Abstract
Drought is an abiotic stress that severely reduces plant growth. Responding to drought, plants would induce a series of physiological and biochemical changes. Colonization by arbuscular mycorrhizal (AM) fungi was reported beneficial in improving plants’ drought tolerance. However, the effect of AM fungi in improving drought tolerance of widely planted Populus spp. was rarely reported. The effect of AM fungus (Rhizophagus irregularis) on malondialdehyde (MDA) content, proline and soluble proteins content, antioxidative enzymes activates, relative water content (RWC) and water use efficiency (WUE), and the aquaporin PIP genes expression of Populus × canadensis ‘Neva’ leaves under well-watered and drought-stressed condition was evaluated. R. irregularis could colonize more than 80 % of poplar roots, reduce MDA and proline content, lower antioxidative enzymes activates, and down-regulate the expression of PIP2;1, PIP2;2. Meanwhile, R. irregularis could increase soluble protein content, increase RWC and WUE, and up-regulate the expression of PIP1;1, PIP1;3, PIP1;4, PIP1;5, PIP2;1, PIP2;2, and PIP2;3. In conclusion, R. irregularis could improve drought tolerance of P. canadensis by increasing RWC via regulation of aquaporin genes expression, and consequently increased WUE, lowered accumulation of osmotic adjustment molecule, reduced ROS accumulation and oxidative damage. Further studies focusing on the influence of AM fungi on specific aquaporin PIP gene location, function and expression in plant responding to drought stress are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is a seriously abiotic stress which reduced plant growth and productivity. Responding to drought, plants induced a series of physiological and biochemical changes (Farooq et al. 2009). One important physiological reaction of plant was the accumulation of soluble substances, such as proline and solute protein, for osmotic adjustment. Proline accumulation could lower the osmotic potential of cell, attract water into cell and help the maintenance of turgor (Lin and Kao 2000). The accumulation of osmotic regulation substances could also improve the stability of cell membrane and the activity of the proteins and enzymes (Hessini et al. 2009).
One important biochemical change under drought stress is the reactive oxygen species (ROS) accumulation, which starts from plant stomatal closure and owes to the over-reduction of the photosynthetic electron chain (Ahmed et al. 2009). Drought often increased the level of ROS, including superoxide anion (O2 −), hydroxyl radical (HO−), hydrogen peroxide (H2O2) and singlet oxygen (1O2), and required protective mechanism to prevent ROS from interrupting normal metabolism and oxidizing proteins, DNA and lipids (Smirnoff 1993; Ahmed et al. 2009). For instance, ROS could stimulate membranous lipid peroxidation and accumulate membranous peroxide product like malondialdehyde (MDA) (Lacan and Baccou 1998). In responding, plants would produce antioxidant enzymes and antioxidant molecules against oxidative stress to protect themselves (Liu et al. 2011). The most important antioxidant enzymes are superoxide dismutase (SOD, EC 1.15.1.1) and peroxidase (POD, EC 1.11.1.7). Cooperation of SOD and POD scavenge O2 − into H2O (Wang et al. 2009; Wu and Zou 2009).
Arbuscular mycorrhizal (AM) fungi from the Glomeromycota could form symbiosis with more than 80 % terrestrial plants and improve their tolerance against biotic and abiotic stresses (Augé 2001; Smith and Read 2008; Augé et al. 2015). Ruiz-Lozano (2003) pointed out that an important mechanism of AM fungi in helping plant under drought stress was the improved ability of plant osmotic regulation, increased stability of cell membrane, and improved antioxidant enzyme activity. Due to the influence of AM fungi, the improved drought tolerance was documented in different plants, e.g., citrus, melon, pistachio, and Knautia arvensis (Huang et al. 2011; Abbaspour et al. 2012; Doubková et al. 2013; Zou et al. 2015).
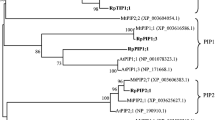
Besides influencing plant physiological and biochemical reactions, AM fungi-regulated plant aquaporin genes expression was also reported to be beneficial for plant under drought stress (Aroca et al. 2007). Aquaporins are water channels that have the capacity of water permeation 10–100 times higher than that of diffusion (Agre et al. 2002). In plants, aquaporins could be separated into five subfamilies: the plasma membrane intrinsic proteins (PIPs), the tonoplast intrinsic proteins (TIPs), the Nodulin 26-like intrinsic proteins (NIPs), small basic intrinsic proteins (SIPs), and the X-intrinsic proteins (XIPs) (Chaumont and Tyerman 2014). Under drought stress, mycorrhizal plants were observed to have better water status (higher root hydraulic conductivity and leaf water potential, and reduced leaf transpiration rate), which might be due to the AM fungi-regulated plant aquaporin genes (Ruiz-Lozano et al. 2006, 2009; Aroca et al. 2008; Maurel et al. 2008). With the help of AM fungi, relative water content of plant under drought stress was usually higher than their counterpart without AM fungi (Wu and Xia 2006; Huang et al. 2011, 2014). Along with improved plant photosynthesis and stomatal closure regulation by AM fungi, mycorrhizal plant usually had promoted water use efficiency (Birhane et al. 2012; Augé et al. 2015). Regulation by AM fungi would overall improve plant water status and drought tolerance (Aroca and Ruiz-Lozano 2009; Zhu et al. 2012).
Populus spp. and their hybrids were used as model plants in forest tree study due to their worldwide cultivation (Cooke et al. 2005). AM fungi could form symbiosis with different poplar species (Pallara et al. 2013; Li et al. 2015), increase their growth and tolerance to abiotic stresses, e.g., heavy metal (Cicatelli et al. 2010) and salinity (Wu et al. 2015). Drought could severely influence growth and physiological characteristics of Populus spp. (Han et al. 2013; Cao et al. 2014). However, the effect of AM fungi on Populus spp. drought tolerance was less investigated. Hence, this study aimed to explore the mechanism by which AM fungi increase drought tolerance of poplar through analyzing the influence of AM fungi on osmotic adjustment, antioxidation and expression of aquaporin genes.
Materials and methods
Growth substrate, plant material and AM fungal inoculum
Growth substrate, Populus × canadensis cuttings, and AM fungal inoculum (Rhizophagus irregularis) were prepared as described by Liu et al. (2015).
Experimental design and growth condition
The experiment consisted of a two-factorial design: (1) inoculation with either R. irregularis (5 g inoculum per pot) or not (5 g autoclaved inoculum with 10 mL inoculum filter drains); (2) water status, well watered (75 % of field capacity) or drought stressed (50 % of field capacity). Soil water content was controlled by controlling pot weights every day. There were four treatments with 30 replicates per treatment.
The growth condition was identical with Liu et al. (2015).
Mycorrhizal colonization
Twenty weeks after inoculation, roots of 6 seedlings from each treatment were collected, washed with tap water, and cut into 1 cm fragments. After staining with trypan blue (Phillips and Hayman 1970), root mycorrhizal colonization was measured by the method of Giovannetti and Mosse (1980).
Malondialdehyde (MDA) concentration, proline content, soluble protein content, POD activity and SOD activity
The fully expanded leaves of randomly selected six plants from each treatment were used to analyze malondialdehyde (MDA) content, proline content, soluble protein content, POD activity and SOD activity. MDA content was measured based on the method of Kramer et al. (1991). Proline content was measured by the method of Bates et al. (1973). Soluble protein contents were measured by the method of Bradford (1976). POD activity was measured by the method of Kwak et al. (1995). SOD activity was measured by the method of Giannopolitis and Ries (1977).
Relative water content (RWC) and water use efficiency (WUE)
The RWC of poplar leaves was measured as described by Liu et al. (2015).
Water use efficiency (WUE) was calculated as the ratio between net photosynthetic rate (Pn) and transpiration rate (E). Each leaf of a seedling was numbered according to a leaf plastochron index (LPI) number, with the most recently appeared leaf (about 2 cm long) being LPI 0 (Larson and Isebrands 1971). Based on the method described by Liu et al. (2015), leaves of LPI 6 of 6 seedlings from each treatment were used to measure Pn and E.
RNA extraction and first-strand cDNA synthesis
Leaf sample was used to extract RNA using polysaccharide and polyphenol total RNA isolation kit (Bioteke Corporation, China) according to the manufacturer’s instructions. RNA quality and quantity were evaluated by the A260/A280 ratio measured with NanoDrop 1000 spectrophotometer (Thermo-scientific, USA). The first-strand cDNA was synthesized using First-Strand cDNA Synthesis Kit (Sangon Biotech, China) from 0.5 μg RNA.
Quantitative real-time PCR (qRT-PCR) analysis
qRT-PCR was performed to analyze the transcript accumulation of PIP family genes using Ubiquitin gene as the reference gene. Primers used are listed in Table 1 (Secchi et al. 2009). qRT-PCR was performed with SYBR Premix Ex Taq™ II (Perfect Real Time) (Takara Biotechnology Co., Ltd, China) according to the manufacturer’s instructions. The amplifications were set at 20 μL reaction system including 10 μL SYBR Premix Ex Taq™ II, 0.8 μL forward and reverse primers, 7.4 μL nuclease-free water, and 1 μL cDNA. The reactions were performed on a Bio-Rad CFX96 real-time PCR instrument and the data were analyzed using Bio-Rad CFX Manager software, version 1.1 (Bio-Rad, USA).
The standard PIP genes PCR amplification was given as follows: initiated at 95 °C for 30 s, followed with 40 cycles of 95 °C for 5 s and 57 °C for 30 s. The fluorescence signal was recorded at each cycle end at 57 °C, and melting curve analysis was performed from 60 to 95 °C with fluorescence signal recorded every 0.5 °C during a 5 s hold. All qRT-PCRs were performed with three biological replications in three technical repetitions, and a maximum difference of 0.5 cycle between the CT of the duplicate samples was considered acceptable. Nuclease-free water was used as template in negative control and non-template controls were conducted for each primer pair to check significant levels of any contaminants.
C T values obtained from qPCR were compared with \(2^{{ - \Delta \Delta {\text{C}}_{T} }}\) method (Livak and Schmittgen 2001) or relative expression software tool (REST©) (Pfaffl et al. 2002).
Statistical analysis
Analysis of variance (ANOVA) was performed by the SPSS 17.0 statistical program (SPSS Inc., IL, USA). Duncan’s test was applied to compare means at 0.05 level of significance.
Results
AM colonization
No colonization was observed in non-mycorrhizal treatment. R. irregularis colonized 85.6 % poplar seedling roots under well-watered (WW) condition and 87.3 % poplar seedling roots under drought-stressed (DS) condition.
MDA
Both AM treatment and DS affected MDA content (P < 0.01). The interaction of AM treatment and DS showed no effect on the MDA content (Table 2). DS significantly promoted the MDA content in poplar leaves, while AM treatment decreased the MDA content in poplar leaves. The AM treatment had significantly reduced the leaves’ MDA content by 34.3 % under WW condition, and 26.8 % under DS condition, compared with non-AM seedlings (Fig. 1a).
Free proline content
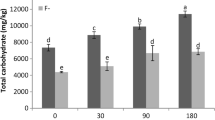
AM treatment affected the free proline content at P < 0.05 level. DS, the interaction of AM treatment and DS showed significant effect on the free proline content at P < 0.01 level (Table 2). Under WW condition, AM seedlings had higher free proline content than non-AM seedlings, but showed no significant difference. Under drought-stressed condition, AM inoculation decreased the free proline content by 13.5 % (Fig. 1b). Drought increased the free proline content of poplar seedlings, 72.5 % in non-AM seedlings while 39.3 % in AM seedlings (Fig. 1B).
Soluble protein content
The soluble protein content was increased by AM treatment and DS (P < 0.01). The interaction of AM treatment and DS showed no effect on the soluble protein content (Table 1). AM seedlings had higher soluble protein content than non-AM seedlings by 36.3 % under WW condition, and by 41.7 % under DS condition (Fig. 1c).
POD activity
AM treatment, DS and the interaction between AM treatment and DS significantly influenced POD activity (P < 0.01) (Table 2). AM seedling leaves had 36.6 % higher POD activity than non-AM seedling leaves under WW condition, while 5.7 % under DS condition. Drought increased the POD activity of poplar leaves, 89.8 % in non-AM seedlings while 47.0 % in AM seedlings (Fig. 1D).
SOD activity
SOD activity was influenced by AM treatment, DS and the interaction between AM treatment and DS (P < 0.01) (Table 2). Results showed that AM treatment had different effects on SOD activity of poplar seedling leaves under different water conditions. Under WW condition, AM treatment promoted the SOD activity by 24.2 %, but under DS condition, AM treatment decreased the SOD activity by 4.7 %. DS increased the SOD activity of non-AM poplar seedlings leaves, while had no effect on AM poplar seedlings leaves (Fig. 1E).
Water use efficiency
AM treatment significantly influenced WUE (P < 0.01) (Table 2). Under WW condition, AM inoculation had no effect on WUE of poplar seedlings. Under DS condition, AM inoculation showed positive effect on WUE of poplar seedlings, and increased 19.3 % compared with non-AM seedlings (Fig. 2a).
Relative water content
RWC was significantly influenced by AM treatment, DS and the interaction between AM treatment and DS (P < 0.01) (Table 1). DS decreased RWC of poplar seedlings. AM treatment had no effect on RWC of poplar seedlings under WW condition. However, the RWC of AM poplar seedlings was 10.2 % higher than non-AM poplar seedlings under DS condition (Fig. 2b).
Gene expression
AM treatment significantly influenced the expression of PIP1;1, PIP1;3, PIP1;4, PIP1;5, PIP2;3 (P < 0.01). DS significantly influenced the expression of PIP1;1, PIP1;3, PIP1;4, PIP1;5, PIP2;1, PIP2;2, PIP2;3 at P < 0.01 level and PIP1;2 at P < 0.05 level. The interaction of AM treatment and DS significantly influenced the expression of PIP1;4, PIP1;5, PIP2;1, PIP2;3 at P < 0.01 level, PIP1;1, PIP2;2 at P < 0.05 level and showed no effect on the expression of PIP1;2, PIP1;3 (Table 2). Under WW condition, AM treatment up-regulated the expression of PIP1;1, PIP1;3, PIP1;5, PIP2;1 and PIP2;3, and had no effect on the expression of PIP1;2, PIP1;4, PIP2;2. Under DS condition, AM inoculation up-regulated the expression of PIP1;1, PIP1;2, PIP1;3, PIP1;4, PIP1;5 and PIP2;3, reduced the expression of PIP2;1 and PIP2;2 (Fig. 3).
Effects of AM fungi on the expression of the aquaporin PIP genes of poplar seedlings under drought. Data are shown as mean ± SD (n = 3). Different letters on which column indicated significant differences at P < 0.05 level. M − W non-mycorrhizal and well-watered treatment; M − D non-mycorrhizal and drought-stressed treatment; M + W mycorrhizal and well-watered treatment; M + D mycorrhizal and drought-stressed treatment
Discussion
Plants developed their own defense systems to protect themselves from drought (Chaves and Oliveira 2004). Forming symbiosis with arbuscular mycorrhizal fungi was reported to be beneficial for plant in drought resistance (Augé 2001). In this study, R. irregularis colonized more than 80 % of poplar seedling roots in 20 weeks. This was consistent with the study of Liu et al. (2014) and set the basis for the study of AM fungus in helping poplar in drought resistance.
Under drought stress, plants accumulate some small molecules, such as proline, for osmotic adjustment (Augé 2001). In this study, drought increased proline content in poplar leaves, but the proline content in mycorrhizal plant was lower than in non-mycorrhizal plant. This was consistent with previous studies (Wu and Xia 2006; Monoharan et al. 2010; Asrar et al. 2012). However, mycorrhizal plants containing higher proline content than non-mycorrhizal plants were also observed in other studies (Fan and Liu 2011; Yooyongwech et al. 2013). Different plant response induced by AM fungi might be due to the different drought stress time. In short-time drought stress, the mycorrhizal plants responded fast and strong by accumulating more proline for osmotic adjustment as AM fungi induced ‘priming’ in response to disease (Jung et al. 2012). In long-time drought stress, AM fungi improved plant leaves’ water status, which was observed in this study, lowered the accumulation of proline. Contrast to the proline content, the soluble protein in mycorrhizal plant leaves was higher than in non-mycorrhizal plant leaves. This might be owed to the fact that AM fungi-improved plant photosynthesis accumulated proteins (Wu and Xia 2006; Wu et al. 2010; Zhu et al. 2012) and AM fungi induced/enhanced specific plant protein under drought stress (Fan and Liu 2011).
Plant could strictly control ROS production and removal under well-watered condition, but lose its control under drought stress, which might result in proteins, DNA and lipids oxidation. Generated by peroxidation of membrane lipid, MDA could reflect the ROS circulation (Lacan and Baccou 1998). In this study, R. irregularis significantly reduced the MDA accumulation which indicated less injury during drought in poplar seedling leaves. This was consistent with studies that analyze the AM fungal influence on plant drought tolerance (Porcel and Ruiz-Lozano 2004; Ruiz-Sánchez et al. 2010). Efficient scavenge of ROS needs the cooperation of several antioxidative enzymes. Increased antioxidative enzymes activity, resulting in limited oxidative damage, had been reported in mycorrhizal plants (Wu and Zou 2009; Huang et al. 2014; Zou et al. 2015). In this study, drought increased the SOD and POD activity in leaves of non-mycorrhizal poplars and increased only POD activity in leaves of mycorrhizal poplars. This indicated that less ROS was accumulated in leaves of inoculated poplars, further confirmed by limited MDA accumulation. In leaves of inoculated poplars, inhibited oxidative damage was probably due to the improved water status observed in this study.
Plant water status and plant photosynthesis, and metabolic processes are closely related. Compared with non-mycorrhizal plants, higher RWC and WUE were observed in mycorrhizal plants (Kaya et al. 2003; Wu and Xia 2006; Huang et al. 2011; Birhane et al. 2012; Huang et al. 2014), and it is believed to be beneficial for stomatal open and high plant metabolism maintenance (Zhu et al. 2012). In this study, AM fungi significantly improved RWC and WUE of poplar leaves under drought stress. This result, on one hand, indicated that R. irregularis could improve poplar seedlings’ water absorption and regulate plant water homeostasis. On the other hand, the improved RWC explained the limited accumulation of antioxidative enzymes, MDA and proline in leaves of inoculated poplars. Improved RWC and WUE by AM fungus might be due to the expanded water absorption area and systemically modified plant gene expression (Augé 2001; Cervantes-Gámez et al. 2016).
Drought stress and AM fungal inoculation-regulated aquaporin genes expression had been documented in previous studies (Porcel et al. 2005, 2006; Bárzana et al. 2012). In this study, R. irregularis differentially regulated poplar PIP genes under drought stress. This result was consistent with previous studies (Aroca et al. 2007; He et al. 2016), and it might be due to the different functions that each PIP genes played from the same gene family (Almeida-Rodriguez et al. 2010). With the help of AM fungi, the water demand might be partially satisfied under drought-stressed condition. In accordance with the improved water status discussed above, 6 PIP genes that were up-regulated in mycorrhizal poplar were used to improve water permeability (Chaumont and Tyerman 2014). Down-regulation of the PIP2;1 and PIP2;2 may be a water protection mechanism to avoid the plant dehydration under drought stress (Alexandersson et al. 2005). Another possibility was the plant-modulated gene localization to facilitate water absorption in other tissue (He et al. 2016). The specific function of each PIP gene under drought stress needed further investigation.
In conclusion, our results indicated that R. irregularis could improve poplar RWC under drought stress via regulation of aquaporin genes expression, and consequently increased WUE, lowered accumulation of osmotic adjustment molecule, reduced ROS accumulation and oxidative damage. Further studies are needed to illustrate the influence of AM fungi on specific aquaporin PIP gene location, function and expression in plant responding to drought stress.
Author contribution statement
Ting Liu carried out the experiment, gathered the data, and drafted the manuscript. Zhen Li gave assistance in qRT-PCR, tables and figures preparation in this manuscript. Hui Chen, Ming Tang and Haoqiang Zhang helped interpretation of the results, prepared and revised the manuscript.
References
Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab M (2012) Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J Plant Physiol 169:704–709
Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S (2002) Aquaporin water channels-from atomic structure to clinical medicine. J Physiol 542:3–16
Ahmed CB, Rouina BB, Sensoy S, Boukhris M, Abdallah FB (2009) Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ Exp Bot 67:345–352
Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59:469–484
Almeida-Rodriguez AM, Cooke JE, Yeh F, Zwiazek JJ (2010) Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii × balsamifera clones with different drought resistance strategies. Physiol Plant 140:321–333
Aroca R, Ruiz-Lozano JM (2009) Induction of plant tolerance to semi-arid environments by beneficial soil microorganisms—a review. Sustain Agric Rev 2:121–135
Aroca R, Porcel R, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 173(4):808–816
Aroca R, Vernieri P, Ruiz-Lozano JM (2008) Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J Exp Bio 59:2029–2041
Asrar A, Abdel-Fattah G, Elhindi K (2012) Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 50:305–316
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11(1):3–42
Augé R, Toler H, Saxton A (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24
Bárzana G, Aroca R, Paz JA, Chaumont F, Martinez-Ballesta MC, Carvajal M, Ruiz-Lozano JM (2012) Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann Bot 109(5):1009–1017
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Birhane E, Sterck FJ, Fetene M, Bongers F, Kuyper TW (2012) Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 169(4):895–904
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao X, Jia J, Zhang C, Li H, Liu T, Jiang X, Polle A, Peng C, Luo ZB (2014) Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol Plant 151(4):480–494
Cervantes-Gámez RG, Bueno-Ibarra MA, Cruz-Mendívil A, Calderón-Vázquez CL, Ramírez-Douriet CM, Maldonado-Mendoza IE, Villalobos-López MÁ, Valdez-Ortíz Á, López-Meyer M (2016) Arbuscular mycorrhizal symbiosis-induced expression changes in Solanum lycopersicum leaves revealed by RNA-seq analysis. Plant Mol Biol Rep 34(1):89–102
Chaumont F, Tyerman SD (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164(4):1600–1618
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55(407):2365–2384
Cicatelli A, Lingua G, Todeschini V, Biondi S, Torrigiani P, Castiglione S (2010) Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann Bot Lond 106:791–802
Cooke JEK, Martin TA, Davis JM (2005) Short-term physiological and developmental responses to nitrogen availability in hybrid poplar. New Phytol 167:41–52
Doubková P, Vlasáková E, Sudová R (2013) Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 370:149–161
Fan QJ, Liu JH (2011) Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol Plant 33:1533–1542
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. In: Sustainable agriculture. Springer, Netherlands, pp 153–158
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500
Han Y, Wang Y, Jiang H, Wang M, Korpelainen H, Li C (2013) Reciprocal grafting separates the roles of the root and shoot in sex-related drought responses in Populus cathayana males and females. Plant Cell Environ 36:356–364
He F, Zhang H, Tang M (2016) Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 26(4):311–323
Hessini K, Martínez JP, Gandour M, Albouchi A, Soltani A, Abdelly C (2009) Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ Exp Bot 67:312–319
Huang Z, Zou Z, He C, He Z, Zhang Z, Li J (2011) Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil 339:391–399
Huang YM, Srivastava AK, Zou YN, Ni QD, Han Y, Wu QS (2014) Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front Microbiology 5:682. doi:10.3389/fmicb.2014.00682
Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38(6):651–664
Kaya C, Higgs D, Kirnak H, Tas I (2003) Mycorrhizal colonisation improves fruit yield and water use efficiency in watermelon (Citrullus lanatus Thunb.) grown under well-watered and water-stressed conditions. Plant Soil 253(2):287–292
Kramer GF, Norman HA, Krizek DT, Mirecki RM (1991) Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 30:2101–2108
Kwak SS, Kim SK, Lee MS, Jung KH, Park IH, Liu JR (1995) Acidic peroxidases from suspension-cultures of sweet potato. Phytochemistry 39:981–984
Lacan D, Baccou JC (1998) High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 204:377–382
Larson PR, Isebrands JG (1971) The plastochron index as applied to developmental studies of cottonwood. Can J For Res 1(1):1–11
Li Z, Wu N, Liu T, Chen H, Tang M (2015) Sex-related responses of Populus cathayana shoots and roots to AM fungi and drought stress. PLoS One 10(6):e0128841
Lin CC, Kao CH (2000) Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul 30:151–155
Liu C, Liu Y, Guo K, Fan D, Li G, Zheng Y, Yu L, Yang R (2011) Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ Exp Bot 71:174–183
Liu T, Wang C, Chen H, Fang F, Zhu X, Tang M (2014) Effects of arbuscular mycorrhizal colonization on the biomass and bioenergy production of Populus × canadensis ‘Neva’ in sterilized and unsterilized soil. Acta Physiol Plant 36(4):871–880
Liu T, Sheng M, Wang CY, Chen H, Li Z, Tang M (2015) Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 53(2):250–258
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Ann Rev Plant Biol 59:595–624
Monoharan PT, Shanmugaiah V, Balasubramanian N, Gomathinayagam S, Sharma MP, Muthuchelian K (2010) Influence of AM fungi on the growth and physiological status of Erythrina variegata Linn. grown under different water stress conditions. Eur J Soil Biol 46:151–156
Pallara G, Todeschini V, Lingua G, Camussi A, Racchi ML (2013) Transcript analysis of stress defence genes in a white poplar clone inoculated with the arbuscular mycorrhizal fungus Glomus mosseae and grown on a polluted soil. Plant Physiol Bioch 63:131–139
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):e36
Phillips J, Hayman D (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158-IN118
Porcel R, Ruiz-Lozano JM (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J Exp Bot 55(403):1743–1750
Porcel R, Gómez M, Kaldenhoff R, Ruiz-Lozano JM (2005) Impairment of NtAQP1 gene expression in tobacco plants does not affect root colonisation pattern by arbuscular mycorrhizal fungi but decreases their symbiotic efficiency under drought. Mycorrhiza 15:417–423
Porcel R, Aroca R, Azcon R, Ruiz-Lozano JM (2006) PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol Biol 60(3):389–404
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317
Ruiz-Lozano JM, Porcel R, Aroca R (2006) Does the enhanced tolerance of arbuscular mycorrhizal plants to water deficit involve modulation of drought-induced plant genes? New Phytol 171:693–698
Ruiz-Lozano JM, del Mar Alguacil M, Barzana G, Vernieri P, Aroca R (2009) Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non mycorrhizal maize plants through regulation of PIP aquaporins. Plant Mol Biol 70:565–579
Ruiz-Sánchez M, Aroca R, Muñoz Y, Polón R, Ruiz-Lozano JM (2010) The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J Plant Physiol 167(11):862–869
Secchi F, Maciver B, Zeidel ML, Zwieniecki MA (2009) Functional analysis of putative genes encoding the PIP2 water channel subfamily in Populus trichocarpa. Tree Physiol 29:1467–1477
Smirnoff N (1993) Tansley review no. 52. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125(1):27–58
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, Cambridge
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Bioch 47(7):570–577
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Wu Q, Zou Y (2009) Mycorrhiza has a direct effect on reactive oxygen metabolism of drought-stressed citrus. Plant Soil Environ 55:436–442
Wu QS, Zou YN, He XH (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32(2):297–304
Wu N, Li Z, Liu H, Tang M (2015) Influence of arbuscular mycorrhiza on photosynthesis and water status of Populus cathayana Rehder males and females under salt stress. Acta Physiol Plant 37(9):1–14
Yooyongwech S, Phaukinsang N, Cha-um S, Supaibulwatana K (2013) Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul 69:285–293
Zhu X, Song F, Liu S, Liu T, Zhou X (2012) Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ 58:186–191
Zou YN, Huang YM, Wu QS, He XH (2015) Mycorrhiza-induced lower oxidative burst is related with higher antioxidant enzyme activities, net H2O2 effluxes, and Ca2+ influxes in trifoliate orange roots under drought stress. Mycorrhiza 25(2):143–152
Acknowledgments
This research was supported by Special Fund for Forest Scientific Research in the Public Welfare (201404217), the National Natural Science Foundation of China (31270639 and 31170567), and the Program for Changjiang Scholars and Innovative Research Team in University of China (IRT1035). We also thank the anonymous reviewers for reviewing the manuscript and offering helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J Zwiazek.
Rights and permissions
About this article
Cite this article
Liu, T., Li, Z., Hui, C. et al. Effect of Rhizophagus irregularis on osmotic adjustment, antioxidation and aquaporin PIP genes expression of Populus × canadensis ‘Neva’ under drought stress. Acta Physiol Plant 38, 191 (2016). https://doi.org/10.1007/s11738-016-2207-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2207-6