbach E (2007) Antifungal Pisum sativum defensin 1 interacts with Neurospora crassa cyclin F related to the cell cycle. Biochemistry 46(4):987–996
Molla KA, Karmakar S, Chanda PK, Ghosh S, Sarkar SN, Datta SK, Datta K (2013) Rice oxalate oxidase gene driven by green tissue-specific promoter increases tolerance to sheath blight pathogen (Rhizoctonia solani) in transgenic rice. Mol Plant Pathol 14:910–922
Molla KA, Karmakar S, Molla J, Bajaj P, Varshney RK, Datta SK, Datta K (2020) Understanding sheath blight resistance in rice: the road behind and the road ahead. Plant Biotechnol J 18:895–915
Nahirñak V, Almasia NI, Fernandez PV, Hopp HE, Estevez JM, Carrari F, Vazquez-Rovere C (2012) Potato snakin-1 gene silencing affects cell division, primary metabolism, and cell wall composition. J Plant Physiol 158:252–263
Nahirñak V, Rivarola M, Almasia NI, Barrios Baron MP, Hopp HE, Vile D, Paniego N, Vazquez Rovere C (2019) Snakin-1 affects reactive oxygen species and ascorbic acid levels and hormone balance in potato. PLoS ONE 14:e0214165
Paranidharan V, Palaniswami A, Vidhyasekaran P, Velazhahan R (2003) Induction of enzymatic scavengers of active oxygen species in rice in response to infection by Rhizoctonia solani. Acta Physiol Plant 25:91–96
Patkar RN, Chattoo BB (2006) Transgenic indica rice expressing ns-LTP-like protein shows enhanced resistance to both fungal and bacterial pathogens. Mol Breed 17:159–171
Pin S, Liao XL, Zhang Y, Huang H (2012) Influencing factors on rice sheath blight epidemics in integrated rice-duck system. J Integr Agric 11:1462–1473
Ribble D, Goldstein NB, Norris DA, Shellman YG (2005) A simple technique for quantifying apoptosis in 96-well plates. Methods Cell Biol 112:361–368
Rong W, Qi L, Wang J, Du L, Xu H, Wang A, Zhang Z (2013) Expression of a potato antimicrobial peptide SN1 increases resistance to take-all pathogen Gaeumannomyces graminis var. tritici in transgenic wheat. Funct Integr Genom 13:403–409
Roxrud I, Lid SE, Fletcher JC, Schmidt ED, Opsahl-Sorteberg HG (2007) GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol 48:471–483
Rubinovich L, Weiss D (2010) The Arabidopsis cysteine-rich protein GASA4 promotes GA responses and exhibits redox activity in bacteria and in planta. Plant J 64:1018–1027
Rubinovich L, Ruthstein S, Weiss D (2014) The Arabidopsis cysteine-rich GASA5 is a redox-active metalloprotein that suppresses gibberellin responses. Mol Plant 7:244–247
Segura A, Moreno M, Madueño F, Molina A, García-Olmedo F (1999) Snakin-1, a peptide from potato that is active against plant pathogens. Mol Plant Microbe Interact 12:16–23
Standard Evaluation System for Rice (2002) International Rice Research Institute, Philippines, pp 1–45
Singh R, Sunder S, Kumar P (2016) Sheath blight of rice: current status and perspectives. Indian Phytopathol 69:340–351
Soares JR, de Melo EJ, da Cunha M, Fernandes KV, Taveira GB, da Silva PL, Pimenta S, Trindade FG, Regente M, Pinedo M, de la Canal L (2017) Interaction between the plant ApDef1 defensin and Saccharomyces cerevisiae results in yeast death through a cell cycle-and caspase-dependent process occurring via uncontrolled oxidative stress. BBA Gen Subj 1861:3429–3443
Sridevi G, Parameswari C, Sabapathi N, Raghupathy V, Veluthambi K (2008) Combined expression of chitinase and β-1,3-glucanase genes in indica rice (Oryza sativa L.) enhances resistance against Rhizoctonia solani. Plant Sci J 175:283–290
Stein N, Herren G, Keller B (2001) A new DNA extraction method for high-throughput marker analysis in a large-genome species such as Triticum aestivum. Plant Breed 120:354–356
Swain DM, Yadav SK, Tyagi I, Kumar R, Kumar R, Ghosh S, Das J, Jha G (2017) A prophage tail-like protein is deployed by Burkholderia bacteria to feed on fungi. Nat Commun 8:1–9
Tao L, Gao N, Chen S, Yu JH (2010) The choC gene encoding a putative phospholipid methyltransferase is essential for growth and development in Aspergillus nidulans. Curr Genet 56:283–296
Thrane C, Olsson S, Harder Nielsen T, Sørensen J (1999) Vital fluorescent stains for detection of stress in Pythium ultimum and Rhizoctonia solani challenged with viscosinamide from Pseudomonas fluorescens DR54. FEMS Microbiol Ecol 30:11–23
van Der Weerden NL, Lay FT, Anderson MA (2008) The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J Biol Chem 283:14445–14452
Wigoda N, Ben-Nissan G, Granot D, Schwartz A, Weiss D (2006) The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. Plant J 48:796–805
Yuan S, Heath IB (1991) A comparison of fluorescent membrane probes in hyphal tips of Saprolegnia ferax. Exp Mycol 15:103–115
Acknowledgements
The work was funded by Department of Biotechnology (DBT), Govt. of India, in the form of DBT Programme Support [Sanction no. BT/PR12656/COE/34/22/2015] and Indian Council of Agricultural Research (ICAR), Govt. of India [Sanction No. CS11/7/2014-1A-IV dated 26.11.2014]. The fellowship [Award Letter: DBT/JRF/14/AL/162/3215 dated 27.6.2014] from the Department of Biotechnology (DBT), Govt. of India, to Kaushik Das is highly acknowledged. We also heartily thank Mrs. Sayani Majumdar for laboratory assistance, Mr. Pratap Ghosh and Mr. Sujoy Mondal for greenhouse work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, K., Datta, K., Sarkar, S.N. et al. Expression of antimicrobial peptide snakin-1 confers effective protection in rice against sheath blight pathogen, Rhizoctonia solani. Plant Biotechnol Rep 15, 39–54 (2021). https://doi.org/10.1007/s11816-020-00652-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-020-00652-3