Abstract
The endogenous minor components from canola, rice bran, sesame and palm oils including selected phospholipids, and various combinations of tocopherol isomers were tested during frying using canola oil triacylglycerols as the frying medium. Thermo-oxidative degradation was assessed by measurement of the total polar components, the rate of volatile carbonyl compounds and 4-hydroxynonenal formation. All the tested minor components protected to a different extent canola triacylglycerides from thermo-oxidative degradation during frying. No significant differences were observed in the protection of the triacylglycerides among all the tested tocopherol isomers and their mixtures. Irrespective of the composition of tocopherol homologous, an increase in the added amounts above 1,000 μg/g did not improve protection. Minor components isolated from rice bran and sesame oils offered better protection during canola triacylglycerides frying than endogenous minor components isolated from canola oil. When 0.2% phosphatidylcholine or phosphatidylethanolamine was added to the canola triacylglycerides, the amount of formed polar components decreased twice as compared to the tocopherol isomers. Accordingly, by optimizing the composition and the concentration of the endogenous minor components, the frying performance of oil can be significantly enhanced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Under frying conditions, a large number of volatile and non-volatile compounds are produced. Not only do these compounds adversely affect the stability of the frying oil, but the food fried in the deteriorated oil acquires a significant amount of decomposition products that may have potentially adverse effects on the safety, flavor, nutritional value, and stability of a fried food. Moreover, several studies have shown that a number of volatile compounds formed during frying exhibit carcinogenic, mutagenic and genotoxic properties [1–3]. In consequence, the thermal stability of frying oils is a vital criterion in the selection of a frying medium [4]. The search for oil with improved frying stability has led to several modifications of the fatty acid composition of many commodity oils [5–9]. On the other hand, a body of research has demonstrated the significant role of endogenous minor components on the frying stability of oil [10–14].
The amount and relative contribution of linoleic, linolenic and other unsaturated fatty acids in canola oil makes it desirable as a good source of essential fatty acids. However, the susceptibility of these acids to oxidation demands measures that will increase the oil’s frying stability without compromising its nutritional value. Previous work in this area focused mainly on the influence of added tocopherols on the frying stability, with relatively fewer reports on the use of other natural antioxidants [14–17]. So far, there has been no report on the effect of endogenous minor components from rice bran, sesame and palm oils on the frying stability of canola oil. Although, a few papers have reported the frying performance of canola oil blends with palm olein, olive and corn, rice bran, and sesame oils [18–22]. These blends had changed fatty acid, triacylglycerols and minor components compositions and make it impossible to assess the effect of the endogenous minor components [23, 24].
In the present study, the effects of selected phospholipids, various combinations of tocopherol homologues, and minor components isolated from canola (CO), rice bran (RBO), sesame (SO) and palm oils (PO) on the frying performance of antioxidant stripped canola oil triacylglycerides were assessed. The vegetable oils chosen for this study contain unique endogenous minor components which are not found in canola oil, namely, tocotrienols and oryzanol in RBO, lignans in SO, and tocotrienols and carotenoids in PO.
Materials and Methods
Materials
Commercially processed regular canola oil was donated by Richardson Oilseed Limited (Lethbridge, Canada). High oryzanol rice bran (RBO) and red palm oils were gifts from Rito, Inc. (Stuttgart, Arkansas, USA) and Golden Jomalina Food Industries (Kuala Langat, Malaysia), respectively. Neutral alumina (70–230 mesh), silica gel 60 Å (70–230 mesh), d-glucose, and potato starch were purchased from VWR (Edmonton, Canada). Phosphatidylcholine (>99%), phosphatidylethanolamine (~98%), β-carotene and 4-hydroxynonenal (HNE) were purchased from Sigma-Aldrich (St. Louis, MO, USA). BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide, TMCS (trimethylchlorosilane), and pyridine were obtained from Supelco (Bellefonte, PA, USA). Standards of tocopherols were purchased from Calbiochem-Novabiochem (San Diego, CA, USA). Standard of γ-oryzanol was provided by Oryza Oil and Fat Chemical Co. Ltd (Ichinomiya-City, Japan). Standards of volatile carbonyl compounds were purchased from Bedoukian Research (Danbury, CT, USA), all were subsequently derivatized to dinitrophenylhydrazones [25].
Preparation of Antioxidant Stripped Canola Triacylglycerides and Isolation of Minor Components
Isolation of endogenous minor components and triacylglycerides from canola and rice bran oils including further purification of fractions is presented in Scheme 1. Canola oil was stripped of its endogenous minor components including antioxidants via adsorption chromatography, following the procedure described by Lampi and Kamal-Eldin [26] with modifications. A slurry of alumina (1 kg; activated at 103 °C for 16 h and at 200 °C for 8 h) in 2 L of hexane was loaded to a glass chromatography column (950 × 50 mm i.d.). The hexane was allowed to flow helping with proper and even packing. Canola oil (500 g) dissolved in 500 mL hexane was loaded into the packed column. The first 800 mL of the eluting hexane was discarded, followed by 3.7 L hexane containing 431 g of purified canola triacyglycerols (CTG). The canola oil minor components (CMC) were recovered from the column by elution with 2.5 L of 10% methanol in methyl tert-butyl ether. Both the column and collection vessels were wrapped in aluminum foil to prevent photo-oxidation. The CTG were stored as hexane solution at −16 °C until used in the experiments. From rice bran oil minor components were similarly isolated forming rice bran triglyceride (RBTG) and minor components fraction (RBMC).
Schematic of isolation and purification of concentrated endogenous minor components from canola and rice bran oils. CC Column chromatography, PTLC preparative thin layer chromatography, MTBE methyl tert-butyl ether, CO canola oil, RBO rice bran oil, CTG canola oil triacylglycerols; CMC and RBMC are minor components isolated by column chromatography from canola and rice bran oils, respectively; TCAN and TRBO are tocopherol fractions isolated from canola and rice bran oils, respectively; SCAN and SRBO are sterol fractions isolated from canola and rice bran oils, respectively. For chromatographic conditions see the text
Purification of Minor Components
The recovered minor components from canola and rice bran oils were further separated into two fractions by adsorption chromatography. Conditioned silica gel was prepared by heating it for 24 h at 160 °C, and the water content adjusted to 5% by addition of a stoichiometric amount. A slurry of 120 g of the conditioned silica gel in 150 mL of hexane was loaded into a glass chromatography column (600 × 45 mm i.d.) allowing constant flow of hexane until the packing was evenly packed. Minor components, 20 g, dissolved in 30 mL of hexane were introduced into the column. The tocopherol fraction was subsequently eluted with 750 mL of 5% methyl tert-butyl ether (MTBE) in hexane while sterols fraction with 750 mL of 50% MTBE in hexane. The purity of fractions was monitored by thin layer chromatography. Collected fractions were concentrated under vacuum using a rotary evaporator (BÜCHI, Flawil, Switzerland) providing 2.5 g of golden oil for tocopherol fraction and 15.3 g of yellow oil for sterol fraction. Both the column and collection vessels were wrapped in aluminum foil to prevent photo-oxidation. The fractions were stored in hexane at −16 °C until used in the experiments.
Purification of Tocopherol and Sterol Fractions
Tocopherol fraction from canola and rice bran oils were further purified by preparative thin layer chromatography (PTLC) using MTBE/hexane (2:8, v/v) as the developing solvent. Bands corresponding to the tocopherols were scraped off and eluted three times with 10 mL of MTBE. Combined extracts were concentrated under vacuum using a rotary evaporator, flushed with nitrogen and kept in hexane at −16 °C until used in the experiments. From 1 g of the starting tocopherol fraction, 157 and 313 mg of purified tocopherols were obtained from canola (TCAN) and rice bran (TRBO) oils, respectively. Sterol fractions from both oils were similarly purified by PTLC using MTBE/hexane (65:35, v/v) as the developing solvent. From 1 g of the initial sterol fraction, 219 and 402 mg of purified sterols fractions was isolated from canola (SCAN) and rice bran (SRBO) oils, respectively.
Solvent Extraction of Endogenous Minor Components
Minor components were extracted directly from rice bran oil according to the Miraliakbari and Shahidi [27] procedure with modifications. Briefly, 200 g of oil was placed in a 500-mL capacity separatory funnel and 200 mL methanol added followed by agitation for 5 min with periodic venting. The mixture was allowed to sit at room temperature for 1 h to allow separation of the oil from the methanol. The extraction was repeated 12 times with the same volume of fresh methanol. Extracts were combined and excess of methanol evaporated under a vacuum using a rotary evaporator. The 9.4 g of light yellow gummy material coded RBOS was flushed with nitrogen and stored at −16 °C until used in the experiments. Minor components from sesame and palm oils were extracted in the same way and coded SOS and POS, respectively.
Formulation of Food for Frying
A mixture of selected food ingredients was prepared to mimic as closely as possible typical frying food providing similar degradation processes as usually happening during French fries frying. A slurry of 4 g of potato starch, 1 g of glucose and 1 g of silica gel in 5 mL of cold distilled water was prepared then to it 15 mL of boiling water was added. The mixture was placed onto a hot plate preset at 110 °C, and heated for 2 min with continuous mixing. The resulting gel was left uncovered to cool to room temperature and used as formulated food (FF) in all frying experiments.
Frying Test
An oil or purified triacylglycerides (12.0 g) were weighed into a clean, acid-washed glass beaker (30 mL, Pyrex, USA). Clean octagonal stir bars (9.5 × 25 mm, Fischer Scientific, USA) was placed into the glass vessel with oil, altering the surface-to-volume ratio of oil to 0.42. The vessel was heated at 185 ± 5 °C for 10 min, then 1.2 g of FF was added. The heating was continued for another 20 min without mixing and continuous stirring initiated at 500 rpm. Heating and stirring were maintained for additional 90 min. About 0.5 g of oil was withdrawn at the 60th, 80th, 100th, and 120th min for analysis. Selected sampling intervals reflect frying time at standard conditions for 1, 3, 5 and 7 days of actual frying using an institutional fryer (General Electric Company, NY, USA) and are based on the amount and composition of polar components formed.
Total Polar Components
The amount of polar components (TPC) was determined by a gravimetric method following AOAC Method 982.27, using column chromatography to separate non-polar from polar fractions as described in the Schulte modification [28, 29].
Tocopherols
Tocopherols were analyzed according to AOCS Official Method Ce 8-89 [30]. Briefly, 50 mg of oil samples were weighed directly into autosampler vials and dissolved in 1 mL hexane. Analyses were performed on a Finnigan Surveyor HPLC (Thermo Electron Corp., Waltham, MA, USA) with a Finnigan Surveyor Autosampler Plus and Finnigan Surveyor FL Plus fluorescence detector, the latter was set for excitation at 292 nm and emission 325 nm. The column was a normal phase Diol column (5 μm; 250 × 4.6 mm; MonoChrom, Varian, CA, USA). Of each sample, 10 µL was injected. Mobile phase consisted of 7% methyl-tert-butyl-ether in hexane with a flow rate of 0.6 mL/min. The amounts of tocopherols were quantified using external calibration for each isomer separately.
Phytosterols
Compositions of phytosterols were analyzed using the procedure described by Rudzińska et al. [31]. Briefly, lipid samples were saponified with 1 M KOH in methanol at room temperature for 18 h, then water was added and unsaponifiables multi extracted with diethyl ether. Dry residues were silylated with BSTFA containing 1% TMCS. Derivatives of the sterols were separated on a Hewlett-Packard 6890 gas chromatograph with an HP-5 capillary column (30 m × 0.32 mm × 0.25 μm; J&W Scientific, Folsom, CA, USA). Split injection with split ratio 1:25 was used. Separation was done isothermally at 290 °C, with a helium flow rate of 1.6 mL/min. The injector and detector temperatures were set at 310 °C. An internal standard, 5-α-cholestane, was used for quantification. Phytosterols were identified by comparison of retention data and by GCMS using a Finnigan Trace 2000 gas chromatograph coupled to a Finnigan Polaris Q quadrupole ion-trap mass spectrometer after separation on a DB-5 capillary column (50 m × 0.2 mm × 0.32 μm; J&W). Helium was used as the carrier gas at a flow rate of 0.6 mL/min. All mass spectra were recorded using electron impact ionization mode at 70 eV and masses were scanned in the range of 100–650 Da. The ion source was held at 200 °C and the injector at 300 °C. A combination of the NIST Mass Spectra Library and collected spectra in the lab were used to identify the sterols.
Gamma-Oryzanol
RBOS and RBMC were analyzed for γ-oryzanol by HPLC as previously reported [32]. A Finnigan Surveyor Plus HPLC system (Thermo Electron, Waltham, MA, USA) was used. A 20 μL sample was injected onto a C18 column (4 μm; 300 × 3.9 mm; Novapak, Waters, MA) held at 30 °C. Separation was achieved by using acetonitrile/water (65:35, v/v) eluant. Finnigan Surveyor photodiode-array detector (PDA) was at 325 nm. The amount of γ-oryzanols is expressed as a sum of all esters separated and quantified by using the external calibration.
Carotenoids
Carotenoids were analysed according to the method of Khachik et al. [33] with modifications. Analyses were carried out on Finnigan Surveyor Plus HPLC system (Thermo Electron, Waltham, MA, USA). A 10 μL sample was injected onto a C18 column (4 μm; 300 × 3.9 mm; Novapak, Waters, MA) held at 25 °C. Gradient system was used as follows: (1) 15% methanol, 75% acetonitrile, 5% methylene chloride and 5% hexane was held for 2 min; (2) at 20 min eluant was changed to 17% methanol, 60% acetonitrile, 11.5% methylene chloride and 11.5% hexane; (3) at 35 min eluant was: 15% methanol, 40% acetonitrile, 22.5% methylene chloride, and 22.5% hexane; followed by returning to the initial eluant composition within 5 min with additional 5 min of equilibration. The flow rate was 0.5 mL/min. Carotenoids were quantified using external calibration using β-carotene as standard.
Analysis of Minor Components Composition
The isolated minor components were separated into lipid classes according to the Silversand and Haux [34] method with modifications. Separation was performed on a Finnigan Surveyor LC (Thermo Electron, Waltham, MA, USA). Components were separated on a normal phase Diol column (5 μm, 250 × 4.6 mm; Monochrom, Varian, CA, USA). The binary gradient was used consisting of: (A) hexane–acetic acid (99:1.0, v/v) and (B) hexane–isopropanol–acetic acid (84:15:1.0, v/v) solvents. The samples of 20 μL were injected and the gradient changed from 0 to 100% of the solvent B within 40 min. The final gradient was kept for 2 min and then returned to the initial composition within 3 min followed by 5 min of equilibration. The flow rate was 0.6 mL/min and the column was kept at 45 °C. Components were detected with a Sedex 75 evaporative light scattering detector (Sedere, Alfortville, France), operated at 30 °C with an air pressure of 1.5 bar. Triolein, diolein, monoolein, oleic acid, and stigmasterol linoleate, were used as standards for external calibration to assess the amount of triacylglycerols (TAG), diacylglycerols (DAG), monoacylglycerols (MAG), free fatty acids (FFA), and sterol esters (SE), respectively.
Quantification of 4-Hydroxynonenal
Isolation and quantification of HNE was carried out according to the method developed in our laboratory and published elsewhere [35]. Briefly, the polar fraction obtained during polar components analysis was utilized. When the non-polar fraction was eluted, the polar fraction was removed from the column with four consecutive elutions using 5 mL of methanol. The combined eluants were subsequently evaporated under gentle stream of nitrogen to 5 mL. The cloudy solution was centrifuged at 2,300 rpm for 5 min, and the clear supernatant analyzed by HPLC.
Analysis of HNE was carried out using a Finnigan Surveyor Plus HPLC (Thermo Electron, Waltham, MA, USA) with a Finnigan Surveyor Autosampler Plus and a Finnigan Surveyor UV–Vis Plus detector. A 20-μL sample was injected onto a C18 column (4 μm; 300 × 3.9 mm; Novapak, Waters, MA). HNE was detected at 223 nm after elution with acetonitrile/water (30:70, v/v) at a constant flow of 0.75 mL/min. Identification of HNE was done by comparison of retention data and by co-elution of HNE standard with selected samples. To further verify the identity of the HNE peak identification was carried out on a QSTAR Elite mass spectrometer (AB SCIEX, Concord, ON, Canada) equipped with an APCI interface operated in a positive mode. Analyst QS 2.0 software was used for data acquisition and analysis. The conditions of mass spectrometric analysis were optimized for 4-hydroxynonenal as follows: the APCI source temperature was set at 450 °C, the curtain gas at 25, the declustering potential at 45 V, the focus potentials at 150 V, and the ion source gas 1 and 2 at 20 and 60 psi, respectively. Quantification of HNE was carried out using external calibration.
Sampling and Quantification of Volatile Carbonyl Compounds
Volatile carbonyl compounds (VCC) generated during the frying experiments was trapped by a Sep-Pak DNPH-Silica cartridge (Waters, Milford, MA) where they were converted to the stable dinitrophenylhydrazones. During trapping the cartridge was connected to a pump with flexible tubing through a flowmeter. The wider end of the cartridge was suspended about 0.5 cm above the upper edge of the beaker used for the frying test, and the samples of vapors were drawn through the cartridge at a flow rate of 350 mL/min. The resulting dinitrophenylhydrazones were eluted from the cartridge with 5 mL of acetonitrile keeping its flow at 2 mL/min.
Dinitrophenylhydrazones were separated and quantified by HPLC using a Finnigan Surveyor Plus HPLC system (Thermo Electron, Waltham, MA, USA). A 20-μL sample was injected onto a C18 column (4 μm; 300 × 3.9 mm; Novapak, Waters, MA) held at 30 °C. Separation was achieved by using the following gradient: (1) 40% acetonitrile and 60% water was held for 5 min; (2) followed by 100% acetonitrile within 40 min and maintained it for 5 min; (3) for 5 min eluant returned to the initial composition followed by 5 min equilibration. The flow rate was 0.5 mL/min, Finnigan Surveyor UV–Vis Plus detector was set at 360 nm. VCC were identified by comparison of retention data with standards and their identity assessed by HPLC–MS (Exactive Bench-Top; Thermo Fischer Scientific, West Palm Beach, FL). The HPLC conditions were similar to the above. The mass spectrometer was equipped with an APCI ion source, operated in the negative mode. The spectra were collected using a mass scan range from 100 to 1,000 Da.
Treatments
Performance of canola oil triacylglycerides was assessed with addition of the following components:
-
1.
Canola oil triacylglycerol (CTG)—control
-
2.
Phospholipids (phosphatidylcholine and phosphatidylethanolamine)
-
3.
Combinations of tocopherol isomers
-
4.
Canola oil tocopherol fraction, TCAN
-
5.
Rice bran oil tocopherol fraction, TRBO
-
6.
Canola oil sterol fraction, SCAN
-
7.
Rice bran oil sterol fraction, SRBO
-
8.
Solvent extracted minor components from rice bran oil, RBOS
-
9.
Minor components isolated by chromatography from rice bran oil, RBMC
-
10.
Solvent extracted minor components from sesame oil, SOS
-
11.
Solvent extracted minor components from palm oil, POS
Statistical Analysis
Results were statistically analyzed by single factor analysis of variance (ANOVA) and regression analyses using Minitab 2000 statistical software (Minitab Inc, PA, ver. 13.2). Statistically significant differences between means were determined by Duncan’s multiple range tests. Statistically significant differences were determined at p ≤ 0.05.
Results and Discussion
Minor Components
In Table 1, the composition of minor components isolated for this study is presented. TCAN and TRBO are fractions rich in tocopherols recovered from canola oil and rice bran oil, respectively. Both fractions contained more than 80% tocochromanols, with an insignificant contribution of sterol esters. The small amounts of sterols are mainly coming from the sterol esters present in oils and having similar chromatographic properties as tocopherols. The corresponding SCAN and SRBO are fractions containing only phytosterols, where the presence of tocopherols was not detected. RBOS and POS are fractions of minor components isolated by solvent extraction from rice bran and palm oils, respectively. Both fractions contained both tocopherols and sterols, reflecting composition of these compounds in the starting oils. In RBOS γ-oryzanols were detected, while as should be expected carotenoids in POS (Table 1). The composition of RBMC is not included in Table 1 because its composition is essentially the combination of compounds present in TRBO and SRBO fractions.
Tocopherols
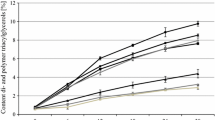
All the indices of performance indicated that tocopherols at all the tested concentrations and homologous compositions protected CTG under frying conditions. For instance, the amount of TPC formed in CTG containing a combination of 100, 450 and 450 μg/g of α-, γ-, and δ-tocopherol, respectively was 28% lower than formed in unprotected CTG at the end of the frying (Fig. 1). A statistically significant increase in the frying performance was observed when the concentration of tocopherol was increased from 500 to 1,000 μg/g (Fig 1). However, a further increase in the tocopherols amount to 2,000 μg/g did not result in a concomitant increase in frying stability of CTG despite the increase in the residual tocopherols amount at the end of frying (Figs. 1, 2). This observation was consistent with the results of previous study by Lampi and Kamal-Eldin [26]. At the concentrations employed in the present work, results indicated that varying the relative proportions of tocopherol homologues in the mixture did not have a significant effect on their ability to protect the CTG during frying. Results by Warner and Moser also showed that varying the ratio of tocopherol homologues has no significant impact on their antioxidant activity when α-, γ-, δ-tocopherols were present together in the oil [36].
Furthermore, as observed for pure tocopherol homologous mixtures (Fig. 1), tocopherols fractions isolated from canola and rice bran oils offered significant level of protection to CTG during frying (Fig. 3). It is interesting that the presence of tocotrienols in rice bran tocochromanol mixtures did not lead to any enhancement in frying performance of CTG fortified with these components over other tested tocopherol compositions. Indeed, Romero et al. [14] found no increase in the antioxidant activity when a mixture of α-tocopherol and α-tocotrienol was added to antioxidants stripped canola oil as compared to when only tocotrienol was added. They, however, reported a reduced activity for the mixture as compared to α-tocopherol alone.
Volatile carbonyl compounds (VCC) are important secondary oxidation products formed during frying causing pollution in frying facility. Twenty-one aldehydes including 9 alkanals, 9 alkenals and 3 alkadienals were detected and quantified in the present study (Table 2; Fig. 4). Propanal and pentenal were the most abundant volatile products formed during thermo-oxidative degradation of linolenic acid, whereas hexanal and pentanal were the major products formed from linoleic acid. Nonanal and octanal, on the other hand, were the major volatile degradation products formed from oleic acid (Table 2). The relatively lower concentrations of 2,4-decadienal and 2,4-heptadienal, the respective oxidation products of linoleic and linolenic acids might be due to further degradation of these compounds to produce other volatile compounds such as ethanal and 2-propenal, under the frying conditions used in the present study [37, 38]. Similarly to TPC, the rate of VCC formation during degradation of unprotected CTG was significantly higher when the tocopherol isomers mixtures were applied. Compared to other tested tocopherol compositions, the system containing 450-α, 450-γ and 100-δ generated the least amount of VCC. An increase in the tocopherol amount from 1,000 to 2,000 μg/g resulted in a significant increase in rate of VCC formation, with the increase more pronounced when higher amounts of α-tocopherol were present (Table 2). Whereas equivalent amounts of tocopherols were used, the rate of VCC formation was lower with TCAN, lowering it further when TRBO was applied. However, the VCC profile was independent of the composition of tocopherol isomers, indicating similar mechanism of degradation [14, 26]. Furthermore, irrespective of the minor components added the CTG, the same trends were observed for the major individual carbonyl compounds such as propanal and hexanal, and the total volatile carbonyl compounds (VCC).
Typical HPLC chromatogram of dinitrophenylhydrazone derivatives of volatile carbonyl compounds. a ethanal, b propenal, c propanal, d 2-butenal, e butanal, f 2-pentenal, g pentanal, h 2-hexenal; i hexanal, j unknown, k 2,4-heptadienal, l 2-heptenal, m heptanal, n 2-octenal, o 2,4-nonadienal, p octanal, q 2-nonenal, r nonanal, s 2,4-decadienal, t 2-decenal, u decanal, v 2-undecenal, w unknown. See the text for HPLC conditions
Results indicated that the formation of HNE was significantly reduced in the presence of tocopherols compared to the control (Fig. 5). At the end of the frying test, the amount of HNE accumulated in oils without tocopherols was 11.7 μg/g, compared to a maximum of 6.2 μg/g in samples containing a mixture of tocopherol isomers. In agreement with the amount of TPC, no significant differences were observed in the amount of HNE formation when all tested tocopherol homologous mixtures were applied (Fig. 5). The ability of tocopherols to restrict the formation of HNE during heating soybean oil at frying temperature has been observed [39].
Phytosterols
Separating tocopherols from sterols furnished a mixture of endogenous phytosterols without tocopherols to study their effect on the frying stability of CTG. Sterol fractions SCAN and SRBO provided a concentration dependent protection for CTG during frying (Fig. 3). When 500 μg/g of SCAN was added, no protective activity was observed. On the contrary, at this low concentration the protection by SRBO was comparable to the observed for endogenous tocopherols isolated from canola or rice bran oils (Fig. 3). This is presumably due to the presence of higher amount of sterols with known antioxidant activity such as an avenasterol in RBO (Table 1). When 3,000 μg/g was added, the protection offered by SCAN was 20% better than tocopherols as measured by the amount of TPC at the end of the frying period (Fig 3). Gertz et al. [4] assessed antioxidative activity using the OSET procedure and reported superior antioxidant activity of canola oil sterols over tocopherols.
CTG fortified with a mixture of SCAN or SRBO and tocopherol isomers accumulated significantly higher amounts of TPC throughout the frying period than those present individually in each of these fractions (results not included). Indeed, whenever tocopherols were added to the sterols fraction, the amount of TPC formed was similar to the amounts accumulated when CTG contained only tocopherols. This indicates lack of synergistic interaction between these components. Thus, the antioxidant effect of sterols might not be realized in the presence of tocopherols, presumably due to the lower activation energy of the latter [26].
The emission rate of VCC was significantly lower when CTG were heated in the presence of sterols at 3,000 μg/g compared to unprotected CTG (Table 2). However, there was no formation of TPC and no significant difference in the emission rate of VCC when CTG were heated with sterols and tocopherols. Furthermore, no significant difference in both the amounts and profiles of volatile carbonyl compounds was produced during heating CTG with SCAN and SRBO fractions at the higher concentration (Table 2; Fig. 4).
The amount of HNE formed during heating CTG fortified with SCAN was 60% lower compared to the control. In agreement with the TPC and VCC formation, no significant differences were observed in HNE formation when SCAN and SRBO fraction were used for protection of CTG (Fig. 6).
Solvent Extracted Minor Components
Fortification of CTG with RBOS, RBMC, SOS and POS at 0.5% resulted in significantly lower amounts of polar compounds formed, indicating lower oxidative degradation (Fig. 7). Compared to RBMC, CTG fortified with RBOS or SOS formed about 19% less TPC under frying. However, no statistically significant differences were observed in the stability of CTG fortified with RBMC, POS, and any of the tocopherol isomers mixtures (Fig 7). Thus, distinctive endogenous minor components such as carotenoids and tocotrienols present in POS and RBMC did not enhance the antioxidant effectiveness of the minor components during frying with CTG. RBOS contained 10.5% γ-oryzanol and 11.8% wax as measured by HPLC and preparative TLC. RBMC, on the other hand, contained insignificant amounts of these minor compounds. Presumably, the significantly higher protective efficiency of RBOS over RBMC could be attributed to the presence of γ-oryzanol and waxes, in addition to tocopherols or potential synergistic effect between these components. Mezouari and Eichner [23] reported a significant antioxidant activity of RBO waxes during frying in sunflower oil. Similarly, the significant protection offered by SOS was possible due to the activity of the sesame lignans such as sesamol, sesaminol, sesamin and sesamolin. The improved frying stability of blends of sesame and canola oils over canola oil alone was attributed to the activity of the sesame oil lignans [20–22]. In a related study, the improved stability of a blend of soybean and sesame oils, and the food fried in it over soybean oil alone was assigned to the sesame oil lignans [40, 41]. According to Hemalatha and Ghafoorunissa [42], the addition of 1.2% of sesame lignans significantly increased the radical scavenging activity and the frying performance of soybean and sunflower oils.
The rate of VCC generation during frying in CTG fortified with RBOS was 1.3 and 2.7 times lower than in CTG containing POS and control, respectively (Table 2). The rate of VCC generation during frying in CTG containing RBOS was 9.8% lower than CTG contained SOS; however, these differences were within the experimental error. No differences were observed in the VCC profiles for CTG fortified with either of RBMC, RBOS, POS and SOS.
The results for HNE formation parallel those of VCC. At the end of the frying period, the amount of HNE detected in CTG fortified with RBOS was 2.7 times lower than the amounts in control (Fig. 6).
Phospholipids
The effects of two phospholipids, namely phosphatidylcholine (PC) and phosphatidylethanolamine (PE) on the frying stability of CTG were examined. As measured by TPC, no significant differences were observed in the ability of both phospholipids to protect CTG during frying (Fig. 8). At a concentration of 500 μg/g, neither PC nor PE offered any protection to CTG. However, at a concentration of 1,000 μg/g, the antioxidant effect of phospholipids became evident (Fig. 8). At the end of the frying test, the amount of TPC accumulated in the control was 2.5 and 2.3 times higher than the amount detected in CTG fortified with PC and PE, respectively (Fig. 8). Furthermore, the results from this study showed that CTG containing 2,000 μg/g PC were 1.9 times more stable than CTG fortified with any tested tocopherol homologous mixtures.
At the low phospholipid concentrations tested, similar effect to sterols was observed when tocopherols were added. However, when the higher amounts of both phospholipids were added, the antioxidant activity was not impeded by the presence of tocopherols. Contrary to some literature reports, lack of synergy was observed between any of the studied phospholipids and tocopherols under the conditions employed in the present study [43, 44]. On the other hand, a statistically significant synergy was observed between the tested phospholipids and RBOS (Fig. 8). This may be due to an interaction between the phospholipids and the phenolic compounds such as γ-oryzanol present in the RBOS. Ramadan [45] reported that quercetin increased the antioxidant activity of soybean lecithin in a triolein model system.
There is rather scanty information available on the application of phospholipids as antioxidants under frying conditions, probably because of their reportedly adverse effects on color and foaming of oils [46]. Kourimska et al. [47] found no significant increase in foaming of olive oil and no effect on the sensory properties of the French fries when lecithin was applied at 0.1%. In the present work, CTG oil darkened at a faster rate when either or both phospholipids were utilized.
Compared to the control, the rate of VCC generation during the frying was 3.1 and 3.5 times slower in the CTG containing PC and PE, respectively (Table 2). Similarly, both PC and PE significantly inhibited the formation of HNE during the frying test. At the end of the frying test, only 2.8 and 2.3 μg/g of HNE were observed in CTG fortified with these phospholipids, respectively, compared to 11.7 μg/g in control (Fig. 6). These results are consistent with the abilities of soybean phospholipids to inhibit HNE formation in fried fish fillets during storage [48]. Despite the higher amount of TPC observed in the samples fortified with PE as compared to PC, the amount of carbonyl compounds, such as VCC and HNE, were generally lower when oil contained PE. This observation was in agreement with previous reports by Hidalgo et al. [49]. Therefore, the possibility of a reaction between the amine group on PE and the carbonyl group is verified, which possibly stimulates drastic color changes [49].
Conclusions
The influence of various endogenous minor components on the frying performance of canola oil triacylglycerols was evaluated. The present study showed that both the composition and the concentration of the minor components exerted a profound influence on the frying performance of the oil. Tocopherols remain the major antioxidants in the frying oils, whereas, the results from the present study indicated that there might be a need to look beyond tocopherols in order to design canola oil with a remarkably improved frying performance. Phosphatidylcholine, phosphatidylethanolamine, and minor components isolated from rice bran and sesame oils particularly enhanced the frying performance of canola oil triacylglycerol.
References
Chiang TA, Wu PF, Ko YC (1999) Identification of carcinogens in cooking oil fumes. Environ Res 81:18–22
Chiang TA, Wu PF, Wang LF, Lee H, Lee CH, Ko YC (1997) Mutagenicity and polycyclic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Mutat Res Fund Mol Mech Mutag 381:157–161
Qu YH, Xu GX, Zhou JZ, Chen TD, Zhu LF, Shields PG, Wang HW, Gao Z (1992) Genotoxicity of heated cooking oil vapors. Mutat Res 298:105–111
Gertz C, Klostermann S, Kochhar PS (2000) Testing and comparing stability of vegetable oils and fats at frying temperature. Eur J Lipid Sci Technol 102:543–551
Clemente TE, Cahoon EB (2009) Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiol 151:1030–1040
Neff WE, El-Agaimy MA (1996) Effect of linoleic acid position in triacylglycerols on their oxidative stability. Food Sci Technol Leb 29:772–775
Neff WE, Mounts TL, Rinsch WM, Konishi H, El-Agaimy MA (1994) Oxidative stability of purified canola oil triacylglycerols with altered fatty-acid compositions as affected by triacylglycerol composition and structure. J Am Oil Chem Soc 71:1101–1109
Fuller G, Kohler GO, Applewhite TH (1966) Fatty acid composition and oxidative stability of cold-pressed edible seed oils. J Food Sci 68:1240–1243
Bhatnagar AS, Prasanth Kumar PK, Hemavathy J, Gopala Krishna AG (2009) Fatty acid composition, oxidative stability, and radical scavenging activity of vegetable oil blends with coconut oil. J Am Oil Chem Soc 86:991–999
Lampi AM, Hopia A, Piironen V (1997) Antioxidant activity of minor amount of γ-tocopherol in natural triacylglycerols. J Am Oil Chem Soc 74:549–554
Abuzaytoun R, Shahidi F (2006) Oxidative stability of alga oils as affected by their minor components. J Agric Food Chem 54:8253–8260
Normand L, Eskin NAM, Przybylski R (2006) Comparison of the frying stability of regular and high-oleic acid sunflower oils. J Am Oil Chem Soc 83:331–334
Normand L, Eskin NAM, Przybylski R (2001) Effect of tocopherols on the frying stability of regular and modified canola oils. J Am Oil Chem Soc 78:369–373
Romero N, Robert P, Masson L, Ortiz J, Gonzalez K, Tapia K, Dobaganes C (2007) Effect of α-tocopherol, α-tocotrienol and Rosa mosqueta shell extract on the performance of antioxidant-stripped canola oil (Brassica sp.) at high temperature. Food Chem 104:383–389
Onal B, Ergin G (2002) Antioxidative effects of alpha-tocopherol and ascorbyl palmitate on thermal oxidation of canola oil. Nahrung 46:420–426
Wanasundara UN, Shahidi F (1994) Canola extract as an alternative natural antioxidant for canola oil. J Am Oil Chem Soc 71:817–822
Shahidi F, Wanasundara UN (1994) Stabilization of canola oil by natural antioxidants. In: Ho CT, Hartman TG (eds) Lipids in food flavors. ACS Symposium Series, Washington, pp 301–314
Xu XQ, Tran VH, Palmer MV, White K, Salisbury P (2000) Chemical, physical and sensory properties of Monola oil, palm olein and their blends in deep frying trials. Food Aust 52:77–85
Farhoosh R, Kenari RE, Poorazrang H (2009) Frying stability of canola oil blended with palm olein, olive, and corn oils. J Am Oil Chem Soc 86:71–76
Farhoosh R, Kenari RE (2009) Anti-rancidity effects of sesame and rice bran oils on canola oil during deep frying. J Am Oil Chem Soc 86:539–544
Alireza S, Tan CP, Hamed M, Che Man YB (2010) Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Inter Food Res J 17:295–302
Serjouie AS, Tan CP, Mirhosseini H, Che Man YB (2010) Effect of vegetable-based oil blends on physicochemical properties of oils during deep-fat frying. Am J Food Technol 5:310–323
Mezouari S, Eichner K (2007) Evaluation of the stability of blends of sunflower and rice bran oil. Eur J Lipid Sci Technol 109:531–535
Kim MA, Matoba T, Hasegawa K (1988) Relationship between the triacylglycerol composition and foaming of mixed coconut oil under deep-fat frying. Agric Biol Chem 52:693–699
Possanzini M, Di Palo V (1995) Determination of olefinic aldehydes and other volatile carbonyls in air samples by DNPH-coated cartridges and HPLC. Chromatographia 40:135–138
Lampi AM, Kamal-Eldin A (1998) Effect of α- and γ-tocopherols on thermal polymerization of purified high-oleic sunflower triacylglycerols. J Am Oil Chem Soc 75:1699–1703
Miraliakbari H, Shahidi F (2008) Oxidative stability of tree nut oils. J Agric Food Chem 56:4751–4759
AOAC (1990) Official methods of analysis of the association of official analytical chemists, 15th edn. AOAC, Arlington, Virginia
Schulte E (2004) Economical micromethod for determination of polar components in frying fats. Eur J Lipid Sci Technol 106:772–776
Firestone D (2009) Official methods and recommended practices of the American Oil Chemists’ Society, 6th edn. AOCS Press, Champaign
Rudzińska M, Muśnicki C, Wąsowicz E (2003) Phytosterols and their oxidized derivatives in seeds of winter oilseed rape. Rośliny Oleiste 24:51–66
Przybylski R, Klensporf-Pawlik D, Anwar F, Rudzińska M (2009) Lipid components of North American wild rice (Zizania palustris). J Am Oil Chem Soc 86:553–559
Khachik F, Goli MB, Beecher GR, Holden J, Lusby WR, Tenorio MD, Barrera MR (1992) Effect of food preparation on qualitative and quantitative distribution of major carotenoid constituents of tomatoes and several green vegetable. J Agric Food Chem 40:390–398
Silversand C, Haux H (1997) Improved high-performance liquid chromatographic method for the separation and quantification of lipid classes: application to fish lipids. J Chromatogr B 703:7–14
Aladedunye F, Przybylski, R. Carbon dioxide blanketing impedes the formation of 4-hydroxynonenal and acrylamide during frying. Eur J Lipid Sci Tech (Accepted)
Warner K, Moser J (2009) Frying stability of purified mid-oleic sunflower oil triacylglycerols with added pure tocopherols and tocopherol mixtures. J Am Oil Chem Soc 86:1199–1207
Frankel EN (2005) Lipid oxidation, 2nd edn. The Oily Press, Dundee
Matthews RF, Scanlan RA, Libbey LM (1971) Autoxidation products of 2,4-decadienal. J Am Oil Chem Soc 48:745–747
Seppanen CM, Csallany AS (2002) Formation of 4-hydroxynonenal, a toxic aldehyde, in soybean oil at frying temperature. J Am Oil Chem Soc 79:1033–1038
Chung J, Lee Y, Choe E (2006) Effects of sesame oil addition to soybean oil during frying on the lipid oxidative stability and antioxidants contents of the fried products during storage in the dark. J Food Sci 71:C222–C226
Chung J, Lee J, Choe E (2004) Oxidative stability of soybean and sesame oil mixture during frying of flour dough. J Food Sci 69:C574–C578
Hemalatha S, Ghafoorunissa (2007) Sesame lignans enhance the thermal stability of edible vegetable oils. Food Chem 105:1076–1085
Kashima M, Cha GS, Isoda Y, Hirano J, Miyazawa T (1991) The antioxidant effects of phospholipids on perilla oil. J Am Oil Chem Soc 68:119–122
Bandara NM, Campos RM, Batista I, Nunes ML, Empis JM (1999) Antioxidant of α-tocopherol and phospholipids. J Am Oil Chem Soc 76:905–913
Ramadan MF (2008) Quercetin increases antioxidant activity of soy lecithin in a triolein model system. LWT Food Sci Technol 41:581–587
Dobarganes C, Marquez-Ruiz G, Velasco J (2000) Interaction between fat and food during deep-frying. Eur J Lipid Sci Technol 102:521–528
Kourimska L, Pokorny J, Reblova Z (1994) Phospholipids as inhibitors of oxidation during food storage and frying. Prehrambeno Technol Biotechnol Rev 32:91–94
Murano Y, Funabashi T, Sekine S, Takeuchi H (2008) Improvement in storage stability of fish fillet using dietary soybean phospholipids. Food Sci Technol Res 14:55–61
Hidalgo FJ, Nogales F, Zamora R (2008) The role of amino phospholipids in the removal of the cito- and geno-toxic aldehydes produced during lipid oxidation. Food Chem Toxicol 46:43–48
Acknowledgments
This work was financed by the Alberta Value Added Corporation, the Agriculture Funding Consortium project #2006F018R and Bioactive Oil Program.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aladedunye, F.A., Przybylski, R. Frying Performance of Canola Oil Triacylglycerides as Affected by Vegetable Oils Minor Components. J Am Oil Chem Soc 89, 41–53 (2012). https://doi.org/10.1007/s11746-011-1887-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1887-8