Abstract
The effect of foliar pretreatment by hydrogen peroxide (H2O2) at low concentrations of 0, 5, 10, and 15 mM on the chilling tolerance of two Zoysia cultivars, manilagrass (Zoysia matrella) and mascarenegrass (Zoysia tenuifolia), was studied. The optimal concentration for H2O2 pretreatment was 10 mM, as demonstrated by the lowest malondialdehyde (MDA) content and electrolyte leakage (EL) levels and higher protein content under chilling stress (7°C/2°C, day/night). Prior to initiation of chilling, exogenous 10 mM H2O2 significantly increased catalase (CAT), ascorbate peroxidase (APX), glutathione-dependent peroxidases (GPX), and glutathione-S-transferase (GST) activities in manilagrass, and guaiacol peroxidase (POD), APX, and glutathione reductase (GR) activities in mascarenegrass, suggesting that H2O2 may act as a signaling molecule, inducing protective metabolic responses against further oxidative damage due to chilling. Under further stress, optimal pretreatments alleviated the increase of H2O2 level and the decrease of turfgrass quality, and improved CAT, POD, APX, GR, and GPX activities, with especially significant enhancement of APX and GPX activities from the initiation to end of chilling. These antioxidative enzymes were likely the important factors for acquisition of tolerance to chilling stress in the two Zoysia cultivars. Our results showed that pretreatment with H2O2 at appropriate concentration may improve the tolerance of warm-season Zoysia grasses to chilling stress, and that manilagrass had better tolerance to chilling, as evaluated by lower MDA and EL, and better turfgrass quality, regardless of the pretreatment applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zoysia, one of the most popular warm-season turfgrasses, is widely used for home lawns, athletic fields, and parks due to its excellent tolerance to heat, drought, and poor soil. Its optimum growth temperature is between 26°C and 32°C, Thus, chilling is a major factor restricting its utilization in subtropical regions that experience low winter temperatures between −5°C and 10°C.
One of the earliest responses of plants to various stress conditions, including chilling, freezing, drought, and heat, among others, is overproduction of reactive oxygen species (ROS) such as superoxide (O −2 ), hydrogen peroxide (H2O2), hydroxyl radicals (OH), and singlet oxygen (1O2). These ROS can be associated with development of oxidative injury and disruption of metabolic functions in plants (Mittler 2002). However, low concentrations of ROS, which are byproducts of normal metabolism, do not lead to oxidative damage, due to the presence of antioxidant systems that can scavenge ROS (Foyer and Noctor. 2000). It is only when production of ROS exceeds the capacity of these scavenging systems that oxidative damage occurs.
During normal metabolism in a plant cell, H2O2 is generated in chloroplast, mitochondria, and peroxisome, and is kept in homeostasis by complicated and effective scavenging systems that have developed over the course of evolution (Zhao and Blumwald 1998). There are two classes of H2O2 defense systems: enzymatic and nonenzymatic. The former class includes enzymes such as catalase (CAT; EC 1.11.1.6), guaiacol peroxidase (POD; EC 1.11.1.7), ascorbate peroxidase (APX; EC 1.11.1.11), glutathione reductase (GR; EC 1.6.4.2), glutathione-dependent peroxidases (GPX; EC 1.11.1.9), and glutathione-S-transferase (GST; EC 2.5.1.18) (Kang and Saltveit 2002). The latter class includes a wide variety of chemical compounds such as ascorbate (AsA), glutathione (GSH), mannitol, and flavonoids, among others.
APX, GR, monodehydroascobate reductase (MDHAR), and dehydroascorbate reductase (DHAR), in combination with ASA and GSH, together form the ascorbate–glutathione cycle. The enhancement of these cycle components has been reported to have a close relationship with acquisition of chilling tolerance; for example, activities of APX, CAT, and GR were found to be higher in chilling-tolerant cultivars, or in plants acclimated to chilling, as demonstrated in a wide range of species (Mittler 2002; Kang and Saltveit 2002; Xu et al. 2006). Alterations in AsA and GSH productions may contribute to increased resistance in plants exposed to abiotic and biotic stresses (Xu et al. 2006). However, changes in the reductive glutathione/glutathione disulfide (GSH/GSSG) ratio or in the amount of GSH bound to proteins appear to be more decisive in determining plant resistance than are overall GSH levels (Kómives et al. 1998).
H2O2 has a long lifespan, is able to cross biological membranes, and rapidly diffuses from cell to cell or can be transported long distances from its sites of origin in plants. Also, its production quickly responds to various environment stimuli (Cheng and Song 2006). Thus, H2O2 has all of the characteristic features of an intercellular signaling molecule and, for this reason, has received increasing attention in recent years (Levine et al. 1994; Alvarez et al. 1998; Neill et al. 2002). H2O2 is an evolutionarily ancient signaling molecule that not only played a key role in inducing evolution of oxygenic photosynthesis but also modulates many physiological events, such as stomatal movement, hypersensitive responses, and programmed cell death (Cheng and Song 2006). Increasing evidence now indicates that H2O2 acts as a local and systemic signal that directly regulates expression of numerous genes. Some of these are involved in plant pathogen defense responses, while others are invoked during adaptation of plants to abiotic stress (Desikan et al. 2001; Wang et al. 2006).
Plants pretreated with low concentrations of H2O2 have shown significantly greater tolerance to abiotic stress than untreated control plants (Gechev et al. 2002). Pretreatment with H2O2 has been shown to induce chilling tolerance in normally chill-sensitive maize seedlings (Neto et al. 2005). Similarly, regenerated potato nodal explants treated with H2O2 became significantly more thermotolerant compared with untreated control plants (Lopez-Delgado et al. 1998). Pretreatment of wheat seeds with H2O2 has been shown to improve the subsequent salt tolerance of the seedlings (Wahid et al. 2007). Arabidopsis leaves pretreated with H2O2 have been shown to develop increased tolerance to excess light (Karpinski et al. 1999). This acquisition of abiotic stress tolerance usually occurs concomitantly with enhanced antioxidative status, as reflected by higher activities and/or protein levels of CAT, APX, POX, and GPX, as well as an increased glutathione pool (Gechev et al. 2002).
However, it is currently unknown whether pretreatment with H2O2 can enhance chilling tolerance of warm-season turfgrasses such as Zoysia grass. Reports on the effect of chilling stress on glutathione metabolism in turfgrass are rare. The aim of this study is therefore to investigate the effects of pretreatment with low concentration of H2O2 on the subsequent chilling tolerance of two Zoysia cultivars, manilagrass and mascarenegrass. Our data indicate that chilling tolerance is invoked by low concentration of H2O2 through regulation of antioxidative systems, including enhancement of activities of antioxidative enzymes and alteration of levels of antioxidants.
Materials and methods
Plants
Manilagrass (Zoysia matrella) and mascarenegrass (Zosia tenuifolia) are two Zoysia cultivars with different tolerance to chilling stress. They were collected from 1-year field plots at the Horticulture Cultivation Center, Nanjing University, and then grown in plastic pots (14 cm diameter, 25 cm long) filled with sand/organic/vermiculite (3/1/1, v/v/v) mixture in a greenhouse at day/night temperature of 32°C/26°C for 1 month. Plants were clipped to height of 10 cm, watered daily, and fertilized weekly with Hoagland solution. Prior to the experiment, plants were transferred to growth chamber for 2 weeks with temperature of 32°C/26°C, relative humidity of ~70%, irradiance of 200 μmol m−2 s−1, and 14-h photoperiod.
Chemical and chilling treatment
In a preliminary experiment, manilagrasses and mascarenegrasses were sprayed with 50 ml of 0, 5, 10, or 15 mM H2O2 and placed in a normal growth chamber for 12 h for full absorption of H2O2 solution. Then these plants were transferred to a chamber with temperature of 7°C/2°C (day/night) and other conditions as above. Leaves were sampled before initiation of chilling (0 days) and after 1, 3, 5, and 7 days of chilling to measure the extent of chilling injury as indicated by malondialdehyde (MDA) content, electrolyte leakage, and protein content.
Based on the preliminary experiment, the optimal concentration for H2O2 pretreatment was found to be 10 mM. Plants of both cultivars pretreated with 50 ml 10 mM H2O2 or distilled water (control) were used in further experiments. Before initiation of chilling and after 3 and 6 days of chilling, turfgrass quality was observed firstly and then leaves were sampled to measure content of H2O2, AsA, DHA, GSH, and GSSG and activity of POD, CAT, APX, GR, GPX, and GST.
Morphological observations
Turfgrass quality was rated visually on a scale from 0 to 9 according to shoot density and color (percentage green leaves) of grass canopy, with 0 being worst (brown, thin, and dead turf), 9 being the best (green, dense, and uniform turf), and 6 being the minimum acceptable level (Larkindale and Huang 2004).
Electrolyte leakage (EL) was measured as described by Nayyar et al. (2005). Samples of leaves were washed with deionized water to remove surface-adhered electrolytes. These were then placed in closed vials containing 10 ml deionized water and incubated at 25°C on a rotary shaker for 24 h. Subsequently, electrical conductivity of the solution (L 1) was determined. Samples were then autoclaved at 120°C for 20 min and the final electrical conductivity (L 2) was obtained after equilibration at 25°C. EL was defined as follows: EL(%) = (L 1/L 2) × 100%.
Protein content was determined by absorbance at 595 nm using bovine serum albumin as standard (Larkindale and Huang 2004).
Physiological assays to determine oxidative damage
Malondialdehyde (MDA) was measured by the thiobarbituric acid (TBA) method as described by Xu et al. (2006). Fresh leaf samples (0.2 g) were homogenized using a mortar and pestle in 5 ml 10% (v/v) trichloracetic acid (TCA), and the homogenate was centrifuged at 12,000g for 15 min. Supernatant (2 ml) was taken out, 2 ml 0.6% (v/v) TBA solution was added, and the mixture was heated in a boiling water bath for 30 min and cooled quickly in an ice bath, before being centrifuged at 12,000g for 15 min. The resulting supernatant was analyzed at 532 nm and 600 nm using an ultraviolet (UV)–Vis spectrophotometer (model M36; Beckman, CA, USA). MAD content was calculated by using an extinction coefficient of 155 mM−1 cm−1 in the following formula: MDA [μmol g−1 fresh weight (FW)] = [(A 532 −A 600)/155] × 103 × dilution factor.
H2O2 content was estimated by forming a titanium hydroperoxide complex (Dagmar et al. 2001). Fresh leaf material (0.2 g) was ground with 5 ml cooled acetone in an ice bath, and the homogenate was centrifuged at 6,000g for 10 min. Supernatant (1 ml) was taken out, and 0.1 ml 5% titanium sulfate and 0.2 ml ammonia were added to this solution. The reaction mixture was centrifuged at 10,000g for 10 min at 4°C. The supernatant was discarded, and the precipitate was dissolved in 5 ml 2 mM H2SO4. The solution was analyzed at 415 nm. H2O2 content was determined using a standard curve plotted with the known content of H2O2.
Extraction and analysis of AsA, DHA, GSH, and GSSG
Treated leaves (0.2 g fresh weight) were homogenized in 5 ml 5% (w/v) cold sulfosalicylic acid in an ice bath, and then centrifuged at 12,000g for 20 min at 4°C. The supernatant was used as the crude extract for AsA, DHA, GSH, and GSSG assays. AsA and DHA were measured according to Kamapfenkel and Montagu (1995), and GSH and GSSG were measured according to Griffith (1980), with minor modification.
To assay AsA and DHA, two 200-μl aliquots of supernatant were removed and neutralized by adding 50 μl 1.84 M triethanolamine. One sample was used for determination of AsA based on reduction of Fe3+ to Fe2+ by AsA and spectrophotometric detection of Fe2+ complexed with 2,2′-dipyridyl. The other sample was used for determination of total AsA by adding 10 mM dithiothreitol (DTT) to reduce DHA to AsA by preincubation for 15 min at 42°C, with excess DTT removed using N-ethylmaleimide (NEM). The reaction solution also included 2.5 mM ethylenediamine tetraacetic acid (EDTA) dissolved in 50 mM phosphate buffer (pH 7.4), 10% TCA, 44% H3PO4, and 4% 2,2’-dipyridyl dissolved in 70% (v/v) ethanol, as well as 3% FeCl3. Absorbance was read at 525 nm using water as a reference. A standard curve prepared by using the known content of AsA. The concentration of DHA was then calculated from the difference of total AsA and AsA (without pretreatment with DTT).
To assay GSH and GSSG, two 100-μl aliquots of supernatant were removed and neutralized by adding 25 μl 1.84 M triethanolamine. One sample was used for determination of total glutathione (GSH + GSSG). The other sample was pretreated with 50 μl 10% 2-vinylpyridine for 60 min at 25°C to avoid measurement of GSH and allow the determination of GSSG alone. Then, both types of samples were mixed with 705 μl 50 mM phosphate buffer (pH 7.4) including 2.5 mM EDTA, 20 μl 10 mM nicotinamide adenine dinucleotide phosphate (NADPH), and 80 μl 12.5 mM 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB). A 20-μl aliquot of glutathione reductase (50 U/ml) was added, and the change in absorbance at 412 nm was monitored for 3 min. A standard curve prepared by using GSSG was used in the calculation of the amounts of total glutathione, GSSG, and GSH (calculated by subtracting GSSG from total glutathione).
Extraction and assays of antioxidative enzymes
Pretreated leaves (0.2 g) were harvested and frozen in liquid nitrogen at different times of treatment and stored at −80°C. After removal from the freezer, samples were immediately ground using a mortar and pestle, in an ice bath, in 5 ml cold phosphate saline buffer (PSB, 100 mM, pH 7.6) containing 2% polyvinylpolypyrrolidone, 1 mM EDTA, and 0.5 mM AsA. The homogenate was then centrifuged at 12,000g at 4°C for 20 min, and the supernatant was used as crude extract for assays of antioxidant enzyme activity.
By the methods of Larkindale and Huang (2004), APX activity was measured by monitoring the rate of oxidation of AsA at 290 nm for 1 min (ε = 2.8 mM cm−1), in 3 cm3 of reaction mixture composed of 50 mM PSB (pH 7.0) containing 0.5 mM AsA, 0.06% H2O2 dissolved in PSB (pH 7.0), and 0.1 ml enzyme extract. POD activity was measured by monitoring oxidation of guaiacol at 470 nm for 1 min, in 3 ml of reaction mixture composed of 50 mM PSB (pH 7.0), 1% guaiacol, 0.3% H2O2, and 0.05 ml enzyme extract. CAT activity was measured according to the decomposition of H2O2 at 240 nm for 1 min, in 3 ml of reaction mixture composed of 50 mM PSB (pH 7.0), 0.06% H2O2, and 0.1 ml enzyme extract. GR activity was measured by the increase of absorbance at 412 nm for 1 min according to Smith et al. (1988). The assayed mixture contained 100 mM PSB (pH 7.5), 0.5 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 2.0 mM NADPH, 2.0 mM GSSG, and 0.1 ml extract in a total volume of 3 ml, and the reaction was started by addition of GSSG. The same volume of PSB replaced the extract in blanks for the measurement of these enzyme activities. One unit of POD, CAT, and GR activity was defined as that causing an absorbance change of 0.01 in 1 min. GPX and GST activity was determined by decrease of GSH content per min (A = 161.11 μM cm−1) using a reagent box provided by Nanjing Jiancheng Bioengineering Institute. GSH reacted with DTNB, forming yellow steady 5′-dithiobis-2-nitrobenzoic acid, and absorbance at 412 nm at the initiation and end (5 min) of the reaction was read to calculate GSH content.
Statistical analysis
Experiments were carried out according to randomized block design (four repeated pots in each block and four replicates in each measurement). All experimental data were subjected to one-way analysis of variance using the SPSS package (1990; SPSS Inc.), and least significant difference at 0.05 probability level was performed.
Results
Selection of the optimal concentration for H2O2 pretreatment
Prior to chilling, MDA content in all pretreated plants was higher than in controls. However, plants of both cultivars pretreated with 5 and 10 mM H2O2 showed lower MDA levels than their controls during chilling stress, and MDA levels in plants pretreated with 15 mM H2O2 were close to controls (Fig. 1). Protein content in controls and pretreatments with 5 and 10 mM H2O2 of both cultivars showed a tendency to increase first then decrease during chilling, reaching maximum levels at the third day. However, protein content in manilagrass and mascarenegrass pretreated with 15 mM H2O2 gradually decreased under stress. In addition, protein content of manilagrass pretreated with 10 mM H2O2 changed insignificantly during stressing time. Controls and all pretreatments of both cultivars showed increased EL during chilling stress. EL in plants pretreated with 5 and 10 mM H2O2 was lower than in controls and in plants pretreated with 15 mM H2O2. Plants pretreated with 10 mM H2O2 exhibited the lowest MDA content and EL, as well as the higher protein content; therefore, the optimal concentration for H2O2 pretreatment was selected as 10 mM. In addition, the optimal pretreatment showed lower MDA and EL and higher protein content in manilagrass than in mascarenegrass.
Effects of H2O2 pretreatment on turfgrass quality and H2O2 levels
Turfgrass quality of all plants was reduced under chilling stress; however, in pretreated plants it was higher than in controls. Grass quality of manilagrass was better than that of mascarenegrass during chilling between controls and pretreatments (Fig. 2a). H2O2 pretreatment significantly increased H2O2 content of both Zoysia cultivars before initiation of chilling (P < 0.05). However, by the third day of chilling, H2O2 levels in pretreated manilagrass and mascarenegrass and their controls were no longer significantly different (P > 0.05). By the sixth day of chilling, H2O2 levels were 58% (manilagrass) and 15% (mascarenegrass) lower than in controls (Fig. 2b).
Turfgrass quality (A) and H2O2 (B) levels in leaves of manilagrass (Zoysia matrella) and mascarenegrass (Zoysia tenuifolia) in control plants (MC manilagrass controls; TC mascarenegrass controls) and H2O2-pretreated plants (MT pretreated manilagrass; TT pretreated mascarenegrass) under chilling stress. Values expressed as mean ± standard deviation (n = 4). Means for each genotype that do not have a common letter are significantly different at P < 0.05
Effects of H2O2 pretreatment on levels of antioxidants
In both cultivars, AsA content in controls and pretreated plants was enhanced during chilling, and showed a significant increase at the sixth day of chilling (P < 0.05). DHA content in controls and pretreated plants of both cultivars showed insignificant change during chilling, except for pretreated mascarenegrass at the sixth day of chilling (Fig. 3). The AsA/total ascorbate ratio in controls and pretreated plants of both cultivars was significantly increased at the sixth day of chilling (P < 0.05). However, these values were not significantly difference between controls and pretreated plants during chilling stress (P > 0.05).
Content of AsA (μmol g−1FW), DHA (μmol g−1FW), and total ascorbate (μmol g−1FW) in leaves of manilagrass (Zoysia matrella) and mascarenegrass (Zoysia tenuifolia) in control plants (MC manilagrass controls; TC mascarenegrass controls) and H2O2-pretreated plants (MT pretreated manilagrass; TT pretreated mascarenegrass) under chilling stress. Values expressed as mean ± standard deviation (n = 4). Means for each genotype that do not share a common letter are significantly different at P < 0.05
H2O2 pretreatment significantly increased the GSSG content in the two cultivars and decreased the GSH content in manilagrass before initiation of chilling (P < 0.05). The GSH level in manilagrass subsequently decreased during chilling; however, it was higher in pretreated plants than in controls at the third day. Levels of GSSH in the two cultivars and of GSH in mascarenegrass reached the highest levels at the third day of chilling, with no differences between pretreated plants and controls (P > 0.05). GSSG and GSH in pretreated mascarenegrass were significantly higher than in controls at the sixth day of chilling (P < 0.05) (Fig. 4). H2O2 pretreatment decreased the GSH/GSSG ratio significantly in manilagrass and insignificantly decreased the ratio in mascarenegrass before initiation of chilling. These ratios were not significantly different between pretreated plants and controls during further chilling stress.
Content of GSH (nmol g−1FW), GSSG (nmol g−1FW), and total glutathione (nmol g−1FW) in leaves of manilagrass (Zoysia matrella) and mascarenegrass (Zoysia tenuifolia) in control plants (MC manilagrass controls; TC mascarenegrass controls) and H2O2-pretreated plants (MT pretreated manilagrass;TT pretreated mascarenegrass) under chilling stress. Values expressed as mean ± standard deviation (n = 4). Means for each genotype that do not share a common letter are significantly different at P < 0.05
Effects of H2O2 pretreatment on activities of antioxidative enzymes
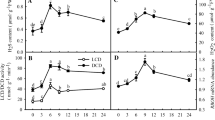
H2O2 pretreatment only significantly increased levels of POD activity in manilagrass at the third day of chilling (P < 0.05). In contrast, in mascarenegrass, there was significant increase in POD activity from the initiation to the end of chilling (P < 0.05) (Fig. 5a). CAT activity of manilagrass was significantly increased during chilling by pretreatment with H2O2, and reached its highest level at the sixth day of chilling (P < 0.05) (Fig. 5b). Pretreatment did not change the CAT activity of mascarenegrass before initiation of chilling, but significantly enhanced it during further chilling (P < 0.05). H2O2 pretreatment significantly increased APX activity in manilagrass during chilling, and in mascarenegrass at the third and the sixth day of chilling stress. APX had the highest activity in both cultivars at the third day of chilling (P < 0.05) (Fig. 5c).
POD (a), CAT (b), APX (c), GR (d), APX (e), and GR (f) activities in leaves of manilagrass (Zoysia matrella) and mascarenegrass (Zoysia tenuifolia) (7/2°C, day/night) in control plants (MC manilagrass controls; TC mascarenegrass controls) and H2O2-pretreated plants (MT pretreated manilagrass;TT pretreated mascarenegrass) under chilling stress. Values expressed as mean ± standard deviation (n = 4). Means for each genotype that do not share a common letter are significantly different at P < 0.05
H2O2 pretreatment did not change GR activity in manilagrass prior to initiation of chilling, but increased it significantly at the third day (P < 0.05) and insignificantly at the sixth day of chilling (P > 0.05). However, in mascarenegrass, H2O2 pretreatment significantly enhanced GR activity, both prior to initiation and at third day of chilling (P < 0.05), and insignificantly increased it at the sixth day (P > 0.05) (Fig. 5d). GPX activity in manilagrass pretreated with H2O2 was significantly higher than in controls during chilling, but gradually decreased with stressing time (Fig. 5e). Pretreatment did not significantly enhance GPX activity in mascarenegrass during chilling, which showed a similar activity pattern to that of untreated controls, with the lowest activity seen on the third day. H2O2 pretreatment significantly increased initial GST activity, but this activity was not different from that of controls on further stressing. GST activities in mascarenegrass were not significantly different between pretreatments and controls (Fig. 5f).
Discussion
Pretreatment with H2O2 at appropriate concentration, such as 5 and 10 mM, improved the tolerance of both investigated cultivars to chilling stress, as shown by lower MDA and EL levels and higher protein content. Chilling stress resulted in considerable oxidative damage to warm-season Zoysia grass. MDA is a product of peroxidation of unsaturated fatty acids in phospholipids (Xu et al. 2006). Electrolyte leakage reflects damage to membranes (Nayyar et al. 2005). Accumulation of soluble protein was also associated with cold acclimation (Kang et al. 2005). Therefore, these are important indicators to assess chilling injury. The lowest MDA content and EL levels and the higher protein content in plants pretreated with 10 mM H2O2 indicate that the optimal concentration for H2O2 pretreatment was 10 mM.
Turfgrass quality reflects visually chilling injury; the leaves of control plants exhibited more severe sear and curliness than did pretreated plants during chilling stress, suggesting that pretreatment with low concentrations of H2O2 alleviated chilling-induced damage. As the stressing time increased, the H2O2 content in controls of the two cultivars continuously increased and was significantly higher than in pretreatments at the sixth day of chilling. Overproduction of H2O2 in controls likely exceeded the capacity of the antioxidative system to eliminate it. However, H2O2 pretreatment reduced production of H2O2 under further chilling stress, postponing oxidative damage. Moreover, manilagrass showed lower MDA levels and EL and better turfgrass quality than mascrarenegrass under stress, regardless of the pretreatment, indicating that manilagrass had better tolerance to chilling stress.
It is now widely recognized that H2O2 can act as a local and systemic signal in plant pathogen defense responses and in adaptation of plants to abiotic stresses (Levine et al. 1994; Vandenabeele et al. 2003; Apel and Hirt 2004; Foyer and Noctor 2005). Transgenic plants with elevated levels of H2O2 due to constitutive overproduction of glucose have been found to be more resistant to pathogens (Wu et al. 1995). Short-term exposure of catalase-antisense plants to high light levels induced increased tolerance to subsequent severe oxidative stress (Vandenabeele et al. 2003). Results from the current study are in agreement with these previous studies. Pretreatment elevated H2O2 content before initiation of chilling stress, when CAT, APX, GPX and GST activities in manilagrass and POD, APX, and GR activities in mascarenegrass were also significantly increased. Improvement of the activities of these enzymes in turn protected plants against subsequent chilling-induced damage. Exogenous H2O2 possibly functioned directly or may have induced intracellular H2O2 generation to act as a signaling molecule under stress. Avsian et al. (2004) have shown that salt stress induced oxidative stress in the form of H2O2, the production of which occurred in intracellular spaces. Its signal transduction pathway, which activated the GPX1 promoter (the Citrus sinensis gene encoding phospholipid hydroperoxide isoform), was different from the pathway induced by extracellularly supplied H2O2.
According to the biochemical pathways of antioxidative reactions in plant (Fig. 6), the antioxidants and antioxidative enzymes tested in our study are the major H2O2-scavenging species. APX catalyzes the first step of the AsA–GSH cycle at the expense of AsA (Foyer and Noctor 2000) (Fig. 6a). In both investigated Zoysia cultivars, APX activities were significantly enhanced from initiation to the end of chilling stress. DHA levels were not changed by chilling in either cultivar, while AsA levels were significantly increased by the sixth day of chilling. AsA is regenerated by utilizing GSH to form GSSG, and GR catalyzes regeneration of GSH from GSSG (Zhao and Blumwald 1998). Our results showed that H2O2 pretreatment significantly increased GR activity in manilagrass at the third day of chilling, and in masarcenegrass throughout chilling stress. GR activity has been reported to be associated with alteration of the GSH/GSSG ratio, which is more decisive in determining plant resistance to abiotic and biotic stresses than is the actual GSH content (Kómives et al. 1998). H2O2 pretreatment decreased the GSH/GSSG ratio in both investigated cultivars prior to initiation of chilling. Previous studies have shown that low temperature can activate redox signaling directly via changes in H2O2 concentration and GSH/GSSG ratio (Kocsy et al. 2001).
Biochemical pathways of antioxdative reaction scavenging of H2O2 in plants. a The ascorbate–glutathione cycle. b The glutathione peroxidase (GPX) cycle. c Catalase (CAT). Superoxide dismutase (SOD) acts as the first line of defense, converting O2 − into H2O2. Ascorbate peroxidases (APX), GPX, and CAT then detoxify H2O2. In contrast to CAT (c), APX and GPX require an ascorbate (AsA) and/or a glutathione (GSH) regenerating cycle (Mittler 2002)
GPX catalyses reduction of H2O2 at the expense of GSH (Kuźniak and Skłodowska 2001) (Fig. 6b). GPX also participates in reduction of lipid hydroperoxides at the expense of reduced glutathione, thus preventing chain oxidation of thylakoid phospholipids and removing lipid hydroperoxides from membranes. Increased GPX activity has been detected in many plants under both biotic and abiotic stresses (Asada 1999; Kuźniak and Skłodowska 1999; Nagalakshmi and Prasad 2001). Similar results were seen in our study, as GPX activities in both investigated cultivars were significantly enhanced by H2O2 pretreatment from initiation to the end of chilling. In yeasts, a glutathione peroxidase, GPX3, can function as a receptor for H2O2 and a transducer of redox signals, to activate gene expression (Delaunay et al. 2002). Further study is needed to understand the actual function of GPX in the observed chilling resistance.
GST catalyzes the conjugation reaction of GSH and protects SH-containing enzymes against oxidation (Kuźniak and Skłodowska 2004). GST is primarily involved in detoxification of xenobiotics. Pathogen attack can also induce an increase in GST messenger RNA (mRNA) in soybean cells (Levine et al. 1994). However, changes in GST activity have rarely been reported in response to abiotic stress. In the current study, GST was significantly enhanced in manilagrass prior to initiation of chilling, but showed no difference in later chilling stress. In mascarenegrass, there were insignificant changes in GST under any condition. This suggests that GST activity may have little relationship to acquisition of chilling tolerance in either investigated Zoysia cultivar.
CAT is indispensable for H2O2 detoxification during stress. Its activity may be inhibited by low temperature (Fadzillah et al. 1996) (Fig. 6c). This was in agreement with our results, where decreased CAT activities were observed in control plants of both cultivars. However, H2O2 pretreatment significantly increased CAT activity in both investigated cultivars. POD activity in both controls was decreased as stressing time increased, and H2O2 pretreatment significantly increased POD activity in masarcenegrass during chilling, and in manilagrass at the third day of chilling. POD activity was possibly related to lignification reactions, which increase the rigidity of cell walls in response to chilling. This type of response would allow pretreated plants to remain upright to overcome the wilting and mechanical weakness imparted by chilling stress (Mäder and Füssl 1982).
In conclusion, pretreatment with H2O2 at appropriate concentration may improve the tolerance of warm-season Zoysia grass to chilling stress. The optimal concentration for H2O2 pretreatment was 10 mM, and manilagrass had better tolerance to chilling stress than mascarenegrass. Exogenous H2O2 may be acting as a signaling molecule that significantly increased CAT, APX, GPX, and GST activities in manilagrass and POD, APX, and GR activities in mascarenegrass prior to initiation of chilling, protecting plants against oxidative damage induced by chilling. Under further stressing, pretreatment alleviated the increase of H2O2 level and the decrease of turfgrass quality, and improved CAT, POD, APX, GR, and GPX activities. These antioxidative enzymes were important for the acquisition of tolerance of the investigated Zoysia cultivars to chilling stress. Especially, APX and GPX activities were significantly enhanced from initiation to end of chilling. GST activity may have little relationship to the chilling tolerance of either investigated cultivar.
References
Alvarez ME, Pennell RI, Meijer PJ, Ishkawa A, Dixon RA, Lamb C (1998) Reactive oxygen species intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Bio 55:373–399
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and issipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Avsian KO, Gueat DY, Lev YS, Gollop R, Ben HG (2004) The salt-stress signal transduction pathway that activates the gpxl promoter is mediated hy intracellular H2O2, different from the pathway induced hy extracellular H2O2. Plant Physiol 135(3):1685–1696
Cheng YL, Song CP (2006) Hydrogen peroxide homeostasis and signaling in plant cells. Sci China Ser C Life Sci 49(1):1–11
Dagmar P, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB (2002) A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111:471–481
Desikan R, Mackerness SAH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol l27:l59–172
Fadzillah NM, Gill V, Finch RP, Burdon RH (1996) Chilling oxidative stress and antioxidant responses in shoot culture of rice. Planta 199:552–556
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gechev T, Gadjev I, Van BF, Inze D, Dukiandjiev S, Toneva V, Minkov I (2002) Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol Life Sci 59(4):708–714
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyriding. Anal Biochem 106:207–212
Kamapfenkel K, Montagu MV (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 255:165–167
Kang HM, Saltveit ME (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol Plant 115:571–576
Kang HM, Park KW, Saltveit ME (2005) Chilling tolerance of cucumber (Cucumis sativus) seedling radicles is affected by radicle length, seedling vigor, and induced osmotic- and heat-shock proteins. Physiol Plant 124:485–492
Karpinski S, Reynolds H, Karpinsk AB, Winsgi EG, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation response to excess excitation energy in Arabidopsis. Science 284:654–657
Kocsy G, Galiba G, Brunold C (2001) Role of glutathione in adaptation and signalling during chilling and cold acclimation in plants. Physiol Plant 113:58–164
Kómives T, Gullner G, Kiraly Z (1998) Role of glutathione and glutathione-related enzymes in response of plants to environmental stress. Stress Life 851:251–258
Kuźniak E, Skłodowska M (1999) The effect of Botrytis cinerea infection on ascorbate-glutathione cycle in tomato leaves. Plant Sci 148:69–76
Kuźniak E, Skłodowska M (2001) Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant Sci 160:723–731
Kuźniak E, Skłodowska M (2004) Differential implication of glutathione, glutathione-metabolizing enzymes and ascorbate in tomato resistance to pseudomonas syringae. J Phytopathol 152:529–536
Larkindale J, Huang BR (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161:405–413
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Lopez-delgado H, Dat JF, Foyer CH, Scott IM (1998) Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J Exp Bot 49:713–720
Mäder M, Füssl R (1982) Role of peroxidase in lignification of tobacco cells. II. Regulation by phenolic compounds. Plant Physio1 70:1132–1134
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Nayyar H, Bainsb TS, Kumar S (2005) Chilling stressed chickpea seedlings: effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ Exp Bot 54:275–285
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53(372):1237–1247
Neto ADA, Prisco JT, Eneas-Filho J, Jand-Venes R, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces saltstress acclimation in maize plants. J Plant Physiol 162:1114–1122
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude homogenates use 5, 5′-dithiol-bis(2-nitrobenzoic acid). Anal Biochem 175:408–413
Vandenabeele S, Der-Kelen KV, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Montagu MV, Zabeau M, Inze D, Breusegem FV (2003) A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc Nat1 Acad Sci U S A 100(26):16113–16118
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164(3):283–294
Wang PC, Du YY, An GY, Zhou Y, Song CR (2006) Analysis of global expression profiles of Arabidopsis genes under abscisic acid and H2O2 applications. J Integr Plant Biol 48:62–74
Wu GS, Shortt BJ, Lawrence EB, Levine EB, Fitzsimmons KC, Shah DM (1995) Disease resistance conferred by expression of a gene encoding H2O2-generating glucoseoxidase in transgenic potato plants. Plant Cell 7(9):1357–1368
Xu S, Li JL, Zhang XQ, Wei H, Cui LJ (2006) Effects of heat acclimation pretreatment on changes of membranelipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285
Zhao SY, Blumwald E (1998) Changes in oxidation-reduction state and antioxidant enzymes in the roots of jack pine seedlings during cold acclimation. Physiol Plant 104:134–142
Acknowledgments
This research was supported by the China projects (863 and 2007 AA10Z231). Thanks to ShineWrite Company for correction of the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Li, J., Wang, J. et al. Exogenous H2O2 improves the chilling tolerance of manilagrass and mascarenegrass by activating the antioxidative system. Plant Growth Regul 61, 195–204 (2010). https://doi.org/10.1007/s10725-010-9470-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9470-0