Abstract
The present study was conducted to investigate the effect of exogenous salicylic acid (SA) added to the nutrient solution on the growth parameters and the functions of the photosynthetic apparatus of rice plants under cadmium (Cd) stress. Our investigations have shown that 10 µM SA has an optimal effect in rice plants grown hydroponically. Pulse amplitude modulated chlorophyll fluorescence, low-temperature chlorophyll fluorescence, oxygen evolution (measured with Clark-type and Joliot-type electrodes) and P700 photo-oxidation measurements were carried out to assess the effect of SA on the activity of the photosynthetic apparatus. The levels of three important parameters associated with oxidative stress (hydrogen peroxide, lipid peroxidation and proline content) were measured. The application of low concentration of SA significantly decreased the levels of hydrogen peroxide, lipid peroxidation and proline under Cd stress. The results revealed that low concentration of SA, applied in plants exposed to 150 µM CdCl2, significantly improves plant growth, photochemical activities of both photosystems, the electron flow from QA to plastoquinone, energetic distribution between pigment-protein complexes and the kinetic parameters of oxygen-evolving reactions. This study suggests that exogenous application of 10 µM SA through the rooting medium has a protective effect against Cd toxicity in rice plants. The possible molecular mechanisms involved in the defence effect of SA on the function of photosynthetic apparatus are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals are one of the major environmental pollutants, whose concentrations have been increasing continuously in the soil and in the water, and their quantities are often sufficient to present a risk to the human health. Plants grown in metal-polluted environments, exhibit altered metabolism, growth and biomass reduction, lower crop yields and metal accumulation (for review see Parmar et al. 2013; Tran and Popova 2013).
Cadmium (Cd) is one of the most toxic heavy metals because of its high solubility in water, its easy absorption through the roots and its accumulation in plant tissues (see Li et al. 2017). This metal strongly influences plant morphology and physiology, and also reduces the effectiveness of photosynthesis (Tran and Popova 2013; Arivazhagan and Sharavanan 2015). It has been found that with the increase of the Cd concentration the number and the size of the chloroplasts decrease, as well as the stacking degree of the thylakoid membranes (Hakmaoui et al. 2007; Moussa and El-Gamal 2010). The changes in the thylakoid membrane organization are a result of membrane lipid peroxidation, which is mediated by the formation of free radicals during heavy metal exposure (Vassilev et al. 2004). A significant degradation of the membrane lipids has been observed under Cd stress, resulting in damage of the pigment-protein complexes of the photosynthetic apparatus in thylakoid membranes (Vassilev et al. 2004; Nouairi et al. 2006). Previous investigations have shown a decrease in the amount of monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), and phosphatidylglycerol (PG), caused by Cd treatment, as well as a decrease in 16:1 trans fatty acid contents, that leads to disorganization of the granal structure (Djebali et al. 2005).
A decrease in the amount of chlorophylls as a result of the replacement of Mg ions with Cd ions in chlorophyll a and chlorophyll b molecules has been found in plants exposed to Cd (Moussa and El-Gamal 2010; Parmar et al. 2013). Reported data about Cd impact on carotenoids are controversial and depend on plant species (for review see Parmar et al. 2013).
It has been proposed that Cd induces changes in both photosystems (Atal et al. 1991; Wodala et al. 2012), which depend on the genotype and the ecotype of the plant species (Prasad 1995). Furthermore, it is well known that under abiotic stress the photosystem II (PSII) complex is more strongly influenced than photosystem I (PSI). Cadmium ions affect both the donor and the acceptor sites of the PSII complex, which leads to an increase of the amount of the inactive centers (Atal et al. 1991). Moreover, the effect of Cd ions on the oxidizing side of PSII leads to uncoupling of the electron transport in the chloroplasts (Atal et al. 1991). It has been also established that Cd displaces Ca ions from the Mn4Ca cluster and thus changes the functions of the oxygen-evolving complex (OEC) on the donor site of PSII, while on the acceptor site it causes some modifications in the secondary quinone acceptor QB and delays the electron flow from QA to QB (Faller et al. 2005). Previous studies have shown that Cd ions also affects the functioning of PSI (Sárvári et al. 2008; Atal et al. 1991). Having in mind the highly toxic effect of Cd, it is of great importance to investigate the different ways to decrease its influence on rice plants and thus to reduce the risk to human health.
Salicylic acid (SA) influences a number of metabolic and physiological processes and thus promotes plant growth and development (Singh et al. 2010). Exogenous application of SA influences a range of developmental and physiological processes, e.g., seed germination and fruit yield (Cutt and Klessing 1992), transpiration rate (Larque-Saavedra 1979), stomatal closure (Rai et al. 1986), membrane permeability (Barkosky and Einhellig 1993), plant growth and photosynthesis (Khan et al. 2003; Khodary 2004; El-Tayeb 2005). In addition, it has been shown that the application of SA reduces root to shoot translocation of Cd and increases the activities of antioxidant enzymes in both roots and shoots of plants exposed to Cd (Wang et al. 2013). Previous studies have shown that exogenous SA plays a key role in promoting plant resistance to various abiotic stress factors (Metwally et al. 2003; El-Tayeb 2005; Guo et al. 2007; Hayat et al. 2010; Tang et al. 2017). Salicylic acid has long been found as one of the endogenous growth regulators that reduces heavy metal toxicity in plants (see Horváth et al. 2007). It has been reported that SA decreases the growth inhibition induced by Cd toxicity in rice (Guo et al. 2007), barley (Metwally et al. 2003), soybean (Drazic and Mihailovic 2005) and wheat (Moussa and El-Gamal 2010). Guo et al. (2007) have shown that pretreatment with low concentration (10 μM) of SA enhanced the antioxidant defence mechanisms in rice roots exposed to Cd, thus alleviating Cd-induced oxidative damages and enhancing Cd tolerance. Therefore, the exogenous SA may indirectly alleviate Cd toxicity through different response mechanisms in plants, some of which include the regulation of the antioxidant machinery and lipid metabolism, leading to maintenance of membrane integrity and protection of membranes (Mishra and Choudhuri 1999; Guo et al. 2007; Tamás et al. 2015). Furthermore, the effect of SA depends on numerous factors such as the species and the developmental stage of plants, the mode of application and its concentration (Horváth et al. 2007; Maslenkova et al. 2009). It has been also suggested that low concentrations of SA might cause enhanced tolerance toward most kinds of abiotic stress factors, while higher concentrations of exogenous SA cause oxidative stress in plants, partially through the accumulation of hydrogen peroxide (Yuan and Lin 2008). Our preliminary investigations have found that 10 µM SA applied on rice seedlings through the nutrient solution has an optimal effect on the growth and the functional activity of the photosynthetic apparatus in rice plants (Yotsova et al. 2018).
Despite the numerous studies, the molecular mechanisms underlying the potential defensive effects of exogenous SA on the photosynthetic apparatus of plants are still not well understood. This study was conducted to assess whether the exogenous application of low concentration of SA through the nutrient solution could reduce Cd toxicity in rice plants, which will contribute to ensuring food safety, grain quality and reducing Cd risk to human health. In particular, in the present study, we investigated the effect of 10 µM SA on the functions of the photosynthetic apparatus and some growth parameters of rice seedlings exposed to 150 µM CdCl2. A detail analysis of the changes in PSI photochemistry as well as in PSII donor and acceptor side has also been made.
2 Materials and methods
2.1 Plant material and growth conditions
Seeds of rice (Oryza sativa L. Galileo) were sterilized and germinated on moisture filter paper in dark at 28 °C for 5 days. After germination, the rice seedlings were grown hydroponically for 14 days and were divided into four groups: (1) control (grown only on half-strength Hoagland’s nutrient solution), (2) rice plants grown on nutrient solution supplied with 10 µM SA, (3) rice plants exposed to150 µM CdCl2 in the nutrient solution and (4) rice plants grown on nutrient solution with 10 µM SA and 150 µM CdCl2. It was used plastic containers (1 L with 60 plants per container) filled with a half-strength Hoagland’s nutrient solution with some modifications: 2.5 mM KNO3, 2.5 mM Ca(NO3)2, 1 mM MgSO4, 0.5 mM NH4NO3, 23 μM H3BO3, 4.5 μM MnCl2, 0.4 μM ZnSO4, 0.2 μM CuSO4, 0.25 μM Na2MoO4 and 20 μM Fe-EDTA (pH 6.0). Considering the assumption for the formation of a complex between Cd and SA, we used GEOCHEM-EZ (Shaff et al. 2010) to calculate the especiation of free Cd2+ in the nutrient solution supplied with 10 µM SA. The results revealed that 88.96% of the added Cd is available as free metal ion. All experiments were conducted in a growth chamber under controlled conditions: light intensity of 150 μmoles m−2 s−1 with a 12 h light/dark photoperiod and relative humidity over 75% for 14 days. During the whole period of cultivation, the nutrient solutions were aerated and changed every 5 days. At the end of the cultivation (at 14 day), the lengths of the shoots and roots were measured. The other measurements were performed on detached leaves or isolated thylakoid membranes. The thylakoid membranes were isolated according to the procedure described by Apostolova et al. (2006). Five independent experiments were made for each treatment, which were analyzed independently.
2.2 Pigment analysis
The pigments were extracted from leaves with ice-cold 80% acetone. The amounts of chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids (Car) were determined spectrophotometrically according to the equations of Lichtenthaler (1987) using Specord 210 Plus (Edition 2010, Analytik Jena AG, Germany).
2.3 Determination of oxidative stress markers
The content of hydrogen peroxide (H2O2) was determined by following the protocol of Alexieva et al. (2001) with minor modifications. 100 mg of leaf tissue were crushed in 1% trichloroacetic acid (TCA) and homogenized at 4 °C and then centrifuged at 14000 g for 20 min. The reaction mixture consisted of 0.5 mL 1% TCA leaf extract supernatant, 0.5 mL of 100 mM Na–K-phosphate buffer (pH 7.6) and 1 mL reagent (1 M KI w/v in fresh double-distilled water). The blank probe consisted of 1% TCA with no leaf extract. The reaction was developed for 1 h in the dark and then the absorbance was measured at 390 nm. The amount of H2O2 was calculated with a molar extinction coefficient of 0.28 μM−1 cm−1 and expressed as nmol per g fresh weight (FW).
The level of lipid peroxidation was determined by estimating malondialdehyde (MDA) using thiobarbituric acid (TBA) reaction (Heath and Packer 1968). MDA is a decomposition product of peroxidized polyunsaturated fatty acid component of membrane lipids. The leaf tissues (100 mg) were homogenized with 1% (w/v) TCA followed by centrifugation at 14 000 g for 20 min. The reaction mixture consisted of 0.5 mL 1% (w/v) TCA leaf extract supernatant, 0.5 mL of 100 mM Na–K-phosphate buffer (pH 7.6) and 1mL reagent [20% (w/v) TCA containing 0.5% (w/v) TBA]. The mixture was heated at 95 °C for 30 min and then cooled in an ice bath to stop the reaction. The tubes were centrifuged at 12000 g for 5 min, and the absorbance of the supernatant was measured at 532 nm. The MDA concentration was determined by its molar extinction coefficient (0.155 μM−1 cm−1) and expressed as nmol per g FW (Heath and Packer 1968).
The proline in the rice leaves was extracted and measured according to the method of Bates et al. (1973) with minor modifications. 100 mg of leaf tissue were homogenized with 3 ml of 3% sulfosalicylic acid and filtered through filter paper (Grade 601 Qualitative Filter Paper). One mL of the extracted solution was reacted with an equal volume of glacial acetic acid and ninhydrin reagent (500 mg ninhydrin in 12 mL of glacial acetic acid and 8 mL of 6 M H3PO4) and incubated at 95 °C for 1 h. The reaction was terminated by placing the samples in ice bath. The reaction mixture was vigorously mixed with 2 mL toluene and the absorbance was recorded at 520 nm. Proline content was calculated from a standard curve and expressed as nmol per g FW.
2.4 Photochemical activity of PSII
The photochemical activity of PSII (PSII-mediated electron transport) was measured on isolated thylakoid membranes with polarographic Clark-type electrode (Model DW1, Hansatech, Instruments Ltd., England) in temperature-controlled cuvette at 22 °C, using saturating white light. The PSII activity was determined by the rate of oxygen evolution in the reaction medium containing: 20 mM MES (pH 6.5), 10 mM NaCl, 5 mM MgCl2, 330 mM sucrose and an exogenous electron acceptor 1,4-benzoquinone (0.4 mM BQ). The chlorophyll concentration in the thylakoid membranes for the measurements of photochemical activity was 25 μg mL−1.
2.5 Kinetic parameters of oxygen evolution
Oxygen flash yields and initial oxygen burst were measured using a self-built polarographic oxygen rate electrode described in Zeinalov (2002). The isolated thylakoid membranes (300 μg Chl mL−1 and 100 μl volume) were suspended in a medium containing: 40 mM HEPES (pH 7.6), 10 mM NaCl, 5 mM MgCl2 and 400 mM sucrose, without artificial electron acceptor. Thylakoid samples formed a thin layer on the platinum electrode. Samples were pre-illuminated with 20 flashes and then dark-adapted for 10 min on the electrode before measurements. Oxygen flash yields were induced by short and saturating periodic flash sequences with 700 ms dark intervals between the flashes. The initial oxygen burst amplitude (A) was recorded after irradiation with continuous white light (400 μmol photon m−2 s−1).
The initial S0–S1 state distribution in the dark, misses (α) and double hits (β) were determined by the least square deviations fitting the experimentally obtained oxygen flash yields to the theoretically calculated yields according to the model of Kok et al. (1970). The parameters SB (the concentration of the blocked PSII centers) and KD (the turnover time constant of the OEC) were obtained using extended kinetic version of the Kok’s model on the base of the measurements by variation of the intervals between the flashes: 1.0, 0.70 and 0.55 s (for details see Zeinalov 2009; Yotsova et al. 2017).
2.6 Pulse amplitude modulated (PAM) chlorophyll fluorescence
The measurements of PAM chlorophyll fluorescence were made using a fluorimeter (H. Walz, Effeltrich, Germany, model PAM 101-103) as described by Stefanov et al. (2017). The leaf discs were dark-adapted for 15 min. The maximal fluorescence levels Fm in dark-adapted state (DAS) and Fm′ in light-adapted state (LAS) were measured with 2500 µmol m−2 s−1 illumination provided by Schott lamp KL 1500 (Schott Glaswerke, Mainz, Germany). The intensity for determination of the minimal fluorescence level (F0) in DAS was 0.02 µmol m−2 s−1. The intensity of the actinic light was 150 µmol m−2 s−1. The following parameters were determined: Fv/Fm—the maximum quantum yield of PSII (Kitajima and Butler 1975); Fv/F0—the ratio of photochemical to nonphotochemical processes (Rohacek 2002); Fv′/Fm′—the effective quantum yield of PSII (Rohacek 2002); ФPSII—the effective quantum yield of photochemical energy conversion of PSII; qP—the photochemical quenching coefficient (Schreiber et al. 1986); ETR—the linear electron transport rate (Genty et al. 1989). The chlorophyll fluorescence decrease ratio, RFd (RFd = Fd/(Fm − Fd)), which correlates with the photosynthetic rate (Lichtenthaler et al. 2005), was measured as in Stefanov et al. (2016). Fd is the fluorescence decrease from Fm to steady state chlorophyll fluorescence.
2.7 Low temperature fluorescence measurements
The low-temperature (77 K) chlorophyll fluorescence emission spectra of thylakoid membranes (chlorophyll concentration 20 µg mL−1) were measured using a Jobin–Yvon (JY3) spectrofluorimeter equipped with a liquid-nitrogen device. The samples were quickly frozen in a cylindrical quartz cuvette by plunging into liquid nitrogen. The chlorophyll fluorescence was excited at 436 nm (for Chl a) with slit widths of 4 nm.
2.8 P700 redox state measurements
The measurements were made in vivo using a PAM-101/103 fluorometer (Walz, Effeltrich, Germany) equipped with an ED-800T emitter-detector as described by Dobrikova et al. (2017). The oxidation–reduction kinetics of P700 was determined by illumination of the dark-adapted (for 15 min at room temperature) detached leaves with far red light supplied by a photodiode (102-FR, Walz, Effeltrich, Germany). The redox state of P700 was assessed by measuring of far red light induced absorbance changes around 830 nm (∆A830).
2.9 Statistical analysis
The mean values for all investigated parameters were calculated from five independent experiments with 3–4 replicates per each treatment. Statistical differences between mean values (± SE) were determined using ANOVA. Values of p < 0.05 was considered as statistically significant differences.
3 Results
3.1 Plant growth and pigment content
Treatment of rice seedlings with 10 µM SA alone increased the growth parameters, and the effect was more pronounced in shoots than in roots (Table 1). The increase of shoots and root lengths was of 45 and 20%, respectively. These SA-induced changes in the shoots elongation were accompanied by an increase in the total Chl content with about 7% (Table 1). The exposure of rice seedlings to 150 µM CdCl2 for 14 days led to a decrease of the shoot and roots lenghts and total chlorophyll content (Table 1). In comparison to the control, Cd reduced the shoot lenght by 72% (Table 1). The contents of Chl and Car reduced by 40% after Cd treatment in comparison to the untreated plants. The inhibitory effects of Cd on the growth parameters and the pigment contents of rice seedlings were alleviated by exogenous SA added to the nutrient solution (Table 1).
3.2 Oxidative stress markers—lipid peroxidation (MDA), hydrogen peroxide and proline levels
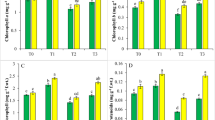
The oxidative damage of the membranes under Cd stress was investigated by monitoring of the MDA content. Our data revealed that Cd caused a significant increase (up to 90%) in the concentration of MDA in comparison to the control, while in the plants treated with SA and Cd the MDA content was lower (the increase was about 20%) than in the plants treated with Cd alone (Fig. 1B). No differences in the levels of lipid peroxidation were observed between the control plants and those treated with SA alone.
Leaf H2O2 content showed an increment of approximately 25% in plants treated with Cd alone, and it was lowered in rice plants treated with SA and Cd combined. There was no difference in the H2O2 content between the control and the rice plants treated solely with 10 µM SA (Fig. 1A).
The highly elevated levels of proline also indicated the toxic effect of Cd (Fig. 1C). Compared to the control, proline concentrations increased approximately three times in plants exposed to Cd. The plants treated with SA and Cd combined exhibited significantly lower proline levels than those treated with Cd alone. There were no differences between control plants and plants treated only with SA.
3.3 Photochemical activity of PSII and photosynthetic oxygen evolution
10 µM SA stimulates the PSII activity by 30%. On the other hand, Cd caused strong inhibition of the PSII activity (about 46%) and the addition of SA to plants exposed to Cd alleviated the inhibitory effect of Cd on the PSII activity.
The maximum amplitude of the flash-induced oxygen yields observed after the third flash (Y) and the oxygen-burst amplitude under continuous illumination (A) were used to assess the impact of SA on the oxygen production in plants exposed to Cd (Fig. 2). The parameter A correlates with the number of all functionally-active PSII reaction centers (i.e., fast and slow operating centers), while the parameter Y characterizes mainly the fast-operating PSII centers situated in grana domains (see in Apostolova et al. 2006). Exogenous application of SA causes a stimulation of the oxygen evolution in thylakoid membranes as shown by the increase of the parameters A and Y (Fig. 2). 150 µM CdCl2 caused a significant reduction in the amplitudes of the oxygen evolution (Y and A) which was diminished in plants grown with SA and CdCl2 together (Fig. 2). SA applied through the rooting medium has a protective effect against Cd-induced inhibition of the oxygen evolution in rice seedlings.
Effects of 10 µM SA and 150 µM CdCl2 on the flash-induced oxygen yields observed after the third flash (Y), the oxygen-burst amplitude under continuous illumination (A) and the photochemical activity of PSII (H2O → BQ), measured in thylakoid membranes isolated from 14-day old rice seedlings. Different letters indicate significant differences at p < 0.05
Cd treatment led to an increase in the amount of the blocked PSII centers (SB), the initial dark distribution of the PSII centres in the S0 state, the misses (α) and the double hits (β), indicating alterations in the Mn cluster and/or damage of the OEC on the PSII donor side (see Yotsova et al. 2017).
10 µM SA alone strongly increased the rate constant of oxygen evolution (KD) with 42%, while Cd treatment led to a 17% decrease in comparison to the thylakoid membranes of control plants (Table 2). Cd treatment resulted in delayed turnover time of the oxygen-evolving centers for a release of one oxygen molecule and increased the amount of the blocked PSII centers by 43%. Furthermore, these Cd-induced alterations in OEC were alleviated by exogenous application of SA (Table 2).
3.4 PAM chlorophyll fluorescence parameters
Cd caused inhibition of ΦPSII as a result of the decrease of the ratio of the photochemical to non-photochemical processes (Fv/F0) (Fig. 3A). Exogenous SA in plants exposed to Cd decreased the Cd-induced changes in ΦPSII—it reached values exhibited by the control plants, and led to an increase of the Fv/F0 ratio. Salicylic acid also stimulated photochemical quenching (qP) and ETR, and increased the fluorescence decay ratio, RFd. Analysis of the PAM chlorophyll fluorescence curves of leaves from Cd-treated plants revealed inhibition of these parameters. The application of SA in plantsexposed to Cd alleviated the inhibition effects of Cd on the following parameters: qP, ETR and RFd (Fig. 3B). The data revealed that the primary photochemistry of PSII (Fv/Fm) is almost unchanged after treatment with SA and Cd together.
Effects of 10 µM SA and 150 µM CdCl2 on PAM chlorophyll fluorescence parameters, measured in leaves of 14-day old rice seedlings: (A) Fv/Fm—the maximum quantum yield of PSII photochemistry; Fv/F0—the ratio of the photochemical to the nonphotochemical processes; ФPSII—the effective quantum yield of the photochemical energy conversion of PSII and (B) qP—the photochemical quenching coefficient; ETR—the linear electron transport rate; the chlorophyll fluorescence decrease ratio, RFd (RFd = Fd/(Fm − Fd)), which correlates with the photosynthetic rate. Different letters indicate significant differences at p < 0.05
The relaxation curves after single saturating pulse in dark-adapted leaves could be fitted by two components (fast and slow component) with rate constants k 1 and k 2 for the fast and the slow components, respectively. Our data showed that Cd leads to a decrease of both constants while no changes were observed after Cd treatment in the presence of SA (Table 3).
3.5 Low-temperature (77 K) chlorophyll fluorescence
The fluorescence emission spectra of rice thylakoid membranes have three bands at 685, 695 and 744 nm. The 685 and 695 nm fluorescence bands originate from PSII, while the band at 744 nm represents the PSI fluorescence (Krause and Weis 1991). Salicylic acid did not influence the energy redistribution between both photosystems, while in plants exposed to Cd the energy transfer from PSII to PSI was increased, i.e., the F744/F685 ratio increased in comparison to untreated plants (Fig. 4). The Cd-induced increase in the ratio F744/F685 was not registered in the presence of SA. In addition, Cd in the presence of SA did not influence the energy transfer between pigment-protein complexes in PSII (Fig. 4), i.e., the F685/F695 ratio was not changed.
Effects of 10 µM SA and 150 µM CdCl2 on the energy transfer in the photosynthetic apparatus determined by low-temperature (77 K) chlorophyll fluorescence: F744/F685—the energy transfer from PSII to PSI, and F685/F695—the energy transfer between the pigment-protein complexes in PSII. The fluorescence ratios are measured for the excitation of Chl a (at 436 nm). Different letters indicate significant differences at p < 0.05
3.6 Oxidation–reduction kinetics of P700
For characterization of PSI photochemistry we have measured the steady-state P700 photo-oxidation (P700+) by far red light-induced absorbance changes around 830 nm (ΔA830). The values of relative amplitudes (∆A/A) from control and treated plants are shown in Table 4. The results showed a 10% increase of PSI photochemistry (ratio ∆A/A) after treatment with SA alone. In contrast, this parameter decreased by 20% after Cd treatment and it was almost not changed in the presence of SA in plants exposed to Cd (Table 4). The post illumination dark-reduction kinetics of P700+ were fitted by two decay exponents with half-times t 1 (for the fast component) and t 2 (for the slow component). The addition of SA alone leads to a 20–25% decrease of both half-times (t 1 and t 2 ), and Cd caused a 47% reduction in t 1 and a 50% decrease in t 2 . After treatment with SA and Cd together the fast half-time t 1 was further reduced (Table 4).
4 Discussion
Salicylic acid has an important role in the protective mechanisms against heavy metal toxicity, activating antioxidant defence system and reducing the generation of superoxide radicals, thus lowering the level of lipid peroxidation and maintaining the stability of the membranes (Guo et al. 2007, Wang et al. 2013; Tamas et al. 2015; Khan et al. 2015). It has been demonstrated that SA pretreatment decreases MDA accumulation, caused by Cd, which confirmed the role of this compound against oxidative damage (see Moussa and El-Gamal 2010). It is known that Cd is involved in the indirect production of reactive oxygen species (ROS), causes peroxidation of membrane lipids in the cellular environment, whose intensity can be estimated by the accumulation of MDA (Schützendübel et al. 2002; Liu et al. 2003; Singh et al. 2006; Guo et al. 2007; Hsu and Kao 2007; Xu et al. 2010; Wang et al. 2011). The increase of MDA amount is an indicator of a high level of oxidative stress (Hou et al. 2007). The main target of the redox-active metals in a plant cell is usually the cell membrane (Yilmaz and Parlak 2011). Our results revealed that the lipid peroxidation in the leaves of rice plants treated with Cd and SA combined was lower than that in plants exposed to Cd alone (Fig. 1B). Similar results were also observed for pretreated with SA pea seeds (Popova et al. 2009) and after applying SA and Cd together through the nutrient solution on perennial rye-grass and common duckweed (Wang et al. 2013; Lu et al. 2018).
Proline accumulates in plants under stress conditions, so it is a relevant indicator of heavy metal stress (Krantev et al. 2008). Our results showed that Cd leads to accumulation of proline in rice leaves (Fig. 1C), which is in agreement with the results reported in previous studies for other plant species: Groenlandia densa (Yilmaz and Parlak 2011), perennial rye-grass (Wang et al. 2013) and Lemna minor (Lu et al. 2018). The decrease in proline concentration in rice seedlings treated with SA and Cd combined suggested a partial alleviation of Cd toxicity by the concentration of SA used in the present study.
Previous studies have shown that Cd can promote the generation of H2O2 in a variety of plants (Maksymiec and Krupa 2006; Rodríguez-Serrano et al. 2006, 2009; Vestena et al. 2011; Zhao et al. 2012). Additionally, Wang et al. (2013) have suggested that H2O2 plays an important role in plant growth inhibition induced by heavy metals in plants. Our data showed that Cd increases H2O2 levels in rice leaves, while adding SA to the solution lowers H2O2 concentrations and reduces the toxic effect of Cd. Similar results were obtained after combined treatments of Cd and SA in the roots of perennial rye-grass and common duckweed (Wang et al. 2013; Lu et al. 2018).
Cadmium is well known to have a harmful effect also on the photosynthesis affecting the chlorophyll metabolism and the chloroplast ultrastructure (Djebali et al. 2005; Hakmaoui, et al. 2007; Arivazhagan and Sharavanan 2015; Parmar et al. 2013). Therefore, the reduction in the pigment content can indicate the toxic effect of Cd on plants. Our data revealed a strong decrease of the chlorophyll and carotenoid contents in leaves of Cd-treated rice seedlings (Table 1), which is most probably a result of inhibition of the chlorophyll biosynthesis (see in Parmar et al. 2013). The Cd-induced reduction in pigment composition was also registered in wheat (Moussa and El-Gamal 2010; Dobrikova et al. 2017), maize (Maurya et al. 2008; Arivazhagan and Sharavanan 2015) and soybean seedlings (Xue et al. 2013). Additionally, Cd-induced changes in the chlorophyll content were accompanied by an increase of the Chl a/b ratio (Table 1). Having in mind that this ratio correlates with the amount of the light harvesting complex of PSII (LHCII) and the degree of membrane stacking (Apostolova et al. 2006, Stoichkova et al. 2006), it could be suggested that Cd treatment caused changes in the organization of the thylakoid membranes, as a result of the increased membrane lipid peroxidation (Fig. 1). All these alterations in the pigment composition were alleviated in the presence of SA in plants exposed to Cd, and the Chl a/b ratio was similar to that in the control plants (Table 1).
The inhibitory effect of Cd on the pigment content corresponds to the reduction of the growth parameters (Table 1), which was alleviated by the exogenous SA applied through the roots. Previous observations by Krantev et al. (2008) on maize plants revealed similar effects. It was suggested that the defensive effect of SA is a result of a very rapid detoxification of ROS, which ensures protection of cells against oxidative damage induced by Cd. A recent study has shown that the addition of SA in the nutrient solution has a protective role against arsenite stress in rice plants, resulting in partial restoration of all plant growth parameters, and especially shoot and root lengths, as well as total chlorophyll content—up to control levels (Singh et al. 2017). One of the possible reasons for the beneficial effect of SA on Cd toxicity could be due to the reduced root-to-shoot translocation of Cd (Wang et al., 2013). Furthermore, the study of Famita et al. (2014) using four rice cultivars has revealed that independently of the ratio of SA (100 µM) to Cd concentrations (100–1500 µM) Cd accumulation in the roots is not influenced, and only a small decrease in Cd content in shoots has been registered.
The Cd-induced changes in the organization of the thylakoid membrane correlated with the observed increase in the energy transfer from PSII to PSI (ratio F744/F685, Fig. 4). This could be a result of the Cd-influence on the LHCII organization, by reducing the amount of the trimers of this complex and eventually the efficiency of light energy utilization (Croce and van Amerongen 2011). Data also revealed that the treatment with SA prevented the Cd-induced increase of the energy transfer from PSII to PSI (ratio F744/F685) (Fig. 4). Our results also indicated that the application of exogenous SA in plants exposed to Cd might be responsible for the decrease of the membrane injuries preventing structural changes and/or swelling of the thylakoid membranes, which is in agreement with previous observations on wheat plants (Moussa and El-Gamal 2010). Moreover, it has been also shown that treatment with SA only does not induce any ultrastructural changes in chloroplasts (Moussa and El-Gamal 2010).
The main targets of Cd ions in the photosynthetic apparatus are electron transfer reactions in PSII complex, whose alterations result in an inhibition of the photosynthetic oxygen evolution (Atal et al. 1991; Chugh and Sawhney 1999; Vassilev et al. 2004). It has been also proposed (Sigfridsson et al. 2004) that Cd exerts multiple effects on both donor and acceptor sides of PSII—it modifies the QB pocket on the acceptor side of PSII and blocks the electron transfer from QA to QB. In this study, we evaluated the impact of Cd on the re-oxidation of Q −A by assessing the relaxation kinetics of chlorophyll fluorescence excitation after single saturating pulse in dark-adapted leaves (see Bukhov et al. 2001; Shirao et al. 2013). Our results showed a reduction of the constants of the chlorophyll fluorescence decay (k 1 and k 2 ) after excitation by a saturating light pulse which suggests a restriction of the electron flow from QA to plastoquinone (Table 3). These Cd-induced alterations in PSII acceptor side correspond to the inhibition of effective quantum yield (ΦPSII), the photochemical quenching (qP), the linear electron transport rate (ETR) and the parameter RFd, which correlates with the net CO2 assimilation (Lichtenthaler et al. 2005) (Fig. 3). Having in mind that chlorophyll a fluorescence is often used by biological researchers as an indicator of changes in plants under different abiotic stress factors (Dąbrowski et al. 2015, 2016; Kalaji et al. 2017), our data suggest a strong influence of Cd on the functions of the photosynthetic apparatus. The Cd-induced inhibition of PAM parameters was diminished by the addition of SA in the nutrient solution. It has been shown that pre-soaking maize seeds with SA has a protective effect on the photosynthesis by diminishing the oxidative damage in plants exposed to Cd (Krantev et al. 2008).
Sigfridsson et al. (2004) have also suggested that Cd rapidly inhibits the functional activity of PSII affecting the donor side between OEC and the first electron donor of P680 (YZ). Our data revealed alterations in the kinetic parameters of the oxygen-evolving reactions suggesting a modification of the Mn4Ca cluster (Table 2), as well as strong inhibition of the oxygen evolution under flashes and continuous illumination (Fig. 2). The damage of the OEC, as well as the increasing of the amount of PSII centers in S0 state and the misses, could be a result of Cd-displacement of the essential Ca2+ cofactor in the Mn4Ca cluster (Faller et al. 2005), as well as a result of a decrease of the active PSII centers (i.e., increased amount of blocked PSII centers, SB) (Table 2). In addition, the observed stronger inhibition of the flash-induced oxygen evolution (Y) in comparison to the oxygen burst (A) under continuous illumination (Fig. 2) suggests that the sets of PSII in grana domain (faster PSIIα centers) are stronger influenced by Cd stress than PSIIβ centers. These results are in agreement with the assumption made by Atal et al. (1991) for Cd-induced reduction of the active PSIIα centers. Previous analysis of the oxygen evolution kinetics has also showed Cd-induced increase in the number of inactive PSII reaction centres, as well as a reduction in the size of the PSII antennae and in the number of active PSIIα reaction centres (in grana domains) in wheat seedlings (Atal et al. 1991; Dobrikova et al. 2017). The addition of exogenous SA in plants exposed to Cd improved the kinetic parameters of oxygen-evolving reactions (Table 2) suggesting the protection of OEC from damage or modifications.
The dark-reduction kinetics of the far red induced absorption changes around 830 nm (ΔA/A) were used to assess the effects of Cd on the PSI photochemical activity (Bukhov et al. 2002; Wodala et al. 2012). Our data showed an increase in the extent of P700 oxidation (P700+, i.e., ΔA/A) in the presence of SA only. Compared to the control plants, Cd decreased the relative amplitudes (ΔA/A) accompanied by some reduction in the half-times t 1 and t 2 (Table 4). It has been proposed that the biphasic kinetics of the dark reduction of P700+ after turning off the far red light are due to a reduction of two different populations of PSI located in different domains of the thylakoid membranes or originate from two electron-donor systems (Albertsson 1995; Bukhov et al. 2002). The combined treatment with SA and Cd led to a strong decrease of the fast half-time t 1 which indicates an increase of the cyclic electron transport around PSI in the stroma lamellae as a defense mechanism. Similar impact of Cd on the photochemistry of PSI has been shown in pea (Wodala et al. 2012) and wheat plants (Dobrikova et al. 2017). Having in mind that both half-times were influenced by Cd (Table 4), it could be supposed that Cd affects both PSI population: in grana margins and in stroma lamellae, while the presence of SA in plants exposed to Cd has a more pronounced effect on the fast time t 1 , i.e, on the cyclic electron transport around PSI situated in the stroma lamellae. The observed changes in the functions of PSI under Cd stress could be a result of the destruction of the iron-sulfur centers and/or of the PSI antenna complex (see Atal et al. 1991; Chugh and Sawhney 1999; Parmar et al. 2013).
5 Conclusions
This study showed that the exogenous application of low concentration of SA (10 µM) through the rooting medium alleviated Cd-induced inhibition of the photosynthetic apparatus in rice seedlings. Our study showed that the lipid peroxidation, the levels of H2O2 and proline were lowered after combining treatments with SA and Cd compared to Cd treatment alone. In addition, our results revealed that the exogenous SA has a beneficial effect on the photosynthetic apparatus of Cd-stressed rice plants maintaining the membrane integrity and increasing the cyclic electron transport around PSI in thylakoid membranes as defense mechanism. The diminished Cd-induced inhibition of the oxygen evolution in the presence of exogenous SA could be a result of its influence on the kinetic parameters of oxygen-evolving reactions preventing Mn-cluster of OEC from damage or modifications.
References
Albertsson PA (1995) The structure and function of the chloroplast photosynthetic membrane—a model for the domain organization. Photosynth Res 46:141–149
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Apostolova EL, Dobrikova AG, Ivanova PI, Petkanchin IB, Taneva SG (2006) Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J Photochem Photobiol B 83:114–122
Arivazhagan V, Sharavanan PS (2015) Effect of cadmium on photosynthetic responses and biochemical contents of maize plants. Am J Environ Eng Sci 2:32–36
Atal N, Saradhi PP, Mohanty P (1991) Inhibition of the chloroplast photochemical reactions by treatment of wheat seedlings with low concentrations of cadmium: analysis of electron transport activities and changes in fluorescence yield. Plant Cell Physiol 32:943–951
Barkosky RR, Einhellig FA (1993) Effects of salicylic acid on plant water relationship. J Chem Ecol 19:237–247
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bukhov N, Samson G, Carpentier R (2001) Non photosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress. The pool size of stromal reductants. Photochem Photobiol 74:438–443
Bukhov N, Egorova E, Carpentier R (2002) Electron flow to photosystem I from stromal reductants in vivo: the size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 215:812–820
Chugh LK, Sawhney SK (1999) Photosynthetic activities of Pisum sativum seedlings grown in presence of cadmium. Plant Physiol Biochem 37:297–303
Croce R, van Amerongen H (2011) Light-harvesting and structural organization of photosystem II: from individual complexes to thylakoid membrane. J Photochem Biochem B Biol 104:142–153
Cutt JR, Klessing DF (1992) Salicylic acid in plants: a changing perspective. Pharm Technol 16:25–34
Dąbrowski P, Pawluśkiewicz B, Baczewska-Dąbrowska A, Oglecki P, Kalaji HM (2015) Chlorophyll a fluorescence of perennial ryegrass (Lolium perenne L.) varieties under long term exposure to shade. Zemdirbyste-Agriculture 102(3):305–312
Dąbrowski P, Baczewska AH, Pawluśkiewicz B, Paunov M, Alexantrov V, Goltsev V, Kalaji MH (2016) Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J Photochem Photobiol B 157:22–31
Djebali W, Zarrouk M, Brouquisse R, Kahoui ES, Limam F, Ghorbel MH, Chaïbi W (2005) Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersicon esculentum) chloroplast membranes. Plant Biol 7:358–368
Dobrikova AG, Yotsova EK, Börner A, Landjeva SP, Apostolova EL (2017) The wheat mutant DELLA-encoding gene (Rht-B1c) affects plant photosynthetic responses to cadmium stress. Plant Physiol Biochem 114:10–18
Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci 168:511–517
El-Tayeb MA (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–224
Faller P, Kienzler K, Krieger-Liszkay A (2005) Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochim Biophys Acta 1706(1–2):158–164
Fatima RN, Javed F, Wahid A (2014) Salicylic acid modifies growth performance and nutrient status of rice (Oryza sativa) under cadmium stress. Int J Agric Biol 16(6):1083–1090
Genty B, Briantals G, Baker N (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Guo B, Liang YC, Zhu YG, Zhao FJ (2007) Role of salicylic acidin alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ Pollut 147:743–749
Hakmaoui A, Ater M, Bóka K, Barón M (2007) Copper and cadmium tolerance, uptake and effect on chloroplast ultrastructure. Studies on Salix purpurea and Phragmites australis. Z Naturforsch C 62:417–426
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68(1):14–25
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast, I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Horváth E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26(3):290–300
Hou W, Chen X, Song G, Wang Q, Chang CC (2007) Effect of copper and cadmium on heavy metal polluted water body restoration by duckweed (Lemna minor). Plant Physiol Biochem 45:62–69
Hsu YT, Kao CH (2007) Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant Soil 298:231–241
Kalaji HM, Schansker G, Brestic M, Bussotti F, Calatayud A, Ferroni L et al (2017) Frequently asked questions about chlorophyll fluorescence, the sequel. Photosyn Res 132:13–66
Khan W, Prithiviraj B, Smith DL (2003) Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol 160:485–492
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Khodary SEA (2004) Effect of salicylic acid on growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int J Agric Biol 6:5–8
Kitajima M, Butler W (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta 376:105–115
Kok B, Forbush B, McGloin M (1970) Co-operation of charges in photosynthetic O2 evolution. I. A linear four step mechanism. Photochem Photobiol 11:457–475
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Biol 42:313–349
Larqué-Saavedra A (1979) Stomatal closure in response to acetylsalicylic acid treatment. Z Pflannzenphysiol 93:371–375
Li H, Luo N, Li YW, Cai QY, Li HY, Mo CH, Wong NH (2017) Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ Pollut 224:622–630
Lichtentaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzym 148:350–382
Lichtenthaler HK, Buschmann C, Knapp M (2005) How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 43:379–393
Liu JG, Liang JS, Li KQ, Zhang ZJ, Yu BY, Lu XL, Yang JC, Zhu QS (2003) Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 52:1467–1473
Lu Q, Zhang T, Zhang W, Su C, Yang Y, Hu D, Xu Q (2018) Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol Environ Saf 147:500–508
Maksymiec W, Krupa Z (2006) The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ Exp Bot 57:187–194
Maslenkova L, Peeva V, Stojnova Zh, Popova L (2009) Salicylic acid-induced changes in photosystem II reactions in barley plants. Biotechnol Biotechnol Equip 23(1):297–300
Maurya R, Prasad SM, Gopal R (2008) LIF technique offers the potential for the detection of cadmium-induced alteration in photosynthetic activities of Zea Mays L. J Photochem Photobiol C 9:29–35
Metwally A, Finkmemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mishra A, Choudhuri MA (1999) Effects of salicylic acid on heavy metal-induced membrane degradation mediated by lipoxygenase in rice. Biol Plant 42:409–415
Moussa H, El-Gamal S (2010) Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol Plant 54:315–320
Nouairi I, Ben Ammar W, Ben Youssef N, Ben Miled Daoud D, Habib Ghorbal M, Zarrouk M (2006) Comparative study of cadmium effects on membrane lipid composition of Brassica juncea and Brassica napus leaves. Plant Sci 170:511–519
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Studies 54:45–51
Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, Janda T (2009) Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem 47:224–231
Prasad MN (1995) Cadmium toxicity and tolerance in vascular plants. Environ Exp Bot 35:525–545
Rai VK, Sharma SS, Sharma S (1986) Reversal of ABA-induced stomatal closure by phenolic compounds. J Exp Bot 37:129–134
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, Del Río LA et al (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueno MC, Del Río LA et al (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic measuring and mutual relationships. Photosynthetica 40:13–29
Sárvári É, Cseh E, Balczer T, Szigeti Z, Záray G, Fodor F (2008) Effect of Cd on the iron re-supply-induced formation of chlorophyll-protein complexes in cucumber. Acta Biol Szeged 52(1):183–186
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Schützendübel A, Nikolova P, Rudolf C, Polle A (2002) Cadmium and H2O2-induced oxidative stress in Populus canescens roots. Plant Physiol Biochem 40:577–584
Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV (2010) GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330:207–214
Shirao M, Takahashi S, Kaneko K, Badger M, Kinjo Y, Forster B, Tsuyama M, Kuroki S (2013) Gymnosperms have increased capacity for electron leakage to oxygen (Mehler and PTOX reactions) in photosynthesis compared with angiosperms. Plant Cell Physiol 54:1152–1163
Sigfridsson KGV, Bernát G, Mamedov F, Styring S (2004) Molecular interference of Cd2+ with Photosystem II. Biochim Biophys Acta 1659:19–31
Singh S, Eapen S, Souza SFD (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62:233–246
Singh PK, Chaturvedi VK, Bose B (2010) Effects of salicylic acid on seedling growth and nitrogen metabolism in cucumber (Cucumis sativus L.). J Stress Physiol Biochem 6(3):102–113
Singh AP, Dixit G, Kumar A, Mishra S, Kumar N et al (2017) A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol Biochem 115:163–173
Stefanov M, Yotsova K, Rashkov G, Ivanova K, Markovska Y, Apostolova E (2016) Effects of salinity on the photosynthetic apparatus of two Paulownia lines. Plant Physiol Biochem 101:54–59
Stefanov M, Yotsova E, Markovska Y, Apostolova EL (2017) Effect of high light intensity on the photosynthetic apparatus of two hybrid lines of Paulownia grown on soils with different salinity. Photosyntetica 55:1–9 (in press)
Stoichkova K, Busheva M, Apostolova E, Andreeva A (2006) Changes in the energy distribution in mutant thylakoid membranes of pea with modified pigment content II. Changes due to magnesium ions concentration. J Photochem Photobiol B 83:11–20
Tamás L, Mistrík I, Alemayehu A, Zelinová V, Bočová B, Huttová J (2015) Salicylic acid alleviates cadmium-induced stress responses through the inhibition of Cd-induced auxin-mediated reactive oxygen species production in barley root tips. J Plant Physiol 173:1–8
Tang Y, Sun X, Wen T, Liu M, Yang M, Chen X (2017) Implications of terminal oxidase function in regulation of salicylic acid on soybean seedling photosynthetic performance under water stress. Plant Physiol Biochem 112:19–28
Tran TA, Popova L (2013) Functions and toxicity of cadmium in plants: recent advances and future prospects. Turk J Bot 37:1–13
Vassilev A, Lidon F, Scotti P, Da Graca M, Yordanov I (2004) Cadmium-induced changes in chloroplast lipids and photosystem activities in barley plants. Biol Plant 48:153–156
Vestena S, Cambraia J, Ribeiro C, Oliveira JA, Oliva MA (2011) Cadmium induced oxidative stress and antioxidative enzyme response in Water Hyacinth and Salvinia. Braz J Plant Physiol 23:131–139
Wang Y, Qian Y, Hu H, Xu Y, Zhang H (2011) Comparative proteomic analysis of Cd-responsive proteins in wheat roots. Acta Physiologiae Plantarum 33(2):349–357
Wang Q, Liang X, Dong Y, Linlin Xu, Zhang Xiuwei, Kong Jing, Liu Shuang et al (2013) Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J Plant Growth Regul 32:721–731
Wodala B, Eitel G, Gyula TN, Ördög A, Horváth F (2012) Monitoring moderate Cu and Cd toxicity by chlorophyll fluorescence and P700 absorbance in pea leaves. Photosynthetica 50:380–386
Xu J, Wang WY, Yin HX, Liu XJ, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Xue XC, Gao HY, Zhang LT (2013) Effects of cadmium on growth, photosynthetic rate and chlorophyll content in leaves of soybean seedlings. Biol Plant 57:587–590
Yilmaz DD, Parlak KU (2011) Changes in proline accumulation and antioxidative enzyme activities in Groenlandia densa under cadmium stress. Ecol Indic 11:417–423
Yotsova EK, Stefanov MA, Dobrikova AG, Apostolova EL (2017) Different sensitivities of photosystem II in green algae and cyanobacteria to phenylurea and phenol-type herbicides: effect on electron donor side. Zeitschrift für Naturforschung C 72(7–8):315–324
Yotsova E, Dobrikova A, Stefanov M, Apostolova E (2018) Impact of salicylic acid on the growth and the activity of photosynthetic apparatus in rice under non-stress conditions. Compt Rend Acad Bulg Sci, http://www.proceedings.bas.bg/
Yuan S, Lin H (2008) Role of salicylic acid in plant abiotic stress. Z Naturforsch 63:313–320
Zeinalov Y (2002) An equipment for investigations of photosynthetic oxygen production reactions. Bulg J Plant Physiol 28:57–67
Zeinalov Y (2009) On the minimum quantum requirement of photosynthesis. Z Naturforsch 64:673–679
Zhao FY, Han MM, Zhang SY, Wang K, Zhang CR, Liu T et al (2012) Hydrogen peroxide-mediated growth of the root system occurs via auxin signaling modification and variations in the expression of cell-cycle genes in rice seedlings exposed to cadmium stress. J Integr Plant Biol 54:991–1006
Acknowledgements
This study was supported by the Project No. 137/12.05.2016 of Program for career development of young scientists, Bulgarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yotsova, E.K., Dobrikova, A.G., Stefanov, M.A. et al. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Physiol. 30, 57–70 (2018). https://doi.org/10.1007/s40626-018-0102-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-018-0102-9