Abstract
Artemisia L. is interesting in cytogenetic research due to having a variety of chromosome numbers and ploidy levels. In this research, the karyomorphological characteristics of nine accessions of French and Russian tarragon (A. dracunculus L.) collected from different locations in Iran were analyzed for the first time. The basic chromosome number was x = 9, with two ploidy levels of 4x (French accessions) and 10x (Russian accessions). The mean of chromosome length and the total haploid chromosome length of the French accessions ranged from 2.32 to 3.39 μm and 40.26 to 61.13 μm, respectively, while these values were 3.99 to 4.22 μm and 179.72 to 190.13 μm, respectively, for the Russian accessions. Chromosome types of French tarragons were determined as metacentric (dominant) and submetacentric, whereas they were metacentric (dominant), submetacentric, and subtelocentric in Russian tarragons. French accessions were classified as 4A, 3B, and 4B according to the Stebbins classification, while all the Russian accessions have a 3B type karyotype. Russian accessions present the most asymmetrical karyotype based on biplot analysis of asymmetry indices. Cluster analysis according to all karyotypic parameters revealed that the French and Russian accessions were placed in two separate groups. Principal components analysis showed that the first two components possessed 95.8% of the total variation. The PCA score plot generated from the first two principal components not only supported the clustering results but also distinguished the French accessions of Arak, Isfahan, and Tehran, which have more symmetrical karyotype, from the remaining French accessions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Artemisia L. is a large and diverse genus of the family Asteraceae. Although the center of diversification of Artemisia is Central Asia, its species grow mainly in temperate areas of the northern hemisphere including North West America, Irano-Turanian, and Mediterranean regions (Zeb et al. 2018). The taxonomy of Artemisia is difficult and complex, and contains about 350–500 species (Shultz 2006). Around 43 species of Artemisia have been identified in Iran (Mozaffarian 2005).
Some species of the genus Artemisia are economically important (Zeb et al. 2018). Artemisia dracunculus L. or tarragon (belongs to subgenus Dracunculus) is a perennial herb known as a medicinal and spice species (Zeb et al. 2018; Ekiert et al. 2021). The essential oil of its aerial part and leaves contains secondary metabolites such as monoterpenoids, flavonoids, phenolic acids, coumarins, and alkamides (Ekiert et al. 2021; Kara and Çağlak 2022). This species has two main varieties: French (or German) tarragon and Russian tarragon (designated as A. dracunculoides L. in some literature) (Zeb et al. 2018). French tarragon is sterile, propagates vegetatively by rhizome cuttings, and is preferred in cooking use for its stronger aroma, while Russian tarragon is fertile, but rarely is used in culinary (Engels and Brinckmann 2014; Ekiert et al. 2021). They are also characterized by a wide range of morphological, anatomical, phytochemical and cytogenetical variabilities (Obolskiy et al. 2011).
Knowledge of chromosome numbers, ploidy levels and karyotypic characteristics is very important in understanding the species’ evolution and their relationships (Abdali and Miri 2020; Ebrahimi et al. 2021), as well as solving classification problems of some closely related taxa (Oroji Salmasi et al. 2019; Rajabi Mazaher et al. 2021). The genus Artemisia has two basic chromosome numbers, x = 8 (less frequent) and x = 9 (most common) (Matoba et al. 2007). Polyploidy has been identified as a common phenomenon in Artemisia species, ranging from 2 × to 6 × for x = 8 and from 2 × to 12 × for x = 9 (Matoba et al. 2007; Hayat et al. 2009). The ploidy level and number of chromosomes of French tarragon are reported as 2n = 4x = 36 and Russian tarragon as 2n = 10x = 90 (Rousi 1969). Although many karyological studies have been carried out on Artemisia species (Ghasemi et al. 2005; Saedi et al. 2005; Matoba et al. 2007; Pellicer et al. 2007, 2008; Naseri et al. 2009; Zhen et al. 2010; Tabur et al. 2012; Dolatyari et al. 2013; Yazdani et al. 2014; Sancar et al. 2021), however to the best of our knowledge, the karyotypic analysis of A. dracunculus remain unknown. Therefore, this study was conducted for the first time to determine the karyotype characteristics of French and Russian tarragon.
Materials and methods
Plant materials
Nine accessions of A. dracunculus including six French tarragons, and three Russian tarragons were investigated. Transplants of French tarragon accessions were collected from natural populations, and deposited at the herbarium of the University of Tehran (UTFH). The seeds of Russian tarragons were collected from the Iranian Biological Resource Center (IBRC) (Table 1).
Chromosome counts
Root tips were immersed in α-bromonaphthalene 1% for 5 h, and fixed in Carnoy’s fixative (3:1 alcohol: glacial acetic acid, v/v) overnight at 4 °C. The root tips were hydrolyzed in 1 N HCl for 25 min at 60 °C, stained by aceto-orcein for 24 h, and then squashed onto slides in acetic acid 45% for observation. At least five metaphase plates per individual and five plants per accession were examined and their average data were used for karyotype analysis. Photos were taken under a Canon digital camera (Powershot SX50 HS) mounted on a CX52 Olympus microscope.
Karyotype analysis
For the numerical characterization of the karyotypes, short arm length (SA) and long arm length (LA) were measured using MicroMeasure 3.3 software. The following parameters were calculated to identify the chromosomal parameters: mean chromosome length (CL = LA + SA), total chromosome length of the haploid complement (HCL = ∑CL), centromeric index (CI = SA/CL), arm ratio (AR = LA/SA), r-value (SA/LA), relative length of chromosome (RL% = (CL/∑CL) × 100) and chromosome type (Levan et al. 1964). Asymmetry indices were calculated using: chromosome form percentage (F% = (SA/∑CL) × 100), total form percentage (TF%; Huziwara 1962), percentage karyotype asymmetry index (AsK%; Arano 1963), intrachromosomal asymmetry index (A1), interchromosomal asymmetry index (A2) (Romero-Zarco 1986), percentage of karyotype symmetry (S% = (CLmin/CLmax) × 100), degree of karyotype asymmetry (A; Watanabe et al. 1999), mean centromeric index (XCI = ∑CL/n), mean centromeric asymmetry (XCA; Peruzzi and Eroğlu 2013), coefficient of variation of chromosome length (CVCL), coefficient of variation of centromeric index (CVCI), asymmetry index (AI) (Paszko 2006), Stebbins’ class asymmetry index (Stebbins 1971), the difference between minimum and maximum relative length of chromosomes (DRL = RL%max–RL%min), and centromeric gradient (CG; Lavania and Srivastava 1992).
Idiograms were drawn using Excel based on chromosome length. The cluster analysis was carried out by the nearest neighbor method using Minitab ver. 16 software. Pearson correlation among some karyotype characters between karyotypic parameters with geographical coordinates was computed by SPSS ver. 23 software. Principal components analysis (PCA) was performed to evaluate the contribution of all karyological variables to the diversity of accessions and the first two principal component scores were plotted to identify the grouping pattern among the accessions using Minitab ver. 16 software.
Results
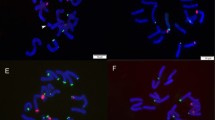
Our results showed that French tarragon accessions were tetraploid with 2n = 4x = 36, while Russian tarragons were decaploid with 2n = 10x = 90 (Table 2). Karyotype characters of the French and Russian tarragons were reported for the first time (Table 2). Their mitotic metaphase chromosomes and haploid ideograms are shown in Fig. 1. All investigated tarragon accessions had small chromosomes (≈ 2 μm or less; Stace 2000). The mean size of the chromosomes in Russian tarragon accessions was higher than that of French. The highest LA (2.4 µm), SA (1.8 µm), CL (4.2 µm), and HCL (190.1 µm) were obtained for RUS-Arak, and the lowest of these values were obtained for FR-Shiraz (1.3 µm, 0.9 µm, 2.2 µm an, 40.2 µm, respectively). The length range of chromosomes was 1.10 to 2.89 µm and 2.10 to 6.33 µm in FR-Shiraz and RUS-Arak, respectively. The highest CI and r-value were observed in FR-Isfahan, while the lowest values were found in RUS-Karaj and RUS-Tehran. In addition, FR-Isfahan (1.2) and RUS-Tehran (1.5) had the lowest and highest values of AR, respectively. Pearson's correlation coefficients showed a significant positive correlation between both parameters of ploidy level and chromosome number with CL (r = 0.84, P < 0.01) and HCL (r = 0.99, P < 0.01).
The presence of satellites was clearly observed in some accessions such as FR-Mashhad and RUS-Karaj, but in other accessions, due to the low resolution of the images of metaphase plates, they could not be reliably detected, so they were not used for chromosome identification and analysis.
According to the nomenclature of chromosomes by Levan et al. (1964), three chromosome types were found in French tarragons (Table 2): metacentric (M and m) in FR-Arak and FR-Tehran, metacentric (M and m) and submetacentric (sm) in FR-Isfahan and FR-Sari, as well as metacentric (m) and submetacentric (sm) in FR-Mashhad and FR-Shiraz. On the other hand, four chromosome types of metacentric (M and m), submetacentric (sm), and subtelocentric (st) were found in all Russian tarragons. Overall, metacentric (m) chromosome pairs were dominant in all tarragon accessions.
The karyotype asymmetry was evaluated according to Stebbins’ classification (Stebbins 1971) and 13 quantitative indices (Table 3). Based on the Stebbins’ class asymmetry index, French tarragon accessions of Isfahan and Mashhad were classified as category 4A, Sari as category 3B, and the other three French accessions as category 4B. All Russian tarragon accessions were located in class 3B. They also presented more asymmetrical karyotypes, as shown in the biplot diagram (Fig. 2), which is indicated by lower values of F%, TF%, S%, A1, DRL, and CG, and higher values of AsK%, XCI, XCA, CVCL, CVCI, AI and A2 (Table 3). FR-Isfahan was the most symmetrical karyotype based on values of F%, TF%, AsK%, XCA, XCA, AI, A1 and A2, while RUS-Tehran was the most asymmetrical karyotype according to F%, TF%, AsK%, S%, XCA, CVCL, CVCI, AI, A1, A2 and CG parameters.
In French accessions, a positive correlation was observed between latitude with chromosome size (LA, SA, CL and HCL) and XCI (Table 4). In addition, a negative correlation was found between longitude with CI and TF%, whereas XCA showed a positive relationship. A negative correlation was achieved between altitude with AR, CVCI and AI. In Russian accessions, latitude and longitude were negatively correlated with S%, while altitude had a positive correlation. In the case of DRL, the opposite of this correlation was found. Longitude and altitude showed a significant positive and negative correlation with A1, respectively.
The results of cluster analysis showed a clear distinction between the studied tarragons, and French and Russian accessions were classified into two major groups (Fig. 3). PCA of all karyological parameters revealed that the first three principal components (with eigenvalues ≥ 1) accounted for 95.8% of the total variance. The first component (64.5%) emphasized the chromosomal parameters, while the second component (22.0%) and especially the third component (9.3%) accentuated the asymmetry indices (Table 5). Based on the results of score plot analysis, the French and Russian accessions were also separated like the cluster analysis, however, accessions FR-Mashhad, FR-Sari and FR-Shiraz (having almost shorter chromosome length and a more symmetrical karyotype) were distinguished from the remaining French accessions (Fig. 4).
Score plot of the nine A. dracunculus accessions for the first two principal components. Symbols as in Table 1
Discussion
Chromosome number
The obtained results indicated that all of the studied tarragon accessions have x = 9, which was confirmed by Rousi (1969), Kreitschitz and Vallès (2003), Eisenman and Struwe (2011) and Pellicer et al. (2013) in Artemisia dracunculus and other species such as A. annua L. (Kreitschitz and Vallès 2003), A. campestris L. (Kreitschitz and Vallès 2003; Tabur et al. 2011), A. abrotanum L., A. absinthium L. (Kreitschitz and Vallès 2003; Tabur et al. 2012), A. armeniaca Lam., A. chamaemelifolia Vill., A. tournefortiana Rchb. and A. arborescens L. (Tabur et al. 2012). In addition, Pellicer et al. (2007) found that the basic chromosome number of A. dracunculus and 18 other studied species is x = 9. However, x = 8 has been reported in some species such as A. scoparia Waldst.et Kit (Tabur et al. 2011) and A. vulgaris L., A. austriaca Jacq., A. incana (L.) Druce, A. splendens Willd., A. caucasica Willd. and A. haussknechtii Boiss. (Tabur et al. 2012). The most common and primitive basic chromosome number of Artemisia is x = 9, while x = 8 is advanced (Zhen et al. 2010).
Genome size is generally correlated with chromosome length and ploidy level (Torrell and Vallès 2001). Our results showed that the mean and total chromosome length of decaploid Russian tarragons were higher than tetraploid French accessions, so with a 2.5-fold increase in ploidy level, the mean chromosome length increased 1.4-fold. Garcia et al. (2007) and Naseri et al. (2009) noted that the total karyotype length in some North American and Iranian Artemisia L. significantly correlated with genome size and DNA content. Mas de Xaxars et al. (2016) also observed that genome size in Alpine Artemisia L. was positively and significantly correlated with ploidy level. Pellicer et al. (2010) found that the increase in genome size in Artemisia polyploids followed a non-linear relationship with saturation behavior. However, some Artemisia species do not follow this pattern (Vallès et al. 2012). These contradictions may be due to the fact that variations in genome size are affected by several factors such as systematic and evolutionary implications, or ecological selection pressures (Torrell and Vallès 2001; Fallahi et al. 2020).
Polyploidy
Polyploidy has played an important role in the evolution, speciation, and biodiversity of higher plants (Afshar 2015; Miri 2020; Shamsolshoara et al. 2020), and is thought to contribute in ecological adaptation and consequently geographical expansion (Vallès et al. 2012; Roughani et al. 2021). It is very prevalent in some Artemisia species, such as A. dracunculus (Vallès et al. 2012). Artemisia dracunculus is a karyotaxonomically interesting species, as Eisenman and Struwe (2011) and Pellicer et al. (2013) have reported the presence of different ploidy levels, from di- to deca-ploidy depending on the origin of the populations. According to our results, Rousi 1969) detected French and Russian tarragon as tetra- and deca-ploid, respectively. Kreitschitz and Vallès (2003) found di- and tetra-ploidy in A. abrotanum and A. absinthium populations and suggested that the increase in polyploidy level is an adaptation mechanism to drought conditions. Similarly, Dolatyari et al. (2013) identified two ploidy levels, 2 × and 4x, in A. oliveriana J.Gay ex Besser accessions, and stated that the doubling of the ploidy level could indicate a speciation process. Russian tarragon is higher vigorous and tolerant (Bown 2001; Tucker and DeBaggio 2009), which could be explained as a result of the polyploidization phenomenon.
Karyotypic variation
Karyotypic variation occurs widely in the genus Artemisia, so the karyotype may vary in different populations or among different individuals (Zhen et al. 2010). Other than the reports on the A. dracunculus karyotypic formula by Wang (2000) and its karyological data by Pellicer et al. (2013), no data have been reported on the karyological characteristics of A. dracunculus so far, which may be due to the small size of chromosomes and high ploidy level. The present study is the first to report the karyomorphology of A. dracunculus. Although Tabur et al. (2012) stated that it is difficult to determine the systematic relationships using karyotypes due to the inter- and intra-specific similarity of Artemisia L. chromosome morphology, however, the results of the karyotypic formula allowed us to compare and differentiate the French and Russian tarragons. Among the accessions studied, the karyotype morphology of French accessions was more homogeneous, as we detected the presence of 16 to 18 metacentric (88.9–100%) and none to two submetacentric (0–11.1%) chromosome pairs. This is while there were 32 to 35 metacentric (71.1–77.7%), 9 to 12 submetacentric (20.0–26.6%) and 1 subtelocentric (2.2%) chromosome pairs in Russian accessions. Wang (2000) reported the karyotypic formula of A. dracunculus as 14m + 4sm. The existence of these karyological differences may be due to the different habitat and climatic conditions of the accessions (Tabur et al. 2012). The genus Artemisia has an almost symmetrical karyotype and most of the chromosomes are metacentric and submetacentric (Vallès et al. 2012). However, Dolatyari et al. (2013) reported that the chromosomes of 28 Artemisia species are mainly meta- or submetacentric except for three species, which have one or two pair(s) of subtelocentric chromosomes, which is similar to the results of Russian tarragon karyotype.
According to Stebbins (1971), higher karyotypic asymmetry can be considered as an evolutionarily derived state. Therefore, it seems that the Russian tarragon originated from the French tarragon. French tarragon accessions were placed into three classes based on Stebbins classification, which these changes in the centromere position and chromosome size may be attributed as an evolutionary trend (Ghorbani Sini and Arzani 2015). Oliva and Vallès (1994) found that the tetraploid karyotype of A. umbelliformis Lam. is more asymmetric than the diploid A. eriantha Ten, and concluded that the latter is at least one of the likely ancestors of the former. These statements agree with the hypothesis of Torrell et al. (2001) that A. campestris (2n = 2x) is the origin of A. campestris (2n = 4x), A. crithmifolia L. (2n = 6x) and A. monosperma Delile (2n = 4x) populations. This shows the importance of karyotype analysis to determine the evolutionary status of different accessions.
Although genome size has often been associated with environmental or ecological variables (Mas de Xaxars et al. 2016), the genus Artemisia has been less evaluated. Several chromosomal parameters and asymmetry indices in the studied tarragons showed significant correlations with geographic coordinates and altitude (as a set of environmental variables), e.g., French accessions collected from higher latitudes had a longer chromosome size or tarragons prepared from higher altitudes had a relatively more symmetric karyotype (based on 2 to 3 asymmetry indices). This relationship was less in Russian accessions, which may be due to the small number of accessions and the proximity of the sample collection site. Hamidi et al. (2018) did not identify any significant correlation between genome size and environmental conditions in 18 populations of A. khorassanica Podlech, and concluded that their 2C DNA amounts were independent of environmental conditions. However, the observed relationships indicate that adaptation to habitat could influence karyotypic characteristics, possibly because different populations encounter different environmental variables that may promote genetic diversity (Fallahi et al. 2020). Oyundelger et al. (2021) in a study on environmental effects on genetic diversity and structure of A. frigida Willd. using SSR markers reported that there are significant correlations between genetic structure and environmental conditions.
Grouping analysis
Cluster analysis and score plot based on karyotypic characteristics were able to separate clearly French and Russian tarragon accessions. The genus Artemisia is one of the most complex genera from a taxonomic classification viewpoint (Dolatyari et al. 2013). The taxonomic delimitation between French and Russian tarragons is ambiguous and they are classified as varieties, cultivars, subspecies, or even species (Obolskiy et al. 2011). These can be distinguished from some morphological characteristics such as height at maturity, branching habit, color and consistency of leaves, hairiness of mature leaves, stems and pedicels, length of the pedicel, the tip of the involucral bracts, the diameter of opening flower head and amount of pollen (Rousi 1969), however, there is a great similarity in the morphology of secretary structures of Russian and French tarragons (Obolskiy et al. 2011). Therefore, in addition to morphological, anatomical and phytochemical characteristics (Werker et al. 1994; Fraternale et al. 2015), karyotype analysis is also clearly able to distinguish between French and Russian tarragons.
Conclusion
In conclusion, we presented the first karyomorphology of French and Russian tarragons, in terms of chromosome formula and asymmetry indices, which could be useful in providing insights into the evolution and systematics of A. dracunculus. The findings of ploidy levels and karyotypic asymmetry indices indicated that Russian tarragons are more evolved than French tarragons. However, we believe that further research on molecular cytogenetics and phylogenetics analyses are necessary to identify the taxonomical relationships and evolution of A. dracunculus.
References
Abdali S, Miri SM (2020) Chromosome counts for six species of Allium (Amaryllidaceae) from Iran. Iran J Bot 26(2):179–187. https://doi.org/10.22092/IJB.2020.343177.1289
Afshar S (2015) Evaluation of cytogenetic variation in different populations of Tanacetum pinatum. Thesis, Islamic Azad University-Karaj Branch, T. partenifolium and T. polycephallum
Arano H (1963) Cytological studies in subfamily Carduoideae (Compositae) of Japan. IX. The karyotype analysis and phylogenic considerations on Pertya and Ainsliaea (2). Bot Mag 76(895):32–39. https://doi.org/10.15281/jplantres1887.76.32
Bown D (2001) Encyclopaedia of herbs and their uses. Dorling Kindersley, London
Dolatyari A, Vallès J, Naghavi MR, Shahzadeh Fazeli SA (2013) Karyological data of 47 accessions of 28 Artemisia (Asteraceae, Anthemideae) species from Iran, with first new reports for Iranian populations and first absolute counts in three species. Plant Syst Evol 299:1503–1518. https://doi.org/10.1007/s00606-013-0813-y
Ebrahimi F, Ghorbani Nohooji M, Miri SM (2021) First karyotype analysis of Nerium oleander populations. Rostaniha 22(2):186–193. https://doi.org/10.22092/BOTANY.2021.354571.1246
Eisenman SW, Struwe L (2011) The global distribution of wild tarragon (Artemisia dracunculus L.; Asteraceae) cytotypes with twenty-seven new records from North America. Genet Resour Crop Evol 58:1199–1212. https://doi.org/10.1007/s10722-010-9653-6
Ekiert H, Świątkowska J, Knut E, Klin P, Rzepiela A, Tomczyk M, Szopa A (2021) Artemisia dracunculus (tarragon): a review of its traditional uses, phytochemistry and pharmacology. Front Pharmacol 12:653993. https://doi.org/10.3389/fphar.2021.653993
Engels G, Brinckmann J (2014) Russian tarragon. HerbalGram 102:1–5
Fallahi M, Mohammadi A, Miri SM (2020) The natural variation in six populations of Calendula officinalis L.: a karyotype study. J Genet Resour 6(1):34–40. https://doi.org/10.22080/JGR.2020.2541
Fraternale D, Flamini G, Ricci D (2015) Essential oil composition and antigermination activity of Artemisia dracunculus (tarragon). Nat Prod Commun 10(8):1469–1472. https://doi.org/10.1177/1934578X1501000839
Garcia S, Garnatje T, Hidalgo O, McArthur ED, Siljak-Yakovlev S, Vallès J (2007) Extensive ribosomal DNA (18S–5.8S-26S and 5S) colocalization in the North American endemic sagebrushes (subgenus Tridentatae, Artemisia, Asteraceae) revealed by FISH. Plant Syst Evol 267:79–92. https://doi.org/10.1007/s00606-007-0558-6
Ghasemi F, Jalili A, Ghamari Zare A, Asri Y, Bakhshi Khaniky GR (2005) Karyotypic investigation of Artemisia spp. from Kashan, Iran region. Iran J Rangel Fore Plant Breed Genet Res 14(1):47–55. https://doi.org/10.22092/IJRFPBGR.2006.115138
Ghorbani Sini F, Arzani A (2015) Karyological studies in Triticum monococcum subsp. aegilopoides and Aegilops cylindrica species grown wild pairwise in West Iran. Rostaniha 16(2):164–173. https://doi.org/10.22092/botany.2016.105985
Hamidi F, Karimzadeh G, Rashidi Monfared S, Salehi M (2018) Assessment of Iranian endemic Artemisia khorassanica: karyological, genome size, and gene expressions involved in artemisinin production. Turk J Biol 42(4):322–333. https://doi.org/10.3906/biy-1802-86
Hayat MQ, Ashraf M, Khan MA, Mahmood T, Ahmad M, Jabeen S (2009) Phylogeny of Artemisia L.: recent developments. Afr J Biotechnol 8(11):2423–2428
Huziwara Y (1962) Karyotype analysis in some genera of Compositae. VIII. Further studies on the chromosomes of Aster. Am J Bot 49:116–119. https://doi.org/10.1002/j.1537-2197.1962.tb14916.x
Kara A, Çağlak E (2022) Phytochemical research and evaluation of tarragon (Artemisia dracunculus L.) as a food additive. Recep Tayyip Erdogan Univ J Sci Eng 3(2):50–60. https://doi.org/10.53501/rteufemud.1160846
Kreitschitz A, Vallès J (2003) New or rare data on chromosome numbers in several taxa of the genus Artemisia (Asteraceae) in Poland. Folia Geobot 38:333–343. https://doi.org/10.1007/BF02803203
Lavania UC, Srivastava S (1992) A simple parameter of dispersion index that serves as a adjunct to karyotype asymmetry. J Biosci 17:179–182. https://doi.org/10.1007/BF02703503
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
Mas de Xaxars G, Garnatje T, Pellicer J, Siljak-Yakovlev S, Vallès J, Garcia S (2016) Impact of dysploidy and polyploidy on the diversification of high mountain Artemisia (Asteraceae) and allies. Alp Bot 126:35–48. https://doi.org/10.1007/s00035-015-0159-x
Matoba H, Nagano K, Hoshi Y (2007) The tendency of chromosomal evolution in some Japanese Artemisia using numerical analysis of karyotypes. Cytologia 72(2):181–188. https://doi.org/10.1508/cytologia.72.181
Miri SM (2020) Artificial polyploidy in the improvement of horticultural crops. J Plant Physiol Breed 10(1):1–28. https://doi.org/10.22034/JPPB.2020.12490
Mozaffarian V (2005) Compositae: Authemideae & Echinopeae. In: Assadi M (ed) Flora of Iran, vol 59. Research Institute of Forests and Rangelands Publications, Tehran, pp 99–260
Naseri HR, Azarnivand H, Jafari M (2009) Chromosomal evolution in some Iranian Artemisia L. using numerical analysis of karyotypes. Cytologia 74(1):55–64. https://doi.org/10.1508/cytologia.74.55
Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M (2011) Artemisia dracunculus L. (tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem 59(21):11367–11384. https://doi.org/10.1021/jf202277w
Oliva M, Vallès J (1994) Karyological studies in some taxa of the genus Artemisia (Asteraceae). Can J Bot 72:1126–1135. https://doi.org/10.1139/b94-138
Oroji Salmasi K, Javadi H, Miri SM (2019) Karyotype analysis of some Allium species in Iran. J Plant Physiol Breed 9(2):115–127. https://doi.org/10.22034/JPPB.2019.10650
Oyundelger K, Herklotz V, Harpke D, Oyuntsetseg B, Wesche K, Ritz CM (2021) Contrasting effects of local environment and grazing pressure on the genetic diversity and structure of Artemisia frigida. Conserv Genet 22:947–962. https://doi.org/10.1007/s10592-021-01375-w
Paszko B (2006) A critical review and a new proposal of karyotype asymmetry indices. Plant Syst Evol 258:39–48. https://doi.org/10.1007/s00606-005-0389-2
Pellicer J, Garcia S, Canela MA, Garnatje T, Korobkov AA, Twibell JD, Vallès J (2010) Genome size dynamics in Artemisia L. (Asteraceae): following the track of polyploidy. Plant Biol 12(5):820–830. https://doi.org/10.1111/j.1438-8677.2009.00268.x
Pellicer J, Garcia S, Garnatje T, Dariimaa S, Korobkov AA, Vallès J (2007) Chromosome numbers in some Artemisia (Asteraceae, Anthemideae) species and genome size variation in its subgenus Dracunculus: karyological, systematic and phylogenetic implications. Chromosom Bot 2(1):45–53. https://doi.org/10.3199/iscb.2.45
Pellicer J, Garcia S, Garnatje T, Hidalgo O, Siljak-Yakovlev S, Vallès J (2008) Molecular cytogenetic characterization of some representatives of the subgenera Artemisia and Absinthium (genus Artemisia, Asteraceae). Collect Bot (Barcelona) 27:19–27. https://doi.org/10.3989/collectbot.2008.v27.2
Pellicer J, Garcia S, Vallès J, Kondo K, Garnatje T (2013) FISH mapping of 35S and 5S rRNA genes in Artemisia subgenus Dracunculus (Asteraceae): changes in number of loci during polyploid evolution and their systematic implications. Bot J Linn Soc 171(4):655–666. https://doi.org/10.1111/boj.12001
Peruzzi L, Eroğlu HE (2013) Karyotype asymmetry: again, how to measure and what to measure? Comp Cytogenet 7(1):1–9. https://doi.org/10.3897/CompCytogen.v7i1.4431
Rajabi Mazaher A, Miri SM, Mohammadi A (2021) A new chromosome number report in Stachys L. species by use of karyological analysis. J Genet Resour 7(1):29–35
Romero-Zarco C (1986) A new method for estimating karyotype asymmetry. Taxon 35:526–530. https://doi.org/10.2307/1221906
Roughani A, Miri SM, Hassandokht MR, Moradi P, Abdossi V (2021) Cytogenetic and micro-morphological studies on several accessions of some Lepidium L. species in Iran. Iran J Sci Technol Trans A Sci 45:417–426. https://doi.org/10.1007/s40995-020-01035-7
Rousi A (1969) Cytogenetic comparison between two kinds of cultivated tarragon (Artemisia dracunculus). Hereditas 52(1–2):193–213. https://doi.org/10.1111/j.1601-5223.1969.tb02229.x
Saedi K, Jalili A, Azarnivand H, Ghamary Zare A (2005) Karyotypic studies of Artemisia L. species in West Azarbaijan province, Iran. Pajouhesh-Va-Sazandegi 67:2–10
Sancar PY, Civelek S, Kursat M (2021) The morphological, karyological and phylo-genetic analyses of three Artemisia L. (Asteraceae) species that around the Van Lake in Turkey. Caryologia 74(3):53–63. https://doi.org/10.36253/caryologia-1139
Shamsolshoara Y, Javadi H, Miri SM (2020) Karyomorphological study of seven species of the genus Astragalus from Iran. Iran J Bot 26(2):172–178. https://doi.org/10.22092/IJB.2020.341321.1271
Shultz LM (2006) Artemisia Linnaeus. In: Editorial Committee (ed) Flora of North America, vol 19. Oxford University Press, New York, pp 503–534
Stace CA (2000) Cytology and cytogenetics as a fundamental taxonomic resources for the 20th and 21st centuries. Taxon 49:451–477. https://doi.org/10.2307/1224344
Stebbins GL (1971) Chromosomal evolution in higher plants. Edward Arnold, London
Tabur S, Civelek Ş, Öney S, Yilmaz Ergün ŞB, Kurşat M, Türkoğlu İ (2012) Chromosome counts and karyomorphology of some species of Artemisia (Asteraceae) from Turkey. Turk J Bot 36:235–246. https://doi.org/10.3906/bot-1010-98
Tabur S, Civelek Ş, Öney S, Yilmaz ŞB, Kurşat M (2011) Chromosome numbers and karyotypes of some taxa of genus Artemisia (Asteraceae, Anthemideae) subgenus Dracunculus (Bess.) Rydb. Caryologia 64(3):335–342. https://doi.org/10.1080/00087114.2011.10589800
Torrell M, Cerbah M, Siljak-Yakovlev S, Vallès J (2001) Étude cytogénétique de trois taxons du complexe d’Artemisia campestris L. (Asteraceae, Anthemideae): Localisation de l’hétérochromatine et de l’ADN ribosomique. Bocconea 13:623–628
Torrell M, Vallès J (2001) Genome size in 21 Artemisia L. species (Asteraceae, Anthemideae): systematic, evolutionary, and ecological implications. Genome 44(2):231–238. https://doi.org/10.1139/g01-004
Tucker AO, DeBaggio T (2009) The Encyclopedia of Herbs. Timber Press, Portland
Vallès J, Pellicer J, Sánchez-Jiménez I, Hidalgo O, Vitales D, Garcia S, Martín J, Garnatje T (2012) Polyploidy and other changes at chromosomal level and in genome size: Its role in systematics and evolution exemplified by some genera of Anthemideae and Cardueae (Asteraceae). Taxon 61(4):841–851. https://doi.org/10.1002/tax.614009
Wang LS (2000) Study on karyotypes of Artemisia sect. Dracunculus Bess. in northeast China. Bull Bot Res 20(4):402–410
Watanabe K, Yahara T, Denda T, Kosuge K (1999) Chromosomal evolution in the genus Brachyscome (Asteraceae, Astereae): statistical tests regarding correlation between changes in karyotype and habit using phylogenetic information. J Plant Res 112:145–161. https://doi.org/10.1007/PL00013869
Werker E, Putievsky E, Ravid U, Dudai N, Katzir I (1994) Glandular hairs, secretory cavities, and the essential oil in the leaves of tarragon (Artemisia dracunculus L.). J Herbs Spices Med Plants 2(3):19–32. https://doi.org/10.1300/J044v02n03_04
Yazdani B, Sohani MM, Bivadi V, Zad MS, Golesorkhi S (2014) Karyotype study among Iranian species of Artemisia subgenera. Int J Agric Crop Sci 7(10):654–660
Zeb S, Ali A, Zaman W, Zeb S, Ali S, Ullah F, Shakoor A (2018) Pharmacology, taxonomy and phytochemistry of the genus Artemisia specifically from Pakistan: a comprehensive review. Pharm Biomed Res 4(4):1–12. https://doi.org/10.18502/pbr.v4i4.543
Zhen L, Chen S, Chen F, Fang W, Li J, Wang H (2010) Karyotype and meiotic analysis of five species in the genus Artemisia. Caryologia 63(4):382–390. https://doi.org/10.1080/00087114.2010.10589750
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Niloufar Jelvehgar, Abdollah Mohammadi and Seied Mehdi Miri. The first draft of the manuscript was written by Seied Mehdi Miri and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jelvehgar, N., Mohammadi, A., Kashi, A. et al. First karyomorphological analysis of French and Russian tarragon (Artemisia dracunculus L.). Biologia (2024). https://doi.org/10.1007/s11756-024-01768-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11756-024-01768-5