Abstract
The oxidative stability of chia oil was evaluated by measuring the effectiveness of the addition of rosemary (ROS) and green tea (GT) extracts, tocopherols (TOC), ascorbyl palmitate (AP) and their blends, and studying the influence of storage conditions. The addition of antioxidants increased induction time, depending on their type and concentration. Considering antioxidants individually, AP at 5,000 ppm was the most effective, whereas ROS + GT at 2,500 and 5,000 ppm provided the best protection among the antioxidant blends. Chia oil peroxide values of 10 mequiv/kg was observed for oils stored at 4 °C while values greater than 10 mequiv/kg were observed between 60 and 120 days when stored at 20 °C. Only AP 2,500 ppm protected oil did not reach 10 mequiv/kg during 225 days at 4 and 20 °C. Similar trends were observed with p-anisidine and Totox values. Differential scanning calorimetry further supported the presence of primary and secondary oxidation. Activation energy of chia oil thermoxidation was 71.9 kJ/mol increasing up to 87.5 kJ/mol when AP was added.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chia (Salvia hispanica L.) seed oil is an interesting source of polyunsaturated fatty acids (PUFA). It contains the highest proportion of α-linolenic acid (~60%) of any known vegetable source [1]. This fatty acid (FA) belongs to the ω-3 family which is essential for the normal growth and development of the human body and plays an important role in the prevention and treatment of coronary artery disease, hypertension, diabetes, arthritis, other inflammatory and autoimmune disorders, and cancer [2]. Chia seed oil also contains a low percentage of saturated fatty acids. Even though the FA profile of chia seed oil is nutritionally favorable, it may result in poor oxidative stability and shelf life. Furthermore, the oxidation of PUFA results in the generation of volatile compounds which many have unpleasant odors and are responsible for the off-flavors in the food industry.
Protection from lipid oxidation is a critical factor in oil quality. Preservation of oils with antioxidants has been adopted as an approach to address this issue [3]. Synthetic antioxidants are widely used and effective. However, they have different toxicological problems [4]. Food safety legislation has gradually become more stringent, requiring the use of toxicity tests for synthetic antioxidants. Additionally, there is a tendency for consumers to use natural products such as antioxidants, as these are perceived as safe and do not require prior testing [5]. The most important natural antioxidants are ascorbic and citric acids and their salts, tocopherols and spice extracts [6]. Among herbs and spices, the focus has been placed on rosemary. The compounds responsible for rosemary antioxidative activity are mainly phenolic diterpenes such as carnosic acid, carnosol, rosmanol, epirosmanol, isorosmanol, methyl carnosate and other phenolic acids, including rosmarinic acid. The α-, β-, γ- and δ-tocopherols are present as mixtures in vegetable oils, being the main natural antioxidants in fats. Green tea extract consists of a complex mixture of catechin gallates which shows different trends in relative antioxidant activity in different lipid systems. Ascorbyl palmitate is a synthetically-derived oil-soluble ester of ascorbic acid that is widely present in vegetables. The mechanisms by which these antioxidants are involved in the control of autoxidation process are different. Rosemary and green tea extracts and tocopherols act as radical scavengers whereas ascorbyl palmitate acts as an oxygen scavenger [7].
Research has evaluated the effectiveness of different natural antioxidants on the stability of a variety of food systems [8]. However, there is little information available regarding the effects of such antioxidants on chia seed oil preservation.
A large repertoire of methods is available to determine oxidative deterioration of food lipids. The usefulness of the results can be evaluated according to chemical and physical measured properties, the specificity and precision of the analysis, and how they relate to shelf life storage of food products [9]. Traditionally, accelerated methods have been employed to estimate the induction period of oxidation in a relatively short period of time [9]. However, accelerated assays have a drawback in that the oxidation process takes place under drastic conditions, quite unlike those typically occurring in oil storage tanks or even during product marketing. As a consequence, methods selected to determine the end-point of the stability assays and the changes observed in oil have no satisfactory correlation with the autoxidation process that takes place at room temperature [9]. Recently, thermoanalytical methods have been used for oil analysis investigation of their thermal autoxidation process. Because autoxidation of fats, fatty acids and lipids is an exothermic process, thermal analysis is valuable for studying thermostability, thermoxidation and autoxidation [10]. Thermal-oxidative decomposition of vegetable oils and fats was studied by the differential scanning calorimetry method (DSC) [11, 12]. Non-isothermal DSC methods are of practical analytical value because they are simple, not time consuming and may be applied to the analysis of small samples (2–10 mg). Thus, more than one measurement must be carried out to obtain a complete picture of the oxidation process.
The objectives of the present work were to study the oxidative deterioration of chia seed oil by using different methods (DSC, Rancimat), to analyze the effectiveness of the addition of different antioxidants (rosemary extract, green tea extract, tocopherols, ascorbyl palmitate) and to evaluate the influence of storage conditions (temperature and time) on the oil oxidative stability.
Experimental Procedures
Materials
Chia seed oil (two different batches, 25 L each) was purchased from Nutracéutica Sturla S.R.L. (Argentina). It was obtained by cold pressing and stored until use at 4 ± 1 °C in darkness using amber glass bottles without head space.
Rosemary extract (Guardian™ 08, oil-soluble), green tea extract (Guardian™ 20S/20 M), and ascorbyl palmitate (Grindox™ 562) were obtained from Danisco (Copenhagen, Denmark). The content of the main rosemary antioxidative components, diterpene phenols, was 4% (wt/wt). Green tea extract was composed of about 20% of catechins, while Grindox™ 562 contained 10% of ascorbyl palmitate. A mix of tocopherols (purity 97%; γ-64%, δ-24%, α-8% and 1% β-tocopherols), was purchased from DSM Nutritional Products Ltd. All of the antioxidants used are classified as GRAS additives in United States. All of the chemicals and solvents used were of analytical grade.
Methods
Characterization of Oil
Fatty acid composition was analyzed by GC according to the IUPAC 2.302 standard method [13]. FAME were prepared using BF3- methanol reagent following the IUPAC 2.301 method [13].
Iodine and saponified values and free fatty acid content were determined according to AOCS recommended practices Cd 1c-85, Ca 6a-40 and Ca 5a-40, respectively [14].
The oil tocopherol content was determined by normal phase HPLC using a Hewlett Packard chromatography system (HPLC Hewlett Packard 1050 Series, Waldbronn, Germany) equipped with a fluorescence detector Agilent 1100 Series (Agilent Technology, Palo Alto, CA, USA) following the procedures described in IUPAC 2.432 [13] and AOCS Ce8-89 [14].
Rancimat Analysis
Rosemary (ROS) and green tea (GT) extracts, tocopherols (TOC), ascorbyl palmitate (AP) and blends containing rosemary extract:tocopherols (1:1) (ROS + TOC), rosemary extract:green tea extract (1:1) (ROS + GT) and green tea extract:tocopherols (1:1) (GT + TOC) were added separately to chia oil aliquots at concentrations of 250, 500, 1,000, 1,500, 2,500 and 5,000 ppm of commercial products.
Oil oxidative stability was evaluated by the Rancimat Mod 679 (Metrohm AG, Herisau, Switzerland) [14] method, using 5 g of oil sample warmed at 98 °C with an air flow of 20 L/h. Oil stability was expressed in terms of induction time (h) and the effectiveness of all tested antioxidants and their blends was expressed as the protection factor (PF):
where tiant is the induction time of the samples treated with antioxidant, and ti0 is the induction time of the control system (without antioxidant).
Differential Scanning Calorimetry (DSC)
A non-isothermal DSC study was carried out in a Q 100 (TA Instruments, Newcastle, USA) differential scanning calorimeter [10, 12]. The apparatus was calibrated with indium, stearic and lauric acids. Antioxidants were added to chia seed oils in the following concentrations: 2,500 ppm of rosemary extract, 2,500 ppm of green tea extract, 1,500 ppm of tocopherols and 2,500 ppm of ascorbyl palmitate. For the corresponding control systems, chia seed oils without antioxidants were used.
Oil samples of 3–5 mg were placed in an aluminum pan and then were heated at constant heating rates (β) of 1, 5, 10, 15 and 20 °C/min from 10 to 350 °C in an oxygen flow of 100 mL/min. Temperatures of the extrapolated start of oxidation (T e) and temperatures of maximum heat flow (T p1 and T p2) were determined from each DSC scan by using the TA Universal Analysis 2000 program (v. 4.2E) (TA Instruments, New Castle, USA).
Storage Experiments
Storage experiments of each pure chia seed oil, and chia seed oils with different antioxidants were carried out at two temperature levels usually used by consumers: room temperature (T = 20 ± 2 °C) and cooling (T = 4 ± 1 °C). Table 1 shows the systems used in these experiments, selected on the basis of Rancimat analyses, and taking into account Codex Alimentarius current legislation [15] for other oils.
Each system was prepared by adding the corresponding antioxidant and then fractionated into 48 amber glass bottles (30 mL each) without head space. Half of the bottles from each system were stored in a cold chamber at 4 ± 1 °C, while the rest were placed in a temperature-controlled room at 20 ± 2 °C, both for 225 days. Two bottles corresponding to each batch of each system stored at the two temperatures were taken periodically (every 15 days) for analysis.
Primary oxidation products—hydroperoxides—were determined by the peroxide value (PV) expressed as milliequivalents of peroxides per kilogram of oil (mequiv peroxide/kg) [14]. Formation of secondary oxidation products was measured by p-anisidine values (p-AV) [14]. Totox values were calculated from the PV and p-AV values of the samples using the Totox value equation = 2PV + p-AV. The free fatty acids contents were determined according to AOCS recommended practice Ca 5a-40 [14].
Fatty acid composition and tocopherol content of the samples were determined as a function of storage time according to IUPAC [13] and AOCS [14] practices.
Statistical Analysis
The data set from accelerated oxidation experiments (Rancimat) was obtained from measurements in triplicate from two individual batches of oil samples. DSC analyses and storage experiments were carried out in duplicate from two batches of oil samples. Statistical analysis was performed by ANOVA at 5% significance level (p ≤ 0.05). Means were separated according to Tukey’s multiple comparison tests (p ≤ 0.05) in all cases. Data was processed using the Statgraphics Plus statistical package (Version 4.0 for Windows, Manugistics Inc., USA).
Results and Discussion
Characterization of Oil
The initial characteristics of chia seed oil used in this study are shown in Table 2. The results obtained agree with those reported in previous works [1, 16, 17]. However, some variations in the fatty acid profile of chia oil obtained from seed grown in different climatic conditions have been recorded [1, 18]. The anti and prooxidant minor components of chia seed oil were not eliminated in order to obtain results with industrial applications.
Rancimat Analysis
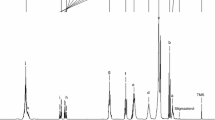
The oxidative stability of chia seed oil and the activity of different antioxidants and their blends were evaluated based on induction time (ti) measurement determined by Rancimat analysis. Figure 1 shows protection factors (PF) (tiant/ti0) for all antioxidants and their blends tested at different concentrations. This factor expresses the effectiveness of the antioxidants, and represents the possibility of blocking the radical chain process by interacting with the peroxyl radicals [19]. The antioxidant addition increased the ti of chia seed oil in all cases. This increase depended on the type and concentration of antioxidant used.
Protection factors of antioxidants and their blends on chia seed oils measured by Rancimat, T = 98 °C, air flow 20 L/h. a Effect of concentration for each antioxidant and antioxidant blends; b Effect of antioxidant and antioxidant blends for each concentration TOC tocopherols, ROS rosemary, GT green tea, AP ascorbyl palmitate Values are the mean of the two independent batches (n = 2) and vertical bars indicate standard deviation. Different letters indicate significant differences (p ≤ 0.05) as follows: a between concentrations for each antioxidant and antioxidant blends and b between antioxidant and antioxidant blends for each concentration, according to Tukey’s (HSD) test
All of the antioxidants and their blends studied, except tocopherols, increased the ti as a function of increasing concentration (Fig. 1a). The stability of chia seed oils treated with tocopherols increased significantly (p ≤ 0.05) up to 1,500 ppm, i.e. concentration at which tiant doubled the value observed for control system. The highest concentrations of tocopherols (2,500 and 5,000 ppm) also produced significantly higher tiant compared to control system, but of lower magnitude, 34 and 32%, respectively, than the 1,500 ppm concentration. The tocopherol concentration is an important factor that influences tocopherol antioxidant activity in bulks oils. In this way, the oxidative stability of soybean oil was optimum at tocopherol concentrations between 400 and 600 ppm. The antioxidant activity of the tocopherol mixture diminished when the tocopherol levels exceeded their optimal concentrations specially with increasing temperature [9, 19].
Rosemary extract addition significantly improved (p ≤ 0.05) oil oxidation stability, leading to protection factors between 1.6 and 3.4. This antioxidant was most effective at the highest concentration studied (5,000 ppm). Concentrations between 250 and 2,500 ppm also showed significant differences (p ≤ 0.05) compared to control oil, although no significant differences (p > 0.05) were found among them. At concentrations ranging between 500 and 5,000 ppm, green tea extract and AP ti values increased significantly between 500 and 5,000 ppm. For both antioxidants, the highest protection factors were found at 5,000 ppm (2.6 and 4.3, respectively). Taking into account the antioxidants individually, the ascorbyl palmitate at 5,000 ppm was found to be the most effective. These results might be attributed to the interaction between ascorbyl palmitate and the natural tocopherols present in chia seed oil. Reische et al. [20] reported that ascorbic acid can scavenge oxygen, shift the redox potential of food systems to the reducing range, act synergistically with chelators, and regenerate primary antioxidants such as tocopherols. These antioxidants can act as synergists with tocopherols by converting oxidized tocopherol back to the reduced form [9].
The effects of antioxidant combinations on ti of chia seed oil are also presented in Fig. 1a. ROS + TOC reached the best tiant at 1,500 and 5,000 ppm, which were 2.3 and 2.4 times the ti of the control system. The other concentrations (250, 500, 1,000 and 2,500 ppm) of this blend were not significantly different (p > 0.05), with protection factors between 1.6 and 1.9. Tocopherol and green tea extract blend (GT + TOC) protection factors of 1.5, 1.8 and 1.7 at the 1,000, 2,500 and 5,000 ppm, respectively, were significantly (p ≤ 0.05) different from the control. ROS + GT antioxidant blend at concentrations above 1,000 ppm and upper had protection factors higher than that of the control sample. The highest protection factors were found at 2,500 and 5,000 ppm.
At 250 ppm, the addition of ROS + TOC had the highest protection factor, which was significantly (p ≤ 0.05) higher than those corresponding to GT and GT + TOC. It was noted that at 500 ppm no significant differences (p > 0.05) were detected among all antioxidants and antioxidant blends. However, at 1,000 and 1,500 ppm, ROS + GT had the highest PF (2.3 and 2.9, respectively), followed by AP and ROS + TOC. AP and ROS + GT had the best antioxidant effects at 2,500 and 5,000 ppm while 5,000 ppm rosemary extract (ROS) exhibited high effectiveness. Thus, the trend of increased antioxidant activity with increasing antioxidant concentrations was observed.
Differential Scanning Calorimetry (DSC)
Several authors reported that DSC is a good technique to determine the kinetic parameters of fatty acid oxidation and those temperatures of the extrapolated start of the oxidation process are useful points [10]. Figure 2 shows DSC curves for chia oil oxidation at five different heating rates: 1, 5, 10, 15 and 20 °C/min. All scans were similar to each another, showing two main peaks. In general, the greater the heating rate, the higher the temperature T e, T p1 and T p2 (Fig. 2; Table 3). According to Litwinienko and Kasprzycka-Guttman [12], an approximated model of the sequential reactions with autocatalytic onset is the best explanation for the DSC signal shape. This model is in accordance with the chain reaction scheme of free radical oxidation of lipids. It shows that the first process observed in the non-isothermal DSC curve is caused by formation of peroxides. The second peak results from the decomposition of the peroxides to further products. Therefore, evaluation of edible oil stability using DSC by the calculation of kinetic parameters of thermoxidation should consider onset temperatures of the process and temperatures of the first peak, rather than the second. These authors suggest that Arrhenius activation energies and rate constants calculated from the onset and first peak may be useful in assessing the susceptibility of edible oils to oxidation. This reaction can be represented by the scheme shown in Eq. 1.
Where the first step, a → b, is the process catalyzed by b. The above interpretation was confirmed experimentally by several other studies including oxidation of partially oxidized oils [21]. DSC curves obtained for chia oil oxidation were similar to those recorded for linseed oil, also characterized by its very high content of α-linolenic acid. Temperatures of the extrapolated start of the exothermic reaction (T e) and temperatures of maximum heat flow (T p1 and T p2) are listed in Table 3. Every temperature value is an average from at least two runs at the same heating rate β. They were used to calculate kinetic parameters by the Ozawa-Flynn-Wall (OFW) method [22, 23]. Activation energies (E a) and pre-exponential factors (A) were calculated from the onset point temperature and from both peaks observed on the DSC curves, T e, T p1 and T p2, respectively.
The plot of log β vs. 1/T, found by the OFW method, shows the linear dependence described by the following equation:
Arrhenius plots with a slope (a = dlogβ/dT−1) calculated by means of the least square method were used to obtain the apparent activation energies of autoxidation (E a) and the pre-exponential factor (A) from Eqs. 3 and 4, as follows:
where R is gas constant and T absolute temperature [21].
The values of kinetic parameters and statistic data calculated for chia seed oil are listed in Table 4. The values of E a obtained from the onset point and the first peak were 71.9 ± 4.3 and 59.9 ± 1.3 kJ/mol, respectively, and these were higher than the activation energies calculated from the second peak (44.4 ± 5.8 kJ/mol). The activation energy of chia seed oil calculated from T e was similar to that reported for pure α-linolenic acid (62–70 kJ/mol) [21], which is the main fatty acid present in chia seed oil, and lower than the activation energy for corn oil (104.3 kJ/mol) [24].
One example of DSC curves obtained for chia oil oxidation with and without the addition of different antioxidants studied at the same heating rate (β = 10 °C/min) is shown in Fig. 3.
DSC curves of thermal oxidation of chia seed oil control and with the addition of different antioxidants at a heating rate of β = 10 °C/min. a chia seed oil control; b chia seed oil + 2,500 ppm of rosemary extract; c chia seed oil + 1,500 ppm of tocopherols; d chia seed oil + 2,500 ppm of green tea extract; e chia seed oil + 2,500 ppm of ascorbyl palmitate
With the addition of the antioxidants, chia seed oil curve shapes are similar to those corresponding to control system, but kinetic parameters calculated from T e to T p1 are different. Arrhenius plot parameters and activation energies for chia oil oxidation with the addition of antioxidants are listed in Table 5.
Chia oils, with the addition of AP and GT had higher E a and A values than the control system. This may be associated with the ability of these compounds to delay the autoxidation. The addition of ROS very slightly increased the value of E a, while A was a bit lower than the control system. Thus, among these compounds, AP had the best antioxidant activity, with values of E a−22% higher than the control system, while GT and ROS caused increases of 13 and 4%, respectively. The kinetic parameters previously mentioned were lower in the TOC oil than those corresponding to the control system, showing their low effectiveness to delay the lipid oxidation process compared to other antioxidants studied. This fact can be explained in terms of the “polar paradox” [9], based on the different antioxidant interfacial properties. Accordingly, tocopherols would be less active in bulk oil because they remain in solution in the oil. Besides, the tocopherol concentration is an important factor that influences tocopherol antioxidant activity in bulk oils. Although, the tocopherol concentration for the DSC study (1,500 ppm) was selected on the basis of Rancimat analyses, it is necessary to consider that the apparent relationship between tocopherol concentration and its antioxidant activity may be influenced by the conditions used to accelerate oxidation in laboratory studies [25].
Chia Seed Oil Storage
For practical purposes, the predictions of the oxidative stability in foods and oils based on the determination of the induction period could be related to the product shelf life. Therefore, it is important to note that the test conditions used should be as similar as possible to those under which the product will be stored [9].
Figures 4 and 5 show the evolution of the PV, p-AV and Totox values, for chia oil oxidation with and without antioxidant addition during storage at 4 ± 1 °C and 20 ± 2 °C, respectively. As expected, storage temperature was a relevant factor in the evolution of the oxidative process of chia oils. Thus, after 225-day storage at 4 ± 1 °C, the control system had PV, p-AV and Totox values of 9.9 mequiv/kg oil, 1.7 and 21.5, respectively, which were lower than the values of the control system stored at 20 ± 2 °C (PV = 16.9 mequiv/kg oil; p-AV = 4.7 and Totox value = 38.5).
None of the samples exceeded the upper limit of PV (10.0 mequiv/kg oil) established by the Codex Alimentarius [15], for oils not covered by individual standards when stored at 4 ± 1 °C. This PV is also considered the upper limit of acceptability for vegetable oils high in polyunsaturated fatty acids (PUFA) [9].
During storage at 4 ± 1 °C, chia oil with added AP had the lowest levels of PV, being significantly lower (p ≤ 0.05) than the control oil after 30 days. After a 150-day storage at this temperature, control chia oil had an increase in the primary oxidation rate. After a 165-day storage, control oil oxidation was significantly (p ≤ 0.05) higher compared to oils with added antioxidants (Fig. 4a).
Secondary oxidation products, determined through p-AV remained practically constant and below 2.0 during storage at 4 ± 1 °C (Fig. 4b). Although some significant differences (p ≤ 0.05) were recorded between the samples at different storage times depending on the type of antioxidant added, differences between them at the end of storage (t = 225 days) were not significant (p > 0.05).
The Totox value provides a measure of both primary and secondary oxidation products. The behavior observed for this index was very similar to that found for PV, as seen in Fig. 4c. The highest Totox values were recorded at the end of the storage period, when the control chia seed oil presented a value significantly higher (p ≤ 0.05) than that of oils with added antioxidants. This difference could be mainly associated with the primary oxidation products (hydroperoxides), determined through the PV, since, as mentioned previously, no differences in the formation of the secondary oxidation products (p-AV) were found. Thus, after the storage period (225 days) at 4 ± 1 °C, the oils studied would be in the earlier stages of oxidation and decomposition of peroxides would not be recorded by that time.
All samples, excepted for chia oil with AP, reached a PV of 10 mequiv/kg oil between day 60 and 120 at 20 ± 2 °C (Fig. 5a). The PV increased from 0.9 to 16.9 mequiv/kg oil after 225 days of storage I the control oil with the highest value, about 20.0 mequiv/kg oil, being observed on day 195. Control oil reached the legal limit (10 mequiv/kg oil) after 60 days of storage at 20 ± 2 °C, and at this time no significant differences (p > 0.05) were found compared to ROS500(20 °C) and GT500(20 °C). However, the addition of a higher concentration (2,500 ppm) of GT and ROS managed to retard the oxidation process in the initial storage period, reaching the legal limit after 90 and 110 days, respectively. Ascorbyl palmitate showed a considerable stabilization effect. PV for AP2500(20 °C) remained practically steady (0.6–1.5 mequiv peroxide/kg oil), and no significant differences (p > 0.05) were found between PV for this system across the storage time studied. If compared to other systems studied, PV in oils with AP2500(20 °C) was significantly different (p ≤ 0.05) after 45 days of storage at 20 ± 2 °C. Thus, PV of oils stored at this temperature suggested the following order in antioxidant effectiveness: AP2500 > ROS2500 > GT2500 > GT500 ~ ROS500.
With respect to p-AN value of oils stored at 20 ± 2 °C, although these values varied in a range wider than those for oils stored at 4 ± 1 °C, these parameters were lower than those reported in soybean oil stored in the dark at room temperature during a similar period (p-AV = 36.1 ± 1.4) [3]. The p-AV of the oil with the addition of AP showed little variation across storage time at 20 ± 2 °C, showing significant differences (p ≤ 0.05) from other systems studied after 60 days of storage. The Totox value exhibited a trend of evolution similar to that of the PV, as observed in oils stored at 4 ± 1 °C. It can be clearly observed that AP exhibited the highest antioxidative activity at room temperature (20 ± 2 °C) among all antioxidants tested.
Table 6 shows the fatty acid composition, free fatty acids and induction time of chia control oil and those to which different antioxidants were added, at the initial time and after 225 days of storage, at both temperatures studied. It can be observed that neither the composition of fatty acids, nor free fatty acid content varied significantly (p > 0.05) after the storage period, indicating that no hydrolytic degradation was present.
Chia oils stored at 20 ± 2 °C had a significant lower (p ≤ 0.05) induction time (t i) after 225 days storage compared to the initial time (Table 6). The t i at the beginning of storage varied depending on the type of antioxidant added. Also, significant differences (p ≤ 0.05) in t i of ROS500, GT500, GT2500 and AP2500 systems after 225 days storage at 4 ± 1 °C were found.
Moreover, at the end of the storage period, the total reduction in the tocopherol content varied between 58 ppm (10%) in sample ROS2500(4 °C) and 136 ppm (24%) in sample GT2500(4 °C) (data not shown). These reductions were similar to those observed by Gómez-Alonso et al. [26] during 21 months storage of virgin olive oil at room temperature, and much smaller than the 50% observed by Okogeri and Tasioula-Margari [27] during the 12-month storage of virgin olive oil in bottles with 3% headspace at room temperature and in darkness.
Conclusion
This study provides an insight into understanding chia seed oil oxidation Results showed that the addition of the different antioxidants increased the induction time of chia seed oil. This increase was related to the type and concentration of the antioxidant and their blends. The best effects were recorded in chia seed oil with the addition of AP (2,500, 5,000 ppm), ROS (5,000 ppm) and its blend (1:1) with GT extract (2,500, 5,000 ppm). DSC thermograms obtained at different heating rates allowed for the calculation of the kinetic parameters of chia oil oxidation. E a values calculated from T e to T p1 were 71.9 ± 4.3, and 59.9 ± 1.3 kJ/mol, which increased by 22.0 and 11.5%, respectively, with the addition of 2,500 ppm of AP. Temperature had a strong influence on oil oxidation. PV of oils stored at 4 ± 1 °C with and without the addition of antioxidants recorded lower PV than the legal limit of 10 mequiv peroxide/kg oil, indicating that relatively low oxidation occurred at low temperature. In contrast, most samples stored at 20 ± 2 °C achieved the legal limit between 60 and 120 days, except the oil with the addition of ascorbyl palmitate, whose PV remained relatively steady during the experiment.
References
Ayerza R Jr, Coates W (2011) Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind Crops Prod 34:1366–1371
Simopoulos AP (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505
Anwar F, Bhanger MI, Kazi TG (2000) Activity of phenolic antioxidants on the storage ability of soybean cooking oil. Pakistan J Biol Sci 3:1333–1335
Amarowicz R, Naczk M, Shahidi F (2000) Antioxidant activity of various fractions of non-tannin phenolics of canola hulls. J Agric Food Chem 48:2755–2759
Mason MJ, Scammon DL, Fang X (2007) The impact of warnings, disclaimers, and product experience on consumers’ perceptions of dietary supplements. J Consum Affairs 41:74–99
Hras AR, Hadolin M, Knez Z, Bauman D (2000) Comparison of antioxidative and synergistic effects of rosemary extract with α-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chem 71:229–233
Shahidi F (1997) Natural antioxidants: an overview. In: Shahidi F (ed) Natural antioxidants. AOCS Press, Illinois, pp 1–11
Erkan N, Ayranci G, Ayranci E (2008) Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 110:76–82
Frankel EN (2005) Lipid Oxidation, 2nd edn. The Oily Press, Bridgewater
Litwinienko G (2007) DSC study of linolenic acid autoxidation inhibited by BHT, dehydrozingerone and olivetol. J Therm Anal Calorim 88:781–785
Rudnik E, Szczucinska A, Szczucinska H, Szulc A, Winiarska A (2001) Comparative studies of oxidative stability of linseed oil. Thermochim Acta 370:135–140
Litwinienko G, Kasprzycka-Guttman T (1998) A DSC study on thermoxidation kinetics of mustard oil. Thermochim Acta 319:185–191
International Union of Pure and Applied Chemistry (IUPAC) (1992) Standard methods for the analysis of oils, fats and derivates, 7th edn. Blackwell Scientific, Oxford
AOCS (1998) Official methods and recommended practices of the AOCS, 5th edn. AOCS Press, Champaign
Codex Alimentarius Commission (1999) Codex Stan 19. Edible fats and oils not covered by individual standards. http://www.codexalimentarius.net/web/standard_list.do?lang=en
Ixtaina VY, Martínez ML, Spotorno V, Mateo CM, Maestri DM, Diehl BWK, Nolasco SM, Tomás MC (2011) Characterization of chia seed oils obtained by pressing and solvent extraction. J Food Compos Anal 24:166–174
Piretti PG, Gai F (2009) Fatty acid and nutritive quality of chia (Salvia hispanica L.) seeds and plant during growth. Anim Sci Technol 148:267–275
Ayerza R (2009) The seed’s protein and oil content, fatty acid composition and growing cycle length of a single genotype of chia (Salvia hispanica L.) as affected by environmental factors. J Oileo Sci 58:347–354
Yanishlieva NV, Kamal-Eldin A, Marinova EM, Toneva AG (2002) Kinetics of antioxidant action of α- and γ-tocopherols in sunflower and soybean triacylglycerols. Eur J Lipid Sci Technol 104:262–270
Reische DW, Lillard DA, Eitenmiller RR (2008) Antioxidants. In: Akoh C, Min D (eds) Food lipids. Chemistry nutrition and biotechnology. CRC Press, Boca Raton, pp 409–433
Litwinienko G (2001) Autoxidation of unsaturated fatty acids and their esters. J Therm Anal Calorim 165:639–646
Ozawa T (1970) Kinetic analysis of derivative curves in thermal analysis. J Therm Anal 2:301–324
Flynn JH, Wall LA (1966) A quick direct method for determination of activation energy from thermogravimetric data. J Polym Sci B Polym Lett 4:323–328
Litwinienko G, Kasprzycka-Guttman T, Jarosz-Jarszewska M (1995) Dynamic and isothermal DSC investigation of the kinetics of thermooxidative decomposition of some edible oils. J Therm Anal 45:741–750
Evans JC, Kodali DR, Addis PB (2002) Optimal tocopherol concentrations to inhibit soybean oil oxidation. J Am Oil Chem Soc 79:47–51
Gómez-Alonso S, Mancebo-Campos V, Salvador MD, Fregapane G (2007) Evolution of major and minor components and oxidation indices of virgin olive oil during 21 month storage at room temperature. Food Chem 100:36–42
Okogeri O, Tasioula-Margari M (2002) Changes occurring in phenolic compounds and α-tocopherol of virgin olive oil during storage. J Agric Food Chem 50:1077–1080
Acknowledgments
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina (PICT 2007-01085), Universidad Nacional de La Plata (UNLP) (11/X502), PIP 1735 CONICET, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Argentina. The authors wish to thank Carmen Mateo, Margarita García, Javier Lecot and Daniel Russo for their technical support and Jorgelina Lastiri for kindly helping with English grammar. Author V. Y. Ixtaina has received a fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ixtaina, V.Y., Nolasco, S.M. & Tomás, M.C. Oxidative Stability of Chia (Salvia hispanica L.) Seed Oil: Effect of Antioxidants and Storage Conditions. J Am Oil Chem Soc 89, 1077–1090 (2012). https://doi.org/10.1007/s11746-011-1990-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1990-x