Abstract
The effects of foliar applied H2O2 on chlorophyll, carotenoids, the non-enzymatic defense system (ascorbic acid), malondialdehyde (MDA) hydrogen peroxide (H2O2) and growth were assessed in roots and shoots of pea (Pisum sativum L.) plants exposed to excess cadmium. In addition, we evaluated the influences of H2O2 spraying on proline, soluble sugars and soluble proteins contents. Excessive cadmium treatment caused reduction in the growth parameters (dry mass, pods and seeds dry weights), chlorophyll and carotenoids contents, roots total free amino acids, roots soluble sugars as well as shoots and roots soluble proteins levels but increased total free amino acids and soluble sugars contents in shoots. Concentrations of hydrogen peroxide and MDA was enhanced under Cd treatment. The foliar treatment of H2O2 alleviated the detrimental effects generated under Cd treatment that represented as increment in pea growth. H2O2 spraying increased photosynthetic pigments, growth characteristics, soluble proteins, and ascorbic acid contents comparing to the control sets not receiving H2O2. Similarly, a higher up-regulation was detected in proline contents of Cd + H2O2 set than Cd group ones at 0.25 mM Cd. Contrarily, malondialdehyde (MDA), soluble sugars and total free amino acids contents of Cd + H2O2 set revealed a lower decrease than Cd group ones especially in roots. The results demonstrated that H2O2 treatment could inverse the harmful effects of cadmium on growth, through inducing the non-enzymatic defense system (ascorbate), proline accumulation, maintenance of chlorophyll in pea leaves and lowering the intensity of H2O2 and lipid peroxidation (MDA).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Naturally, plants are subjected to many adverse environmental circumstances like abiotic and biotic stresses. Trace element stress is of great interest which has remarkable harmful effects on crop growth and productivity (Gill 2014). The increased agricultural dependence on sewage wastewater irrigation, chemical fertilizers and rapid development of industry have increased amount of toxic metals in agricultural soils resulting in detrimental effects on soil–plant environment system (Gadallah and Sayed 2014; Jali et al. 2016).
Cadmium is a poisonous metal and is regarded with a major environmental concern to the agricultural system. The divalent cation (Cd2+) is almost exclusively present in fertilized soils and accompanied with inorganic and organic complexes. Many studies have now found that the free Cd2+ ion or Cd complexes by inorganic ligands is the predominant Cd species exist in the soil solution of most soils. Furthermore, Cd is constantly, cumulated in soil, throughout anthropogenic and natural resources, for example, weathered cadmium-rich rocks, smelting, mining, the application of sewage sludge or excessive use of phosphate fertilizers, and metal contaminated water for crop irrigation (Alloway 2013; Hooda 2010; Kabta-Pendias 2011).
Cd is absorbed rapidly via plant roots and accumulates in plants to concentrations that could potentially cause animal toxicity. Cadmium uptake by plants is affected by array of variables—plant cultivar, pH of soil, salinity, mineralogy and organic matter content, cation interchange ability and concentrations of other nutrients, especially N, P and Zn (Burzynski et al. 2005; Clemens et al. 2013; Migocka et al. 2011; Plaza et al. 2015; Verbruggen et al. 2013). Cadmium solubility is greatest in acidic soil. The toxicity of Cd due to its high solubility and mobility within the ecosystem (Groppa et al. 2012; Jali et al. 2016) affects plant growth, induces necrosis and chlorophyll degradation and changes nutrient absorption, protein metabolism, carbon fixation and membrane functioning (Abdallah et al. 2015; Ahmad et al. 2015; Jali et al. 2016; Khan et al. 2013; Shah et al. 2017; Singh and Prasad 2014). Furthermore, cadmium enhances the activeness of antioxidant enzymes (Peng et al. 2017) and has high affinity towards the sulfhydryl groups of enzymes (Mendoza-Cozatl et al. 2005). Ultimately, cadmium prompts oxidative stress through its elevated affinity for carboxyl, SH and amine groups of the proteins (DalCorso et al. 2008).
Hydrogen peroxide is a paramount cellular molecule, performs numerous functions in metabolism, development and constancy of aerobes (Bienert et al. 2006). It’s generation increased is due to various stress conditions (Neill et al. 2002). Hydrogen peroxide acts as an essential reactive oxygen species in signal transmission paths which activates plant defences against different imposed ecological stresses (Xu et al. 2011). In plants, H2O2 is the mostly stable ROS and can regulate vital metabolic pathways, as defence, development as well as, acclimation (Ślesak et al. 2007) and guard cell signaling (Song et al. 2014).
H2O2 is relatively stable molecule, more diffusible through membranes, considered as a long distance signal component (Vranová et al. 2002), can trigger Ca2+ influxes, protein alterations and gene expression (Bienert et al. 2006).
During the last decades, the acclimatory role of this component in plant has progressively become an interested fact. External H2O2 applications concurrently stimulated multi-tolerance mechanism towards cold, heat, drought and salinity stresses in Zea mays seedlings (Gong et al. 2001). AzevedoNeto et al. (2005) and Uchida et al. (2002) explained that addition of H2O2 to nutrient solution inducts acclimatization to salt stress in maize and rice seedlings. Also, Ismail et al. (2015) reported that H2O2 have regulatory impacts on plant growth, evolution and nutritional value of fruits. Hossain et al. (2015) indicated that H2O2 pretreatment improves abiotic oxidative stress acclimation. On Brassica napus, the hydrogen peroxide pretreatment mitigates cadmium stimulated oxidative stress damage (Hasanuzzaman et al. 2017). Khan et al.(2017) established that the seedlings of Brassica subjected to water-deficient condition that were supplied with H2O2 and Ca2+ recovered from chlorosis, overcoming water loss in plant, and the plants were able to grow normally. The exogenous H2O2 application has been accompanied by an increasing in its endogenous production (Terzi et al. 2014).
Considerable scientists confirmed the detrimental impacts of Cd on the outgrowth of plants, however; publications’ concerning the ameliorating effects of H2O2 in cadmium-stressed plants is scarce. In addition, responses of plants to H2O2 foliar application and Cd stress are not still recognizable. Our study was performed with the assumption that H2O2 application able to modulate the adverse influence of cadmium stress on pea growth. Therefore, we investigated effects of foliar H2O2 spraying on improving cadmium stress tolerance of Pisum sativum and whatever the protecting effect was associated with some metabolic regulation in the shoots and roots tissues.
Materials and methods
Growth conditions and treatments
Seeds of Pisum sativum L. (cultivar Master B) were achieved from the Agricultural Research Center, Giza, Egypt. Plants was cultivated in plastic pots holding 4 kg of clean and air dry soil (clay/sand 2:1) in the experimental greenhouse in normal field conditions of humidity, temperature, light, and day/night pattern at Botany and Microbiology Department, Faculty of Science, Assiut University (Egypt). Extract of this soil records an electrical conductivity (EC) and pH as 0.876 mS cm−1 and 7.83, respectively. Three plants in each pot were allowed to grow for 5 weeks; water content of the soil kept at field capacity. Plants irrigated twice with 500 cm3 full strength nutrient solution (Down and Hellmers 1975). The stock nutrient solution composited (mM) of: KNO3 100; Ca (NO3)2 100; MgSO4·7H2O 100; NH4H2PO4 100; KCl 50; H3BO3 25; MnSO4·H2O; ZnSO4·7H2O2; CuSO4·5H2O 0.5; H2MoO4 0.5 and Fe·EDTA 20 mM.
Various treatments applied for pot experiments were classified to control (C), Cd stress (Cd) and, Cd stress combined with H2O2 (Cd + H2O2). Five-week-old plants were irrigated with (900 ml/pot) 0.00, 0.125, 0.250, 0.500 and 1 mM CdCl2·2.5 H2O. Soil was irrigated 5 times at 3 day intervals with these solutions. Cadmium solution was applied without nutrient solution. After 2 weeks of Cd supplying one set of the plants (0.0, 0.125, 0.250, 0.500 and 1 mM CdCl2·2.5 H2O) was foliar sprayed with distilled water, the second set was foliar sprayed with 1 m M H2O2 solution, and the third set was foliar sprayed with 2 m M H2O2 solution. Foliar applications were done three times at 3 day intervals. H2O2 solutions were prepared from stock solution (1 M/100 ml distilled water). All treatments took place at the same time (at the end of the day). The concentration of Cd and H2O2 were chosen from the preliminary results. Randomly, five replicates were allocated to each treatment combination at each application. Seven days following preceding foliar (three times at 3 days intervals) H2O2 applications, the plants were analyzed.
Determination of soil electric conductivity (EC) and pH value
Electric conductivity (EC) of the soil was measured employing conductivity meter (model 4310 JEN WAY), as stated by the methods from Jackson (1967). Soil water extracts (1:5) was prepared by shaking 40 g of dry soil with 200 ml distilled water for 2 h, then filtrated to obtain a clear filtrate. Soil reaction of the filtrate was measured using electric pH-meter (model pH-206, Lutron).
Determination of photosynthetic pigments
Chlorophylls (a and b) and carotenoids were extricated from fresh leaves (0.25 g in 10 ml 95% ethyl alcohol) and absorbance readings measured spectrophotometrically (Unico UV-21 00, Unico, USA). The absorption was measured at 645 nm (Chl a), 663 nm (Chl b) and 470 nm (carotenoids). Chlorophylls and carotenoids concentrations (as mg g−1 FW) were estimated using equations as cited by Wellburn (1994).
Hydrogen peroxide (H2O2) determination
H2O2 content was determined by crushing 0.5 g fresh tissues of plants with 5 ml of trichloroacetic acid (TCA 0.1%) and centrifuged at 12,000×g for 15 min at 4 °C. To 0.5 ml of the supernatant, 0.5 ml of 10 mM potassium phosphate buffer (pH = 7.0) and 1 ml of 1 M KI were added. Absorbance was measured at 390 nm (Unico UV-21 00, Unico, USA). Concentration of H2O2 estimated as μmol g−1 FW (Velikova et al. 2000).
Determination of malondialdehyde (MDA)
A lipid peroxidation level was assessed by determination of malondialdehyde (MadhavaRao and Sresty 2000). 0.2 g fresh tissues sample of plants was crushed in 5 ml 0.1% TCA and centrifuged at 10,000×g for 5 min. 4 ml of 20% TCA containing 0.5% thiobarbituric acid (TBA) was added to 1 ml of the supernatant aliquot. Mix was incubated at 95 °C for 15 min and immediately cooled. The non-specific absorbance of the supernatant at 600 nm was deducted from the maximal absorbance at 532 nm utilizing spectrophotometer (Unico UV-21 00, Unico, USA). The concentration (μmol g−1 FW) of malonydialdhyde was recorded using (ϵ = 155 mM−1 cm−1).
Determination of ascorbic acid content
Ascorbic acid concentration (μmol g−1 FW) assayed as designated by Mukherjee and Choudhuri (1983) through mingling 2 mol l−1 Folin-Ciocalteu reagent and 10% TCA with 20% of fresh tissue homogenate. After 10 min of centrifugation, the blue colour established in the supernatant was measured at 760 nm (Unico UV-21 00, Unico, USA). Ascorbic acid concentration was determined from a standard curve using different concentrations of ascorbic acid.
Determination of soluble carbohydrates and nitrogen metabolites
Contents of soluble sugars, total free amino acids and soluble proteins were recorded spectrophotometrically in hot water plant extract of both root and shoot tissues.
Content of soluble sugars was measured using phenol-sulfuric acid procedure of Dubois et al. (1956). One ml of 5% (v/v) phenol followed by 5 ml of sulfuric acid was appended, respectively, to a known volume of plant sample. The previous mixture was stirred and cooled in room temperature for 15 min. Absorbance was registered at 490 nm. Calibration curve using glucose was constructed.
Amino acids and soluble protein contents were determined utilizing the ninhydrin reagent (Lee and Takahashi 1966) and folin–phenol reagent (Lowry et al. 1951) procedures. Calibration curves using glycine and bovine serum albumin as standard was, respectively, constructed.
Proline content determination
Proline extracted from plant fresh tissue samples, its concentration was recorded following the methods of Bates et al. (1973). Fresh tissue samples were powdered in 3% sulphosalicylic acid; centrifuged at 3000×g for 20 min. The supernatant reacted with glacial acetic acid, ninhydrin reagent, boiled for 1 h and cooled. The developed colour was detached in toluene stratum and the absorbance estimated at 520 nm spectrophotometrically (Unico UV-21 00, Unico, USA). Proline was stated as μmol g−1 FW.
Statistics
Analysis of variance (ANOVA) with post hoc Duncan (1955) Multiple Comparison test was performed applying SPSS of Windows (Ver. 13.0, SPSS Inc., USA). Significance concerning the means among control and treatments were estimated using probability level p < 0.05. The values of H2O2 treatment were compared with those of Cd at each Cd level.
The relative role of each factor on the entire influence of treatment combination was calculated from the coefficient of determination (η2).
Experimental results
Growth
Results in Table 1 indicated that increased cadmium concentrations lowered shoots and roots biomass. Pods and seeds dry weights decreased progressively with rising cadmium concentrations. Number of seeds showed significant decrease with increasing Cd concentrations. The H2O2 foliar spray increased the yield characteristics in the Cd-stressful plants. Effectively it increases dry weight of shoots, roots, seeds and pods.
Photosynthetic pigments
Content of chlorophylls (a and b) was declined gradually with rising cadmium concentrations (Table 2). Cadmium at the concentrations 0.500 and 1 mM diminished considerably the content of the carotenoids in comparison with control.
However, the H2O2 foliar treatment could decrease the negative influence of imposed Cd on photosynthetic pigments. Supplementation with H2O2 increased Chlorophylls a, b and carotenoids content at Cd-stressed plants over cadmium concentration range from 0.250 to 1 mM Cd but decreased its contents in Cd-unstressed (0 mM) and low stressed plants (0.125 mM Cd).
Soluble sugars
Cadmium stress enhanced soluble sugars content in shoots but reduced the contents in roots (Table 3). Treatment with H2O2 reduced the contents of soluble sugars in roots of Cd-stressed and unstressed plants. Shoots showed similar response at Cd concentration of 0.250 and 0.500 mM Cd and opposite response was found in unstressed (0 Cd) and highly stressed (1 mM Cd) plants. On the other hand H2O2 foliar application increased soluble sugars content in shoots, especially at higher Cd concentrations (0.5 and 1.00 mM).
Soluble proteins and total free amino acids
Soluble proteins amounts in roots and shoots decreased progressively during increasing cadmium concentration (Table 3). Total free amino acids content showed a similar response in roots but opposite trend was found in shoots where shoots of Cd-stressed plant had higher total amino acids contents than plants without Cd. Foliar spraying with H2O2 increased soluble proteins content in shoots of Cd unstressed and stressed plants. In roots, the same response was noticed at higher Cd concentrations (0.500 and 1 mM Cd), however, in Cd free plants or those received low Cd concentrations (0–0.250 mM Cd) soluble proteins was lower than those not supplemented with H2O2.
In the existence or deficiency of Cd, amino acids contents in each of shoots or roots showed low concentrations in plant sprayed with H2O2 (Cd-unstressed and Cd stressed plants at 0.500 mM were exceptions).
Ascorbic acid, hydrogen peroxide and MDA
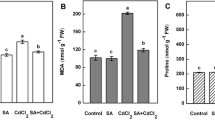
Data in Table 4 revealed cumulative amounts of ascorbic acid and as well as peroxidized lipids in roots and shoots of growing pea plants in response to Cd exposure. Lower concentration of Cd2+, decreased hydrogen peroxide content in shoots, whereas the higher Cd levels (0.500 and 1 mM cadmium) increased the content. In roots, hydrogen peroxide content increased gradually with increasing cadmium concentration.
Foliar supplementation of H2O2 increased ascorbic acid content in the shoots. In roots low (1 mM) H2O2 concentration decreased ascorbic acid content but the concentration of 2 mM H2O2 induced slightly increase in ascorbic acid content in Cd-supplied plants. Two used H2O2 concentrations decreased malondialdehyde (M DA) content in roots and shoots of Cd-untreated as well as treated plants, except for shoot in Cd untreated plants. In shoots, MDA content was increased with elevation of Cd concentration (compared to control). H2O2 application (1 mM and 2 mM) decreased the concentration of MDA contents especially at high Cd concentration (compared to their controls at each H2O2 treatment). Furthermore, the content of MDA was suppressed in roots treated with H2O2 (1 mM and 2 mM) compared to their controls (at each Cd level).
Hydrogen peroxide content (Table 4) enhanced as a result of H2O2 treatment within roots of plants without Cd as well as in Cd applied plants. In shoots, its content increased at Cd concentrations 0, 0.125 and 0.250 m M Cd and down-regulated at higher Cd levels.
Proline
Proline content (Table 4) decreased gradually with increasing Cd concentration in both shoots and roots. The foliar supplementation with H2O2 increased proline contents in shoots of Cd treated plants over Cd concentration range from 0.250 to 1 mM. Opposite response was observed in roots at 0.125 and 0.25 mM Cd for both H2O2 concentrations used.
Role of Cd and H2O2 in plant stress and their interactions
Statistical analysis present in Table 5 indicated that the effects of cadmium (Cd), hydrogen peroxide, (H2O2) and the interaction (Cd × H2O2) were significant for most variables tested as indicated by F values. Further analysis of data through computation of the coefficient of determination (η2) that represented the proportional share of (Cd), (H2O2) and (Cd × H2O2) on the total influence of treatment combination (Table 5) signified to those: (1) Cadmium was predominant in affecting shoots and roots dry weights, fruit (pods) dry weight, shoot soluble proteins, Chl a and carotenoids. (2) Hydrogen peroxide had dominant effects on roots TAA, SP and SS and number of seeds. (3) The share of interaction (Cd × H2O2) was dominant in affecting shoots TAA and SS and shoots and roots proline, ascorbic acid, H2O2 and Chl b content. (4) Cd and Cd × H2O2 interaction had equal dominant share in affecting MAD contents in shoots and roots. (5) Cd, H2O2 and the interaction (Cd × H2O2) seem to play duality share in their subordinate influence.
Discussion
Plants perform several mechanisms to compete against the adverse effects of pollution. These mechanisms may be enhanced by the addition of chemicals to plants (Gadallah 1995). Adaptation of Pisum sativum plants to toxic effect of Cd as expressed in various metabolic changes and growth improvement was enhanced by exogenously added H2O2. Generally, the H2O2 applied as foliar spray affected positively the growth and yield aspects in the cadmium suffered plants. This positive effect could be attributed to an increased in photosynthetic pigments.
Hydrogen peroxide treatment mitigates the injuries of the several abiotic stressors like cadmium stressor in rice (Bai et al. 2011; Hu et al. 2009). Guzel and Terzi (2013) reported that hydrogen peroxide increases water content, growth, mineral concentration, total sugar content, soluble protein and proline content compared to their relative (H2O2 free) sets in young maize plants.
In our work, H2O2 supplying increased photosynthetic pigments that permitted high photosynthetic activities and increased shoots dry matter content (Khandaker et al. 2012). In addition, the lower H2O2 accumulation induced by the H2O2 supplementation at higher Cd concentration is evidence that plants of Pisum sativum could regulate oxidative injuries generated by ROS in the photosynthetic apparatus and retain leaf gas exchange (Gondium et al. 2013). The prevented chlorophyll degradation due to H2O2 addition may be assigned to retain lower hydrogen peroxide content and higher leaf relative water content in leaves under abiotic stresses (Chakraborty et al. 2012; Gondium et al. 2013; Khan et al. 2017).
Though, accurate metabolism of the defensive action of H2O2 treatment at low doses against different stressors particularly Cd stress is still un-interpreted.
Generally, Pisum sativum plants supplemented with H2O2 had low contents of soluble sugars in their shoots and roots could be due to the enhancement of sugars utilization for the formation of new cells and tissues.
Data of present study indicated that cadmium-induced loss of soluble protein and stimulation of amino acids accumulation disappeared when the Pisum sativum plants were treated with H2O2 could be due to decrease oxidative stress of proteins by H2O2, retaining of the structure of proteins or/and an increased protein synthesis.
Supplementation of H2O2 increased proline contents in the tissues of cadmium stressed pea. Together, our data and results of Yang et al. (2009) showed that external H2O2 application caused a significant accumulation of proline in radicles and coleoptiles of maize seedlings. Proline accumulation recognized as a monitor of a biotic stress and regarded as essential protecting agent (Gadallah 1999). This increase in this amino acid content could be due to: (a) an increment in proline biosynthesis (Charest and Phan 1990), (b) a reduction in proline degradation, (c) the induction of a proline-producing enzyme and the inhibition of the catabolic enzyme proline oxidase (Nayyar 2003) and (d) a decrease in proline utilization.
These increment in proline content might possibly due to its several functions i.e. redox-regulation, osmo-regulation, protection against damage by ROS and metal chelation (Guzel and Terzi 2013). Hyat et al. (2013) reported that the foliar treatment of Cicer arietinum with proline caused the mitigation of the negative effects initiated by cadmium introduction. Accordingly, our data pointed to the inducement of ROS scavenging bio components e.g. proline in pea plants treated by hydrogen peroxide under cadmium stress.
Several studies showed that the addition of H2O2 at low doses might benefit plant resistance to heavy metal exposure (Bai et al. 2011; Gondim et al. 2010; Hu et al. 2009; Lin et al. 2004, 2012; Xu et al. 2011). The enhanced tolerance towards metallic stress might due to stimulated antioxidant defence mechanism following treatment by H2O2 in rice plants (Bai et al. 2011; Hu et al. 2009).
Amongst all undesirable effects stimulated by cadmium, malondialdehyde formation is the most detrimental as it can imply to cell membrane deterioration (Nazar et al. 2012). In this study malondialdehyde (MDA) significantly increased after treatment with Cd. Similar upward trend in MDA was indicated in cotton suffered from Cd toxicity (Khan et al. 2013). Conversely, H2O2 application induced downward regulation in MDA contents in shoots and roots in cadmium-treated Pisum sativum plants compared to their controls at each Cd level. The high content of malondialdehyde and increasing activity of antioxidant enzymes is an ideal detector in determining cadmium tolerance in Fragaria x ananassa plant (Muradoglu et al. 2015).
Endogenous hydrogen peroxide content raised in response to H2O2 treatment at roots of pea plants (without Cd as well as in Cd-exposed plants) and in shoots (in plants exposed to low Cd concentrations) that was in agreement with results of Xu et al. (2011). Terzi et al. (2014) found that endogenic hydrogen peroxide concentration lightly increased in hydrogen peroxide pretreated seedlings comparing to H2O2 free. This increase resulted from permeation of externally applied H2O2 to maize leaves. On the other hand, H2O2 application decreased H2O2 content at elevated Cd concentration (compared to their H2O2 sprayed controls at 1 mM and 2 mM) in pea shoot. Hasanuzzaman et al. (2017) and Hossain et al. (2015) noticed that hydrogen peroxide treatment mitigates Cd-induced oxidative stress via regulation of the antioxidant protective and glyoxalase mechanism in Brassica napus L. They concluded that the increment of both the enzymatic and non-enzymatic antioxidants benefit in decrement the oxidative injury as cleared by reduced amounts of MDA as well as H2O2.
Exogenous application of H2O2 improved the content of important reactive oxygen species scavenge components, GSH, AsA as well as the antioxidant enzyme activities that stimulated ROS scavenge pathway (Hasanuzzaman et al. 2017; Hossain et al. 2015). Supplementation with 2 mM H2O2 increased the contents of ascorbic acid in highly Cd-treated Pisum sativum plants that are related directly to hydrogen peroxide scavenge metabolism (Ashraf 2009; Blokhina et al. 2003; Gill and Tuteja 2010). Xu et al. (2011) noticed that hydrogen peroxide stimulated up-regulation of ascorbic acid and metabolism related to aluminum acclimation in Triticum aestivum L. plants. According to Noctor and Foyer (1998), the ascorbate able to react directly reactive oxygen species hence stimulate oxidative defense contra various stressful conditions. Furthermore, it was observed that the ascorbic acid and the alterations in ascorbate redox status are instantly related to stress acclimation in different plant species (Wang et al. 2010; Xu et al. 2011).
The present data indicated significant interactions between cadmium stress and H2O2 and their effects on the variables examined as indicated by F values. So as, in natural habitats the plants not only respond to the environmental factors as separate factors, however, also affected by their interactions. At certain cases e.g. shoots TAA, shoots SS, shoots and roots proline content, ascorbic acid, H2O2 and leaf chlorophyll b content, the relative impact of the interaction between the single factors was dominant but the role of separate factors was subsidiary or the minor one, though even significant.
Conclusions
Results illustrated that treatment with H2O2 enables Pisum sativum plant to endure the injurious effect of cadmium, causing improvement in growth, seeds and pods quality. The cadmium resistance prompted by H2O2 foliar treatment is due to decrease in endogenous MDA and H2O2 contents at higher Cd levels and enhancement of the non-enzymatic defense system (ascorbate), accumulation of proline especially in shoot and maintenance of chlorophyll content in Pisum sativum leaves. These characteristics promote oxidative protection against Cd stress and allow Pisum sativum plants to sustain increment of metabolic rates in cadmium stressful condition and ameliorate the growth. Finally, it can be concluded that H2O2 treatment in sub-lethal doses can exert an ameliorative effect and helped Pisum sativum plants to grow successfully in the areas subjected to cadmium pollution, such as in mining or smelting area.
Author contribution statement
SS and MG have a same contribution towards experiment design, attainment of data, analysis and performance data and prearranging of the manuscript. The ultimate manuscript was read and established via SS and MG.
Abbreviations
- AsA:
-
Ascorbic acid
- Chl:
-
Chlorophyll
- LP:
-
Lipid peroxidation
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SS:
-
Soluble sugars
- SP:
-
Soluble proteins
- TAA:
-
Total free amino acids
- TCA:
-
Trichloroacetic acid
References
Abdallah EF, Hashem A, Alqarawi AA, Alwathnani HA (2015) Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscularmycorrhizal fungi. Pak J Bot 47:785–795
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LP (2015) Alleviation of cadmium toxicity in Brassica juncea L (Czern & Coss) by calcium application involves various physiological and biochemical strategies. PLoS ONE 10(1):e0114571
Alloway BJ (2013) Trace metals and metalloids in soils and their bioavailability. Environmental pollution, vol 22, 3rd edn. Springer, Dordrecht
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
AzevedoNeto AD, Prisco JT, Enéas-Filho J, Medeiros JR, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol l 162:1114–1122
Bai XJ, Liu LJ, Zhang CH, Ge Y, Cheng WD (2011) Effect of H2O2 pretreatment on Cd tolerance of different rice cultivars. Rice Sci 18:29–35
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994–1003
Blokhina O, Virolainen E, Fagerstedt V (2003) Antioxidants, oxidative damage and oxygen deprivation stress. A review. Ann Bot 91:179–194
Burzynski M, Migocka M, Klobus G (2005) Cu and Cd transport in cucumber (Cucumis sativus L.) root plasma membranes. Plant Sci 168:1609–1614
Chakraborty K, Sairam RK, Bhattacharya RC (2012) Differential expression of salt overly sensitive pathway genes determines salinity stress tolerance in Brassica genotypes. Plant Physiol Biochem 51:90–101
Charest C, Phan CT (1990) Cold acclimation of wheat (Triticum aestivum) properties of enzymes involved in proline metabolism. Physiol Plant 80:159–168
Clemens S, Aarts MG, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18(2):92–99
DalCorso G, Farinati S, Maistri S, Furini A (2008) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50:1268–1280
Down RJ, Hellmers H (1975) Environment and experimental control of plant growth. Acad Press, London, New York, San Francisco, p 145
Dubois M, Gilles KA, Hamilton JK, Rabers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356
Duncan DB (1955) Multiple ranges and multiple F-test. Biometrics 11:1–42
Gadallah MAA (1995) Effect of cadmium and kinetin on chlorophyll content, saccharides and dry matter accumulation in sunflower plants. Biol Plant 37:233–240
Gadallah MAA (1999) Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol Plant 42:249–257
Gadallah MAA, Sayed SA (2014) Impacts of different water pollution sources on antioxidant defense ability in three aquatic macrophytes in Assiut Province, Egypt. J Stress Physiol Biochem 10:47–61
Gill M (2014) Heavy metal stress in plants: a review. Int J Adv Res 2(1043):1055
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gondim FA, Gomes-Filho E, Lacerda CF, PriscoJ T, André D, AzevedoNetoA D, Marques EC (2010) Pretreatment with H2O2 in maize seeds: effects on germination and seedling acclimation to salt stress. Braz J Plant Physiol 22:103–112
Gondium FA, Miranda RD, Gomes-Filho E, Prisco JT (2013) Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theor Exp Plant Physiol 25(4):251–260
Gong M, Chen B, Li ZG, Guo LH (2001) Heat-shock-induced cross adaptation to heat, chilling, drought and salt in maize seedlings and involvement of H2O2. J Plant Physiol 158:1125–1130
Groppa MD, Ianuzzo MP, Rosales EP, Vazquez SC, Benavides MP (2012) Cadmium modulates NADPH oxidase activity and expression in sunflower leaves. Biol Plant 56:167–171
Guzel S, Terzi R (2013) Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot Stud 54:26
Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BHN, Fujita M (2017) Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci 8(115):1–10
Hooda PS (ed) (2010) Trace elements in soils. Wiley, Chichester
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li Hong-Yu, Burritt DJ, Fujita M, Tran L-SP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:1–19
Hu Y, Ge Y, Zhang C, Ju T, Cheng W (2009) Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Reg 59:51–61
Hyat S, Hayat Q, Alyemeni MN, Ahmad A (2013) Proline enhances antioxidative enzyme activity, photosynthesis and yield of Cicer arietinum L. exposed to cadmium stress. Acta Bot Croat 72:323–335
Ismail SZ, Khandaker MM, Mat N, Boyce AN (2015) Effects of hydrogen peroxide on growth, development and quality of fruits: a review. J Agron 14(4):331–336
Jackson ML (1967) Soil chemical analysis. New Delhi, Prentice-Hall of India, Private limited New Delhi, p 498
Jali P, Pradhan C, Das AB (2016) Effects of cadmium toxicity in plants: a review article. Sch Acad J Biosci 4(12):1074–1081
Kabta-Pendias A (2011) Trace elements in soils and plants. CRC Press, Taylor and Francis Group, Boca Raton
Khan DM, Mei L, Ali B, Chen Y, Cheng X, Zhu SJ (2013) Cadmium-induced up regulation of lipid peroxidation and reactive oxygen species caused physiological, biochemical, and ultrastructural changes in upland cotton seedlings. Bio Med Res Int 2013:10
Khan A, Anwar Y, Hasan MM, Iqbal A, Ali M, Alharby HF, Hakeem KR, Hasanuzzaman M (2017) Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 6(2):20
Khandaker M, Boyce AN, Osman N (2012) The influence of hydrogen peroxide on the growth, development and quality of wax apple (Syzygium samarangense, [Blume] Merrill and L.M. Perry var. jambumadu) fruits. Plant Physiol Biochem 53:101–110
Lee YP, Takahashi T (1966) An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem 14:71–77
Lin Q, Chen Y, Wang Z, Wang Y (2004) Study on the possibility of hydrogen peroxide pretreatment and plant system to remediate soil pollution. Chemosphere 57:1439–1447
Lowry OH, Resbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin–phenol reagent. J Biol Chem 193:265–275
MadhavaRao KV, Sresty TV (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Mendoza-Cozatl D, Loza-Tavera H, Hernandez-Navarro A, Moreno-Sanchez R (2005) Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protests and plants. FEMS Micro Biol Rev 29:653–671
Migocka M, Papierniak A, Kosatka E, Klobus G (2011) Comparative study of the active cadmium efflux systems operating at the plasma membrane and tonoplast of cucumber root cells. J Exp Bot 62(14):4903–4916
Mukherjee SP, Choudhuri MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and H2O2 in Vigna seedlings. Plant Physiol 58:166–170
Muradoglu F, Gundogdu M, Ercisli S, EncuT Balta F, Jaafar HZE (2015) Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res 48:11
Nayyar H (2003) Variation in osmoregulation in differentially drought-sensitive wheat genotypes involves calcium. Biologia Plant 47:541–547
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NF (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signaling. Curr Opin Plant Biol 5:388–395
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol 49:249–279
Peng Q, Chen W, Wu L, Bai L (2017) The uptake, accumulation, and toxic effects of cadmium in barnyard grass (Echinochloa crus–galli). Pol J Environ Stud 26(2):779–784
Plaza S, Weber J, Pajonk S, Thomas J, Talke IN, Schellenberg M, Pradervand S, Burla B, Geisler M, Martinoia E, Krämer U (2015) Wounding of Arabidopsis halleri leaves enhances cadmium accumulation that acts as a defense against herbivory. Biometals 28(3):521–528
Shah K, Mankad AU, Reddy MN (2017) Cadmium accumulation and its effects on growth and biochemical parameters in Tagetes erecta L. J Pharmacogn Phytochem 6(3):111–115
Singh A, Prasad SM (2014) Effect of agro-industrial waste amendment on Cd uptake in Amaranthus caudatus grown under contaminated soil: an oxidative biomarker response. Ecotoxicol Environ Saf 100:105–113
Ślesak I, Libik M, Karpinska B, Karpinski S, Miszalski Z (2007) The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim Pol 54:39–50
Song Y, Miao Y, Song CP (2014) Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol 201:1121–1140
Terzi R, Kadioglua A, Kalaycioglua E, Saglamb A (2014) Hydrogen peroxide pretreatment induces osmotic stress tolerance by influencing osmolyte and abscisic acid levels in maize leaves. J Plant Interact 9:559–565
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523
Velikova V, Yordanov L, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants, protective role of exogenous polyamines. Plant Sci 151:59–66
Verbruggen N, Juraniec M, Baliardini C, Meyer CL (2013) Tolerance to cadmium in plants: the special case of hyperaccumulators. Biometals 26(4):633–638
Vranová E, Inzé D, Breusegem FV (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Wang Y, Li JL, Wang JZ, Li ZK (2010) Exogenous H2O2improves the chilling tolerance of manila grass and mascarene grass by activating the antioxidative system. Plant Growth Reg 61:195–204
Wellburn AR (1994) The spectral determination of chlorophyll a and b, as well as total caretenoids, using various solvent with spectrophotometers of different solution. J Plant Physiol 144:307–313
Xu FJ, Jin CW, Liu W, Zhang YS, LinX Y (2011) Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J Integr Plant Biol 54:44–53
Yang SL, Lan SS, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166(15):1694–1699
Acknowledgements
We express our gratitude to the respectful reviewers for helpful, purposeful comments to improve the manuscript and present this work in its fullest required form.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
The authors stressed that they have not any kind of conflict concerning interest.
Additional information
Communicated by G. Klobus.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sayed, S., Gadallah, M. Hydrogen peroxide supplementation alleviates the deleterious effects of cadmium on photosynthetic pigments and oxidative stress and improves growth, yield and pods quality of pea (Pisum sativum L.) plants. Acta Physiol Plant 41, 113 (2019). https://doi.org/10.1007/s11738-019-2901-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2901-2