Abstract
The influence of arbuscular mycorrhiza (AM) and drought stress on aquaporin (AQP) gene expression, water status, and photosynthesis was investigated in black locust (Robinia pseudoacacia L.). Seedlings were grown in potted soil inoculated without or with the AM fungus Rhizophagus irregularis, under well-watered and drought stress conditions. Six full-length AQP complementary DNAs (cDNAs) were isolated from Robinia pseudoacacia, named RpTIP1;1, RpTIP1;3, RpTIP2;1, RpPIP1;1, RpPIP1;3, and RpPIP2;1. A phylogenetic analysis of deduced amino acid sequences demonstrated that putative proteins coded by these RpAQP genes belong to the water channel protein family. Expression analysis revealed higher RpPIP expression in roots while RpTIP expression was higher in leaves, except for RpTIP1;3. AM symbiosis regulated host plant AQPs, and the expression of RpAQP genes in mycorrhizal plants depended on soil water condition and plant tissue. Positive effects were observed for plant physiological parameters in AM plants, which had higher dry mass and lower water saturation deficit and electrolyte leakage than non-AM plants. Rhizophagus irregularis inoculation also slightly increased leaf net photosynthetic rate and stomatal conductance under well-watered and drought stress conditions. These findings suggest that AM symbiosis can enhance the drought tolerance in Robinia pseudoacacia plants by regulating the expression of RpAQP genes, and by improving plant biomass, tissue water status, and leaf photosynthesis in host seedlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black locust (Robinia pseudoacacia L.) is a fast-growing woody legume originating in southeastern USA, and it has been planted ubiquitously in temperate terrestrial ecosystems (Barrett et al. 1990). Robinia pseudoacacia has great economic value: The leaves are important source of livestock feed, the flowers are available for wild honey production, and the wood is often used for lumber, buildings, and vehicles (Dickerson 2002). Robinia pseudoacacia shows high resistance to water deficit (Mantovani et al. 2014), and it is one of the most widely distributed forest trees in the world (Garlock et al. 2012). It has been widely planted to prevent soil erosion and desertification in the Loess Plateau, located in western China, for the past three decades (Jin et al. 2011). Therefore, it is most important to improve the growth and increase the drought tolerance of Robinia pseudoacacia seedlings in arid and semi-arid regions.
Water is the vital regulator of plant growth, and drought hampers water uptake (Ouziad et al. 2006). Under drought stress, plants undergo a series of physiological, physical, and morphological changes for long-term adaptations and short-term effects. Such changes are ultimately regulated by aquaporins (AQPs) (Luu and Maurel 2005), a class of membrane intrinsic proteins (MIPs) that mediate water transport across membranes following an osmotic gradient (Li et al. 2014). Plant AQPs comprise multiple homologous genes, with several tens of members in different species, such as rice (Nguyen et al. 2013), Arabidopsis (Maurel et al. 2008), and Populus trichocarpa (Gupta and Sankararamakrishnan 2009). According to amino acid sequence similarities, plant AQPs are divided into five subfamilies: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), NOD26-like MIPs or NOD26-like intrinsic proteins (NIPs), small basic intrinsic proteins (SIPs), and the newly identified X (or uncategorized) intrinsic proteins (XIPs). TIPs and PIPs are the most abundant AQPs in plant vacuolar and plasma membranes, respectively (Laur and Hacke 2014).
Plant AQPs have been shown to be associated with various physiological processes, and their sophisticated mechanisms for functional regulation have been extensively researched over the past decade. AQPs participate in plant responses to various environmental stresses, including drought, salinity, and heavy metals (Ligaba et al. 2011; Sreedharan et al. 2013). They are water-selective channels involved in plant tolerance to drought and have been used in bioengineering studies to improve crop resistance and yield (Zhang et al. 2008). However, in some cases, the overexpression of an AQP gene is not beneficial to plant growth and can even exert a deleterious effect by accelerating plant wilting under drought stress (Aharon et al. 2003; Wang et al. 2011). Plants may limit their symplastic water transport via AQPs, and hence their transpiration rate as a defensive mechanism to prevent faster wilting under water stress (Aharon et al. 2003). However, the roles of plant AQPs in water transport under drought stress and favorable growth conditions, which may be mediated by plant species, growth conditions, and growth stage, remain a matter of debate (Aharon et al. 2003; Guo et al. 2006; Sreedharan et al. 2013; Wang et al. 2011; Zhang et al. 2008), and further studies are needed to elucidate specific functions of AQP proteins in plant cells.
The symbiotic relationship between arbuscular mycorrhizal (AM) fungi and higher plants is widely established in almost all terrestrial ecosystems (Yang et al. 2014). Plants obtain nutrients and water via this symbiosis to survive under adverse conditions (Smith and Read 2008). AM symbiosis can enhance plant resistance to drought, for example in Phaseolus vulgaris (Aroca et al. 2007), Zea mays (Bárzana et al. 2012), Helianthus annuus (Gholamhoseini et al. 2013), and Robinia pseudoacacia (Yang et al. 2014). Plant root cells undergo extensive morphological alterations to accommodate the presence of the AM fungal symbiont, and most of the changes occur in vacuolar or cytoplasmic membrane systems (Bárzana et al. 2014). Therefore, changes in the expression patterns of genes encoding membrane-associated proteins such as AQPs can be expected in AM plants (Aroca et al. 2007; Bárzana et al. 2014; Krajinski et al. 2000).
Since AM symbiosis has a remarkable capacity to alter the hydraulic properties of plant roots, it is not surprising that AM fungi can regulate plant AQP gene expression (Aroca et al. 2007; Bárzana et al. 2014) and that the significance of AQPs for nutrient and water exchanges during symbiosis between plants and mycorrhizal fungi is discussed (Maurel and Plassard 2011). Thus far, AQPs have been isolated and characterized in several plant species, including rice (Nguyen et al. 2013), Arabidopsis (Maurel et al. 2008), and Populus trichocarpa (Gupta and Sankararamakrishnan 2009). However, no AQP gene sequences of Robinia pseudoacacia are available, and the effect of AM fungi on the expression of Robinia pseudoacacia AQP genes (RpAQPs) has not been examined under drought stress.
AM fungi colonize many plant species, including key model plants such as Populus trichocarpa, Lotus japonicas, and Medicago truncatula (Smith and Read 2008). Rhizophagus irregularis is among the most studied of AM fungi and is the prime ingredient in several commercially available inocula (Hart et al. 2015). Moreover, Rhizophagus irregularis is the only AM fungal species for which spores are available commercially in pure form in large quantities (Tisserant et al. 2012). In the present study, the effect of Rhizophagus irregularis on AQP gene expression, water status, drought resistance, and photosynthesis in Robinia pseudoacacia seedlings was evaluated under different soil water conditions. Results provide new insights into the role of AM in alleviating drought stress to Robinia pseudoacacia.
Materials and Methods
Plants, growth substrate, and AM fungal inoculum
Seeds of Robinia pseudoacacia L. were purchased from a local market (Yangling, China). Seeds were surface-sterilized in 10 % NaClO for 10 min and washed with sterile water eight times, before pre-germination on wet filter paper in petri dishes at 25 °C. Ten-day-old seedlings were transplanted into plastic pots, with three plants per pot. After 15 days, one seedling of uniform growth was kept in each pot.
The plant growth substrate contained sand and soil (1:1, v/v). The soil was collected from the top soil layer (5–25 cm) on the Northwest A&F University campus (Yangling, China) and passed through a 2-mm sieve. Soil available phosphorus was extracted with 0.5 M sodium bicarbonate (NaHCO3; pH 8.5) for 30 min and determined colorimetrically with a spectrophotometer (Hitachi, UV2300, Japan) at 660 nm (Page 1982). Available nitrogen was estimated by a micro-diffusion technique after alkaline hydrolysis (Page 1982). Available potassium was extracted with 1 M ammonium acetate (CH3COONH4; pH 7) and determined using a Perkin-Elmer flame photometer (Perkin-Elmer Corp., USA) (Bao 2000). Soil organic matter content was assayed by dichromate oxidation and titration with ferrous sulfate (Nelson and Sommers 1982). Soil pH was determined with a digital pH meter (Leici PHS-3D, Shanghai, China) by using a 1:5 (w/v) soil/water suspension (Bao 2000). The basic soil physicochemical properties were as follows: available phosphorus 13.78 mg kg−1, available nitrogen 33.98 mg kg−1, available potassium 152.54 mg kg−1, organic matter 16.21 g kg−1, and pH 7.59. Sand was collected from Wei River near the campus and washed ten times with tap water. The sand/soil mixture was autoclaved at 121 °C for 2 h.
The AM inoculum of Rhizophagus irregularis (Bank of Glomales in China, No. BGC BJ09) was provided by Beijing Academy of Agriculture and Forestry Sciences (Beijing, China). The inoculum consisted of spores (∼50 spores per gram), hyphae, colonized root fragments, and sand.
Experimental design
Pot experiments were conducted at Northwest A&F University from March to June 2014. Seedlings were planted in pots (top diameter 170 mm; depth 160 mm) filled with 1.5 kg of sand/soil substrate and grown in a greenhouse at 12–35 °C with a 14-h light/10-h dark cycle. The experimental layout had two factors: inoculation status (Rhizophagus irregularis inoculation, AM; no Rhizophagus irregularis inoculation, NM) and water status (well-watered, WW; drought stress, DS). Forty pots were inoculated with 10 g of AM fungal inoculum each, and another forty pots received 10 g of sterilized AM inoculum (autoclaved at 121 °C for 2 h) with 10 mL of inoculum filtrate (<20 μm) to provide a normal microbial population free of AM propagules (Porcel et al. 2006).
Pots were arranged in a completely randomized block design. Volumetric soil moisture was determined using a ML2 ThetaProbe (Delta-T Devices Ltd., Cambridge, UK). Field capacity (14.5 % volumetric soil moisture) was measured by using a pressure plate apparatus to apply one third the atmospheric pressure for 48 h and followed by determination of volumetric soil moisture (Porcel and Ruiz-Lozano 2004). In total, eighty pots were initially well-watered and kept at 75–80 % of field capacity (10.7–11.4 % of volumetric soil moisture) for 75 days. Next, half of the inoculated and non-inoculated pots (20 each) were drought-stressed and kept at 35–40 % of field capacity (5–5.7 % of volumetric soil moisture) for 14 days. Meanwhile, the remaining half of the inoculated and non-inoculated pots (20 each) were well-watered (controls) and kept at 75–80 % of field capacity for 14 days. Water was supplied to each pot daily at 17:00 to maintain field capacity in the designated range throughout the experimental period. At the end of the experiment, three plants were harvested and stored in liquid nitrogen at −80 °C before gene cloning and expression analysis. Five plants were randomly selected to determine AM fungal colonization, electrolyte leakage, dry mass, water saturation deficit, and gas-exchange parameters.
AM fungal colonization
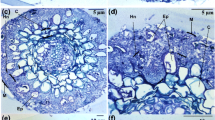
AM fungal colonization was measured using the method of Phillips and Hayman (1970) with minor modifications. Fresh roots were collected immediately after the seedlings were harvested. The roots were gently washed with running tap water and cut into 1-cm pieces, before clearing with 10 % KOH at 90 °C for 30 min, bleaching with alkalized H2O2 for 20 min, acidifying with 1 % HCl at room temperature for 30 min, staining with trypan blue solution (450 mL water, 500 mL glycerol, 50 mL 1 % HCl, and 0.05 % trypan blue) at 90 °C for 5 min, and finally destaining with lactic acid–glycerin (1:1, v/v) at room temperature. AM fungal colonization was estimated by using the grid line intersection method under a light microscope (Giovannetti and Mosse 1980).
Plant growth and hydraulic parameters
A precision straight edge was used to measure plant height. For water saturation deficit (WSD) determination, and the fresh mass (FM) of root, stem, and leaf, samples were measured immediately after collection. The turgid mass (TM) was estimated by soaking the samples in distilled water for 24 h, and then dry mass (DM), including root dry mass (RDM), stem dry mass (SDM), and leaf dry mass (LDM), was estimated by drying in a hot air oven (80 °C) to constant weight. WSD (Farissi et al. 2013), aboveground dry mass, and total dry mass were calculated as follows:
To measure electrolyte leakage (EL), ten fresh young leaf discs (6 mm in diameter) were cut before harvest from the uppermost fully expanded leaves for each treatment and thoroughly rinsed five times with deionized water. The clean leaf discs were placed in closed vials containing 10 mL of deionized water and incubated at 25 °C for 24 h on a rotary shaker (100 rpm) before determination of the initial electrical conductivity (EC 0). Samples were then autoclaved at 121 °C for 20 min and the final electrical conductivity (EC t) was obtained after attaining equilibrium at 25 °C. EL was calculated with the following formula (Nedjimi 2014):
Leaf physiological parameters
Net photosynthetic rate (P n), stomatal conductance (G s), transpiration rate (T r), and intercellular CO2 concentration (C i) were measured before harvest using a LI-6400 portable open flow gas-exchange system (LI-COR Inc., Lincoln, NE, USA). Measurements (n = 5) were made on the fifth youngest leaf of each seedling from 08:30 to 11:30 a.m. (Zhu et al. 2014). Prior to the measurement, the instrument was warmed up for 30 min and calibrated in the ZERO IRGA mode. Two steps of calibration were taken: initial zeroing for the built-in flow meter and second zeroing for the infrared gas analyzer (Jaafar et al. 2012). Automatic measurements were made under optimal conditions: 1000 μmol m−2 s−1 photosynthetically active irradiation, 60 % relative humidity, 0.5 dm3 min−1 air flow rate, 25 °C leaf temperature, and 400 μmol mol−1 CO2 concentration in the sample chamber. Data were stored in the LI-6400 console and analyzed by the Photosyn Assistant software (Version 3, Lincoln Inc., USA). Water use efficiency (WUE) was calculated as
RNA isolation and cDNA synthesis
Frozen root samples of Robinia pseudoacacia were homogenized in liquid nitrogen. Total RNA was extracted with the Trizol RNA isolation kit (Sangon, Shanghai, China) according to the manufacturer’s protocol. RNA integrity was checked by electrophoresis in 1.0 % agarose gels, and quantitative determination was carried out by spectrophotometric analysis with a Nano Drop 2000 (Thermo scientific, Pittsburgh, PA, USA). RNA purity was estimated by calculating the A260/A280 ratio. RNA was reverse-transcribed to complementary DNA (cDNA) using a PrimeScriptTM RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China).
RpAQP gene cloning and sequence analysis
The cDNA obtained from Robinia pseudoacacia root RNA was used as template in PCR reactions. Degenerate primers were designed according to the conserved regions from a ClustalX (version 1.8 software package, available at http://www-igmbc.u-strasbrg.fr/BioInfo/clustalx,ftp://ftp-igmbc.u-strasbrg.fr) alignment of published AQP sequences from M. truncatula, Phaseolus vulgaris, Glycine max, Lotus japonicus (http://www.ncbi.nlm.nih.gov/protein) (Supplementary Table S1). Six pairs of degenerate primers (Supplementary Table S2), designed in Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA), were used to clone and screen the putative conservative fragment from the AQP family. cDNA sequences were amplified with a C1000 thermocycler (BioRad Laboratories, Hercules, CA, USA) in a 20-μL reaction containing 0.5 μL of each primer, 1 μL cDNA, 8 μL ddH2O, and 10 μL Premix Taq® Version 2.0. PCR conditions included an initial denaturation for 3 min at 95 °C, followed by 25 cycles of 95 °C denaturation for 30 s, 56 °C annealing for 30 s, 72 °C elongation for 1 min, and a final 72 °C extension for 10 min (Navarro-Ródenas et al. 2013).
PCR products were purified and cloned into the pMD 18-T vector and then transformed into competent Escherichia coli DH5α. The transformants were selected on LB agar plates containing ampicillin (50 μg mL−1), X-Gal (0.002 %), and IPTG (0.2 mM). Ten randomly chosen clones with inserts were sequenced by GenScript USA Inc. (Nanjing, China). The sequences obtained were blasted on NCBI (www.ncbi.nlm.nih.gov), translated into amino acid sequences using the ExPASy Translate Tool (http://www.expasy.org/tools/dna.html), and subjected to a BLASTp search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) against the NCBI GenBank database (www.ncbi.nlm.nih.gov/genbank/).
The conservative fragment sequences identified above were used to design gene-specific primers (Supplementary Table S2) for amplifying the 5′ and 3′ end sequences. PCR amplification was performed using rapid amplification of cDNA ends (RACE) with the SMARTerTM RACE cDNA Amplification Kit (Clontech Laboratories Inc., Mountain, CA, USA). Amplicons were purified, cloned, sequenced, and blasted against the GenBank database as described above. AutoAssembler (Perkin Elmer) was applied to assemble the conservative fragment with 5′ and 3′ end sequences to acquire the complete sequences. Specific primers (Supplementary Table S2) were then designed based on the obtained complete sequences to amplify the full-length DNA of six RpAQP genes. PCR reactions were prepared and PCR products were purified, cloned, and sequenced as previously described (Navarro-Ródenas et al. 2013). The deduced amino acid sequences were deposited in GenBank and accession numbers are listed in Table 4.
Bioinformatics and phylogenetic analysis of full-length RpAQP genes
Molecular weight (kDa) and isoelectric point were analyzed by the PROTPARAM program (Gasteiger et al. 2005). Probable subcellular localization was predicted using the TARGETP program (Emanuelsson et al. 2000) with the default parameters. Sequences were aligned using the MultAlin program (http://multalin.toulouse.inra.fr/multalin/multalin.html). Protein sequence alignments and phylogenetic tree construction were performed using MEGA 5.0 (Tamura et al. 2011).
Expression analysis of six RpAQP genes in different tissues and treatments
Total RNA was extracted from roots, stems, and mature Robinia pseudoacacia leaves as described above. First-strand cDNA was synthesized using the PrimeScriptTM RT reagent Kit with gDNA Eraser (TaKaRa). Primer Premier 5.0 was used to design primer pairs for quantitative real-time PCR (qRT-PCR) analysis (Supplementary Table S2). The qRT-PCR reaction contained 12.5 μL of SYBR Premix Ex Taq II (TaKaRa), 0.4 μL of each primer, 2 μL of diluted cDNA template, and 9.7 μL ddH2O. qRT-PCR reactions were performed using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories) with the following amplification procedure: initial denaturation for 3 min at 95 °C, followed by 20 cycles at 95 °C for 30 s, 59 °C for 20 s, and 72 °C for 20 s, and a final extension step at 72 °C for 5 min (Navarro-Ródenas et al. 2013).
PCR contamination was not detected in negative controls for each analysis. The experiment was replicated three times (biological replicates) with three technical replications for each gene. Relative quantification was assessed using the 2−∆∆CT method (Livak and Schmittgen 2001), and the expression levels of all genes were normalized to that of the 18S rRNA gene (Chen et al. 2013).
Statistical analysis
Data are means ± standard deviation. The physiological data were analyzed on five biological replicates. Gene expression was analyzed on three biological replicates. SPSS 17.0 (IBM SPSS Statistics, Chicago, IL, USA) was used for two-way ANOVAs and Duncan’s test (P = 0.05).
Results
AM fungal colonization and plant growth
Rhizophagus irregularis colonization of Robinia pseudoacacia seedling roots was significantly (P ≤ 0.001) influenced by AM fungal inoculation, DS, and their interactions (Table 1). No AM fungal colonization was detected in the NM plants. Rhizophagus irregularis-inoculated plants showed 77 % root colonization under WW, while water deficit under DS provoked a slight reduction in root colonization (70 %).
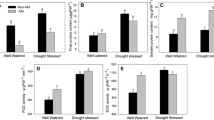
Plant height of AM plants was higher than that of NM plants under WW (68.1 %) and DS (69.3 %) conditions. DS caused a large reduction in aboveground (63.9 and 80.8 %), belowground (70.0 and 89.1 %), and the total dry mass (64.9 and 82.4 %) of AM and NM seedlings, respectively. All the three parameters were higher in AM plants than in NM plants under different soil water conditions, although the differences in aboveground and belowground dry mass were not significant between AM and NM plants under DS condition (Table 1).
DS influenced WSD of roots, stems, and leaves as well as EL of Robinia pseudoacacia seedlings (Table 2). AM and DS showed an interaction on WSD of roots and EL of seedlings. Under WW, Rhizophagus irregularis inoculation brought a significant reduction in root (43.7 %) and leaf (32.5 %) WSD (P ≤ 0.05). Under DS, AM plants had lower root and leaf WSD, and EL than NM plants (P ≤ 0.05), although DS markedly increased stem and leaf WSD, and EL in both NM and AM seedlings.
Photosynthetic and hydraulic parameters
AM formation and DS significantly (P ≤ 0.05) influenced P n, G s, T r, C i, and WUE of Robinia pseudoacacia seedlings (Table 3). DS led to a decrease in P n (43.5 and 42.5 %), G s (14.8 and 11.1 %), T r (25.6 and 26.8 %), and WUE (25.0 and 22.2 %) in AM and NM seedlings, respectively, but an increase in C i (7.0 and 24.4 %). Rhizophagus irregularis inoculation improved P n, G s, T r, and WUE under different soil water conditions. The differences were significant between AM and NM plants except for WUE under DS.
RpAQP gene isolation and identification
Six RpAQP genes were successfully cloned and sequenced. The deduced amino acid sequences have been deposited in GenBank, and their accession numbers are listed in Table 4. A neighbor-joining tree was constructed based on amino acid sequences of AQP proteins from M. truncatula, Arabidopsis thaliana, Z. mays and those deduced from the cloned Robinia pseudoacacia genes (Fig. 1). BLAST searches showed that the identity between the full-length sequences of six RpAQP genes and the reference sequences retrieved from GenBank database is 77–97 %. RpAQP genes (RpTIP1;1, RpTIP1;3, RpTIP2;1, RpPIP1;1, RpPIP1;3, and RpPIP2;1) share the highest sequence identity (91, 92, 90, 95, 97, and 89 %) with M. truncatula genes.
Neighbor-joining tree based on amino acid sequences of six Robinia pseudoacacia aquaporins (in bold) from the present study and closely related relatives retrieved from the GenBank database (accession numbers in parentheses). The plant plasma membrane intrinsic proteins (PIPs) form a divided cluster representing the PIP1 and the PIP2 subfamilies. The plant tonoplast intrinsic proteins (TIPs) form a divided cluster representing the TIP1 and the TIP2 subfamilies. Bootstrap values shown at nodes are for frequencies at or above a 50 % threshold (1000 bootstrap resampling). Bar indicates 0.2 sequence variance
Tissue expression levels of six Robinia pseudoacacia aquaporin (RpAQP) genes. RpAQP transcript abundance in roots, stems, and leaves was analyzed by quantitative real-time PCR. Relative expression levels were assessed using the 2−ΔΔCT method and 18S rRNA as reference gene. Data are means ± SD (n = 3 biological replicates). Different lowercase letters (a to c) within each stacked column indicate significant differences at P ≤ 0.05
The full-length sequences of the RpAQPs genes are 825–1201 bp, with 750–870-bp open reading frame encoding 251–289 amino acids. The predicted protein molecular weights are between 25.37 and 31.06 kDa, and the isoelectric points are in the range of 5.09–8.96. Subcellular localization prediction indicates that RpAQPs are probably localized in the tonoplast (RpTIP1;1, RpTIP1;3, and RpTIP2;1) or plasma membrane (RpPIP1;1, RpPIP1;3, and RpPIP2;1) (Table 4). The complete amino acid sequences contained typical conserved residues, including NPA motifs and Ar/R (aromatic/arginine) selectivity filter (H2, H5, LE1, and LE2) and Froger’s position (P1–P5) (Supplementary Fig. S1). All RpAQPs contain the MIP family signature sequence SGXHXNPAVT, and three RpPIPs contain the signal region (XGGA NXXXX GY and TGXNPARSXGAAXIXN) of plasma membrane.
Tissue expression profiles of RpAQP genes
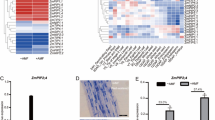
The RpAQP genes were differentially expressed in roots, stems, and mature leaves of Robinia pseudoacacia seedlings (Fig. 2). Generally, roots showed higher transcription levels of RpAQPs. Four genes, RpTIP1;3, RpPIP1;1, RpPIP1;3, and RpPIP2;1, were highly expressed in roots; however, the remaining two genes, RpTIP1;1 and RpTIP2;1, were more abundant in leaves. RpAQP mRNA levels were lower in stems, except for RpTIP1;3.
Root (a), stem (b), and leaf (c) expression patterns of Robinia pseudoacacia aquaporin (RpAQP) genes. WW, well-watered; DS, drought stress; NM, non-mycorrhizal plants; AM, Rhizophagus irregularis inoculated plants. Relative expression of RpAQPs in various tissues was assessed after 14 days of drought stress using the 2−ΔΔCT method and 18S rRNA as reference gene. The average values of three biological replicates were used to produce a heat map using R software. The intensity value bars are shown at the right edge of the heat map. Red represents high expression and green denotes low expression
RpAQP expression responses to AM and water regime
Root, stem, and leaf expression of the six RpAQPs was measured in mycorrhizal and non-mycorrhizal Robinia pseudoacacia seedlings (Fig. 3a–c and Supplementary Table S3). Under well-watered condition (WW), root and stem expression of RpTIP1;1, RpTIP2;1, and RpPIP2;1 was induced by AM, whereas the expression of RpTIP1;3 and RpPIP1;3 was downregulated in response to AM. Under drought stress condition (DS), root expression of RpTIP1;1 and RpPIP1;3 was downregulated by AM; however, no significant differences were observed in root expression of RpTIP1;3/RpPIP1;1 between NM and AM plants. In contrast, the expression levels of RpTIP2;1 and RpPIP2;1 in AM were 1.3- and 1.8-fold those in NM plants. There was no difference in stem expression of RpTIP1;3 between AM and NM plants under DS. Nonetheless, stem expression levels of RpTIP1;1, RpTIP2;1, and RpPIP2;1 were 1.2-, 1.8-, and 1.2-fold, while RpPIP1;1 and RpPIP1;3 expression levels were 37.0 and 85.5 % lower in AM than those in NM plants under DS.
Under WW, no difference was found in leaf expression of RpTIP1;1, RpTIP2;1, or RpPIP2;1 between AM and NM plants. Nonetheless, leaf expression of RpPIP1;1 in AM was increased by 46.3 % compared with NM plants under WW. Under DS, leaf expression levels of RpTIP2;1 and RpPIP2;1 were 1.4- and 1.6-fold in AM than those in NM plants. The opposite trend was found in the response of leaf expression of RpTIP1;3 and RpPIP1;3 to AM under DS. The differences in leaf expression of RpTIP1;1 and RpPIP1;1 were not significant between NM and AM seedlings under DS.
Compared with WW, DS led to an elevation in leaf expression of RpTIP1;3, RpTIP2;1, and RpPIP1;3 and root expression of RpTIP1;1 in both AM and NM plants. The opposite trend was observed in root and stem expression of RpTIP2;1, RpPIP1;1, and RpPIP2;1, and leaf expression of RpTIP1;1 and RpPIP1;1 in NM and AM seedlings. Generally, both AM and NM plants responded in the same way to drought stress in the root, stem, and leaf expression of RpTIP1;1, RpTIP2;1, and RpPIP1;1. The exceptions were RpTIP1;3, RpPIP1;3, and RpPIP2;1 that responded differently to drought stress, to some extent, depending on AM fungal inoculation (Fig. 3a–c and Supplementary Table S3).
Consequently, both AM and DS markedly affected root, stem, and leaf expression of RpTIP1;1, RpTIP1;3, RpTIP2;1, RpPIP1;3, and RpPIP2;1 in the plants (P ≤ 0.05). The interaction between AM and DS was significant for root, stem, and leaf expression of RpPIP1;1 and RpPIP1;3 (P ≤ 0.001) (Supplementary Table S3).
Correlations
Correlations were observed in AM plants between gene expression of specific RpAQPs and gas-exchange parameters such as P n and G s (Table 5). Most of the correlations were negative, indicating that the lower the P n or G s, the higher the expression of certain RpAQPs. In AM plants under DS, expression of RpTIP1;3 in roots and RpPIP1;1 in stems was negatively correlated with P n (P ≤ 0.05); however, RpTIP1;1 expression in leaves was positively correlated with G s (P ≤ 0.05). Two correlations were observed for AM plants under WW: G s similarly showed a significantly negative correlation with the expression of RpPIP1;3 in roots, while it was positively correlated with the expression of RpPIP2;1 in leaves (P ≤ 0.05). NM plants did not show any correlation with RpAQP expression under different soil water conditions.
Discussion
The results from the pot experiment in this study showed that Robinia pseudoacacia seedlings subjected to drought stress had slightly lower Rhizophagus irregularis colonization than well-watered plants. This is in agreement with previous reports of a negative effect of drought stress on AM fungal colonization in black locust (Robinia pseudoacacia) (Yang et al. 2014), melon (Cucumis melo), (Huang et al. 2011) and poplar (Populus spp.) (Liu et al. 2015). Water deficit can inhibit spore germination (Estaun 1990) and hyphal growth of AM fungi in soils, or limit the spread of root colonization from spores (Jacobson 1997).
The results from the present study also confirm the beneficial effect of AM fungi on host plant water status under drought stress (Zhao et al. 2015). Rhizophagus irregularis inoculation of Robinia pseudoacacia seedlings prevented tissue dehydration as revealed by the markedly lower WSD, and indicator of the degree of water deficit in plants (Akhter et al. 2013), in roots and leaves of AM plants compared with those of NM plants under different soil water conditions. This positive mycorrhizal effect may be due to an increase in root surface area for water uptake produced by the external hyphae of AM fungi (Asrar et al. 2012), or a regulation of stomatal behavior by G s (Augé et al. 2015).
A positive effect of Rhizophagus irregularis was found for the biomass of Robinia pseudoacacia under different soil water conditions, consistent with data previously reported for this tree species (Yang et al. 2014). Such an effect has been attributed to the improvement of water uptake (Gholamhoseini et al. 2013) and P n (Augé et al. 2015) in other AM plants. Rhizophagus irregularis colonization also significantly decreased electrolyte leakage in leaves of Robinia pseudoacacia under drought stress. Reduction in electrolyte leakage is an indicator of enhanced resistance to drought and of cell membrane stability (Ortiz et al. 2015).
AM plants of Robinia pseudoacacia maintained relatively high leaf net photosynthetic rate (P n) and stomatal conductance (G s) compared to NM plants under different water regimes, in agreement with previous reports of AM-positive effect on plant photosynthesis (Liu et al. 2015), a critical process of primary metabolism and essential to plant growth (Pinheiro and Chaves 2011). Additionally, the results suggest that AM plants maintained a higher level of gas exchange in leaves by reducing stomatal resistance and increasing the period of stomatal opening under the experimental conditions (Zhu et al. 2012). Consequently, it is not surprising that there were significant differences in P n, G s, T r, and C i between AM and NM plants under drought stress.
AQPs are channel proteins regulating plant water homeostasis and the transcellular movement of various molecules such as boron, small alcohols, and compatible solutes (Diehn et al. 2015). Here, the gene sequences and regulation patterns of six AQPs from Robinia pseudoacacia are reported for the first time. The identified RpAQPs have been assigned to two subfamilies and form four groups, reflecting the diversity and complexity of RpAQPs. Given the large protein family of AQPs comprising several tens of genes in plants (Bárzana et al. 2014), a larger number of unknown AQP genes probably exist in Robinia pseudoacacia. RpAQP expression was detected in all the tissues of Robinia pseudoacacia investigated but seemed to be preferentially expressed in leaves and roots. RpTIP1;1 and RpTIP2;1, for example, were more abundantly transcribed in leaves than in roots and stems, reflecting transport of substances by these tissues for leaf development, while RpPIPs had higher expression levels in roots, signifying that they may play a pivotal role in plant transport of water and solutes from soil. A similar gene expression pattern has been observed in other plant species. For instance, a PIP gene (ZmPIP1-5b) in maize was found abundant in roots but rare in mature and young leaves (Gaspar et al. 2003). The above data suggest that, in water homeostasis maintenance, R. pseudoacacia roots, stems and leaves need the coordination of certain RpPIP and RpTIP proteins or share a relatively redundant system (Guo et al. 2006). Some AQPs in plants present a close relationship with stomatal closure (Moshelion et al. 2014; Pou et al. 2013); however, the RpPIPs and RpTIP1;3 of Robinia pseudoacacia exhibited lower expression in leaves than in roots and stems. Interestingly, RpTIP1;1 and RpTIP1;3, classified as TIP1 subclass, share similar amino acid sequences, but the two genes displayed different expression patterns, indicating distinct functions.
Drought stress affected RpAQP gene expression patterns in the tested tissues of Robinia pseudoacacia. The gene RpTIP2;1 was downregulated in roots but highly expressed in leaves under drought stress, irrespective of Rhizophagus irregularis inoculation. The downregulation of this gene may limit the loss of cellular water during water deficit. However, another TIP gene, RpTIP1;1, was markedly upregulated in roots of both AM and NM plants during drought stress. This may contribute to root tolerance of water deficit since elevated osmotic and hydrostatic hydraulic conductance of roots has been correlated with upregulated TIP expression during water deficit (Bárzana et al. 2014). Although multiple AQP genes, especially TIPs and PIPs, can be markedly upregulated by water stress in roots (Aroca et al. 2006; Nguyen et al. 2013), only RpTIP1;1 (AM and NM roots) and RpPIP1;3 (NM roots) genes were upregulated by a drought condition in the present study on Robinia pseudoacacia. The higher expression of these two genes may mediate osmotic root hydraulic conductance and partly compensate for AQP gating that is blocked by root zone drying (McLean et al. 2011; Yue et al. 2014).
AM symbiosis can cause substantial changes in plant aquaporin activity (Aroca et al. 2007; Bárzana et al. 2014; Porcel et al. 2006), and plant AQPs may be significant for AM responses. In the present study, expression of RpPIP1;3 was downregulated in roots, stems, and leaves of Robinia pseudoacacia seedlings by Rhizophagus irregularis inoculation, compared with NM plant tissues, under different soil water conditions. This may contribute to a regulatory mechanism to decrease membrane water permeability, limit water loss, and allow cellular water conservation (Porcel et al. 2006). Data on WSD in various tissues of Robinia pseudoacacia showed that AM plants had a markedly lower root and leaf WSD than NM plants. Decrease in RpAQP expression after mycorrhization might also be associated with lower free exuded sap flow rate and osmotic root hydraulic conductance in AM plants (Bárzana et al. 2014). The genes RpTIP2;1 and RpPIP2;1 were, on the contrary, upregulated by AM formation in roots, stems, and leaves of Robinia pseudoacacia under drought stress condition, suggesting that these RpAQP genes may play a role in the plant response to mycorrhization by transporting various compounds of physiological importance for the host plant during water deficit.
Most interesting was the downregulation (RpTIP1;3 and RpPIP1;3) and upregulation (RpTIP2;1 and RpPIP2;1) of RpAQP expression in leaves of AM plants compared with NM plants under drought stress. These results suggest that the transcriptional regulation of RpAQPs in leaves of Robinia pseudoacacia is complicated and may be mediated by plant transpiration. Mycorrhiza exerts a certain control over transpiration (Ruiz-Lozano 2003), and upregulation of RpTIP2;1 and RpPIP2;1 in AM leaves may be correlated with mycorrhization. AM fungal inoculation can promote stomata closure and result in higher osmosis in leaves subjected to drought stress (Ruiz-Lozano 2003). Downregulation of the RpTIP1;3 and RpPIP1;3 genes in leaves of AM Robinia pseudoacacia has the potential to reduce membrane permeability, further preventing water loss from mesophyll cells and improving WUE in mycorrhizal plants (Ruiz-Lozano 2003).
The negative correlations observed with gas-exchange parameters of Robinia pseudoacacia indicate a possible control of the expression of certain RpAQPs genes by P n or G s. In other words, when P n decreases, an increase in RpAQP expression for certain genes may be considered as a compensatory mechanism. However, although leaf expression of RpTIP1;1 (treatment DS+AM) and RpPIP2;1 (treatment WW+AM) appeared to be positively regulated by G s in AM plants regardless of water treatment, caution must be taken about the correlations between leaf RpTIP1;1/RpPIP2;1 expression and G s because the role of AQPs is not just about facilitating water flow across cellular membranes (Wudick et al. 2009), and they may also be involved in either osmoregulation or CO2 transport (Pou et al. 2013). For example, a TIP (ZmTIP1;1) from maize exhibited a high capacity not only for transporting water but also H2O2 (Bárzana et al. 2014), the HaTIP1;1 and HaPIP2;1 genes from Helianthemum almeriense showed divergent roles in the transport of CO2, water and NH3 in Saccharomyces cerevisiae (Navarro-Ródenas et al. 2013). In the absence of a clear explanation for the positive correlations between stomatal conductance and expression of RpAQPs genes in mycorrhizal Robinia pseudoacacia, Augé et al. (2015) proposed that higher G s in AM plants is associated with higher water status and hence more open stomata. Thus, improved G s and water status in AM plants might be due to the apoplastic, symplastic, and cell-to-cell pathways for water movement at least partly through effects on host plant AQP expression and/or activity of the fungal symbiont (Bárzana et al. 2012; Ruiz-Lozano and Aroca 2010).
While the exact roles of RpTIP1;1 and RpPIP2;1 in transporting different compounds (e.g., CO2, water, and H2O2) are unknown, the amino acid sequences of RpTIP1;1 and RpPIP2;1 contain a Val residue and conserved Arg, respectively, which are generally considered important for transport functions (e.g., for water or glycerol) in TIP1 and PIP aquaporins (Forrest and Bhave 2007). In vivo expression in Xenopus oocytes and transgenic techniques will help to elucidate the specific functions of AQP proteins in Robinia pseudoacacia cells. Furthermore, more information is needed on their cellular and membrane location for a better understanding of their role. For example, a location of RpTIP1;1 and RpPIP2;1 in the mesophyll could indicate a close coupling between gene expression regulation of RpTIP1;1/RpPIP2;1 and stomatal control (Pou et al. 2013).
The present study on Robinia pseudoacacia analyzed only a few RpAQP genes within an AQP superfamily that presumably contains numerous members. More RpAQPs need to be identified for their expression and contribution to transmembrane water transport in Robinia pseudoacacia seedlings, as well as AM fungal AQPs, in order to acquire a general AQP-based interpretation of physiological parameters in AM plants. In addition, water exchange across cell membranes may be governed by AQPs in three ways: (1) gene expression level, (2) cellular trafficking, and (3) channel gating (the opening or closing of AQPs) (Heinen et al. 2009). Gene expression, as studied here, is one of the most important ways of AQP regulation, and knowledge about their expression level is highly relevant to a better understanding of their physiological role (Heinen et al. 2009). Future objectives are the study of cellular trafficking and channel gating to obtain a comprehensive understanding of the physiological functions of RpAQPs with respect to whole plant hydraulics and responses to drought stress and AM symbiosis in Robinia pseudoacacia.
In conclusion, regulation of the expression of RpAQP gene in Robinia pseudoacacia seedlings by Rhizophagus irregularis inoculation depends on soil water condition and plant tissues. Under drought stress and well-watered conditions, AM symbiosis downregulated RpPIP1;3 gene expression in roots, stems, and leaves of Robinia pseudoacacia seedlings, which might be a way to minimize water loss in host tissues. During drought stress, AM symbiosis upregulated root, stem, and leaf gene expression of RpTIP2;1 and RpPIP2;1, which could be a way to increase water flow to specific plant tissues important for host survival during drought stress. AM symbiosis acts on RpAQPs in a manner to alter Robinia pseudoacacia water relations and physiology, allowing the host plant to cope better with adverse environmental conditions. In this respect, AM plants exhibited lower water saturation deficit and electrolyte leakage under drought stress. AM symbiosis also protected Robinia pseudoacacia seedlings against drought stress by improving a series of physiological parameters, including plant growth, total dry mass, and leaf photosynthesis. Further studies are needed to elucidate the specific functions of each RpAQP gene regulated by AM symbiosis, in order to reveal the exact mechanism of AM symbiosis to alter plant adaptation under drought conditions.
Abbreviations
- AM:
-
Arbuscular mycorrhiza
- NM:
-
Non-mycorrhizal plants
- AQP:
-
Aquaporin
- RpAQP :
-
Robinia pseudoacacia aquaporin gene
- PIPs:
-
Plasma membrane intrinsic proteins
- TIPs:
-
Tonoplast intrinsic proteins
- NIPs:
-
NOD26-like MIPs or NOD26-like intrinsic proteins
- SIPs:
-
Small basic intrinsic proteins
- XIPs:
-
Newly identified X (or uncategorized) intrinsic proteins
- WW:
-
Well-watered
- DS:
-
Drought stress
- WSD:
-
Water saturation deficit
- EL:
-
Electrolyte leakage
- EC:
-
Electrical conductivity
- P n :
-
Net photosynthetic rate
- G s :
-
Stomatal conductance
- T r :
-
Transpiration rate
- C i :
-
Intercellular CO2 concentration
- WUE:
-
Water use efficiency
References
Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15:439–447
Akhter N, Hossainn F, Karim A (2013) Influence of calcium on water relation of two cultivars of wheat under salt stress. Inter J Env 2(1):1–8
Aroca R, Ferrante A, Vernieri P, Chrispeels MJ (2006) Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann Bot 98:1301–1310
Aroca R, Porcel R, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 173:808–816
Asrar AA, Abdel-Fattah GM, Elhindi KM (2012) Improving growth, flower yield, and water relations of snapdragon (Antirrhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 50:305–316
Augé RM, Toler HD, Saxton AM (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24
Bao SD (2000) Soil and agricultural chemistry analysis. China Agriculture Press, Beijing
Barrett RP, Mebrahtu T, Hanover JW (1990) Black locust: a multi-purpose tree species for temperate climates. Advances in new crops. Timber Press, Portland, pp 278–283
Bárzana G, Aroca R, Paz JA, Chaumont F, Martinez-Ballesta MC, Carvajal M, Ruiz-Lozano JM (2012) Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann Bot 109:1009–1017
Bárzana G, Aroca R, Bienert GP, Chaumont F, Ruiz-Lozano JM (2014) New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol Plant Microbe Inter 27:349–363
Chen HY, Chou MX, Wang XY, Liu SS, Zhang FL, Wei GH (2013) Profiling of differentially expressed genes in roots of Robinia pseudoacacia during nodule development using suppressive subtractive hybridization. PLoS One 8, e63930
Dickerson J (2002) Plant fact sheet: black locust (Robinia pseudoacacia L). USDA NCRS, Washington
Diehn TA, Pommerrenig B, Bernhardt N, Hartmann A, Bienert GP (2015) Genome-wide identification of aquaporin encoding genes in Brassica oleracea and their phylogenetic sequence comparison to Brassica crops and Arabidopsis. Front Plant Sci 6:166
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300:1005–1016
Estaun MV (1990) Effect of sodium chloride and mannitol on germination and hyphal growth of the vesicular-arbuscular mycorrhizal fungus. Agr Ecosyst Environ 29:123–129
Farissi M, Ghoulam C, Bouizgaren A (2013) Changes in water deficit saturation and photosynthetic pigments of alfalfa populations under salinity and assessment of proline role in salt tolerance. Agric Sci Res J 3:29–35
Forrest KL, Bhave M (2007) Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Funct Integr Genomics 7:263–289
Garlock RJ, Wong YS, Balan V, Dale BE (2012) AFEX pretreatment and enzymatic conversion of black locust (Robinia pseudoacacia L.) to soluble sugars. Bioenerg Resour 5:306–318
Gaspar M, Bousser A, Sissoëff I, Roche O, Hoarau J, Mahé A (2003) Cloning and characterization of ZmPIP1-5b, an aquaporin transporting water and urea. Plant Sci 165:21–31
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, Totowa, NJ, pp 571–607
Gholamhoseini M, Ghalavand A, Dolatabadian A, Jamshidi E, Khodaei-Joghan A (2013) Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric Water Manage 117:106–114
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Guo L, Wang ZY, Lin H, Cui WE, Chen J, Liu MH, Chen ZL, Qu LJ, Gu HY (2006) Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res 16:277–286
Gupta AB, Sankararamakrishnan R (2009) Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9:134
Hart M, Ehret DL, Krumbein A, Leung C, Murch S, Turi C, Franken P (2015) Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 25:359–376
Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. J Exp Bot 60:2971–2985
Huang Z, Zou ZR, He CX, He ZQ, Zhang ZB, Li JM (2011) Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil 339:391–399
Jaafar HZE, Ibrahim MH, Mohamad Fakri NF (2012) Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), maliondialdehyde (MDA) and photosynthetic responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 17:7305–7322
Jacobson KM (1997) Moisture and substrate stability determine VA-mycorrhizal fungal community distribution and structure in an arid grassland. J Arid Environ 35:59–75
Jin T, Liu G, Fu B, Ding X, Yang L (2011) Assessing adaptability of planted trees using leaf traits: a case study with Robinia pseudoacacia L. in the Loess Plateau, China. Chinese Geogr Sci 21:290–303
Krajinski F, Biela A, Schubert D, Gianinazzi-Pearson V, Kaldenhoff R, Franken P (2000) Arbuscular mycorrhiza development regulates the mRNA abundance of Mtaqp1 encoding a mercury-insensitive aquaporin of Medicago truncatula. Planta 211:85–90
Laur J, Hacke UG (2014) Exploring Picea glauca aquaporins in the context of needle water uptake and xylem refilling. New Phytol 203:388–400
Li GW, Santoni V, Maurel C (2014) Plant aquaporins: roles in plant physiology. BBA-Gen Subjects 1840:1574–1582
Ligaba A, Katsuhara M, Shibasaka M, Djira G (2011) Abiotic stresses modulate expression of major intrinsic proteins in barley (Hordeum vulgare). CR Biol 334:127–139
Liu T, Sheng M, Wang CY, Chen H, Li Z, Tang M (2015) Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 53:250–258
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408
Luu DT, Maurel C (2005) Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ 28:85–96
Mantovani D, Veste M, Freese D (2014) Effects of drought frequency on growth performance and transpiration of young black locust (Robinia pseudoacacia L.). Int J For Res. 10.1155/2014/821891.
Maurel C, Plassard C (2011) Aquaporins: for more than water at the plant fungus interface? New Phytol 190:815–817
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
McLean EH, Ludwig M, Grierson PF (2011) Root hydraulic conductance and aquaporin abundance respond rapidly to partial root-zone drying events in a riparian Melaleuca species. New Phytol 192:664–675
Moshelion M, Halperin O, Wallach R, Oren R, Way DA (2014) Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: crop water-use efficiency, growth and yield. Plant Cell Environ. doi:10.1111/pce.12410
Navarro-Ródenas A, Bárzana G, Nicolás E, Carra A, Schubert A, Morte A (2013) Expression analysis of aquaporins from desert truffle mycorrhizal symbiosis reveals a fine-tuned regulation under drought. Mol Plant Microbe Inter 26:1068–1078
Nedjimi B (2014) Effects of salinity on growth, membrane permeability and root hydraulic conductivity in three saltbush species. Biochem Syst Ecol 52:4–13
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL (ed) Methods of soil analysis: part 2, agronomy monograph 9, 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 539–579
Nguyen MX, Moon S, Jung KH (2013) Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 238:669–681
Ortiz N, Armada E, Duque E, Roldán A, Azcón R (2015) Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J Plant Physiol 174:87–96
Ouziad F, Wilde P, Schmelzer E, Hildebrandt U, Bothe H (2006) Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ Exp Bot 57:177–186
Page AL (1982) Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America, p 1159
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882
Porcel R, Ruiz-Lozano JM (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J Exp Bot 55:1743–1750
Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM (2006) PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol Biol 60:389–404
Pou A, Medrano H, Flexas J, Tyerman SD (2013) A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ 36:828–843
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies Mycorrhiza 13:309–317
Ruiz-Lozano JM, Aroca R (2010) Modulation of aquaporin genes by the arbuscular mycorrhizal symbiosis in relation to osmotic stress tolerance. In: Seckbach J, Grube M (eds) Symbioses and stress: joint ventures in biology, cellular origin, life in extreme habitats and astrobiology. Springer Science+Business Media, Dordrecht, pp 359–374
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London, p 787
Sreedharan S, Shekhawat UK, Ganapathi TR (2013) Transgenic banana plants overexpressing a native plasma membrane aquaporin MusaPIP1;2 display high tolerance levels to different abiotic stresses. Plant Biotech J 11:942–952
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, Colard A, Martin F (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol 193:755–769
Wang X, Li Y, Ji W, Bai X, Cai H, Zhu D, Sun XL, Chen LJ, Zhu YM (2011) A novel Glycine soja tonoplast intrinsic protein gene responds to abiotic stress and depresses salt and dehydration tolerance in transgenic Arabidopsis thaliana. J Plant Physiol 168:1241–1248
Wudick MM, Luu DT, Maurel C (2009) A look inside: localization patterns and functions of intracellular plant aquaporins. New Phytol 184:289–302
Yang YR, Tang M, Sulpice R, Chen H, Tian S, Ban YH (2014) Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J Plant Growth Regul 33:612–625
Yue C, Cao HL, Wang L, Zhou YH, Hao XY, Zeng JM, Wang XC, Yang YJ (2014) Molecular cloning and expression analysis of tea plant aquaporin (AQP) gene family. Plant Physiol Bioch 83:65–76
Zhang YX, Wang Z, Chai TY, Wen ZS, Zhang HM (2008) Indian mustard aquaporin improves drought and heavy-metal resistance in tobacco. Mol Biotechnol 40:280–292
Zhao RX, Guo W, Bi N, Guo JY, Wang LX, Zhao J, Zhang J (2015) Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl Soil Ecol 88:41–49
Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X (2012) Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ 58:186–191
Zhu XQ, Wang CY, Chen H, Tang M (2014) Effects of arbuscular mycorrhizal fungi on photosynthesis, carbon content, and calorific value of black locust seedlings. Photosynthetica 52:247–252
Acknowledgments
This research was supported by Special Fund for Forest Scientific Research in the Public Welfare (201404217), the National Natural Science Foundation of China (31270639, 31170567), and the Program for Changjiang Scholars and Innovative Research Team in University of China (IRT1035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, F., Zhang, H. & Tang, M. Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 26, 311–323 (2016). https://doi.org/10.1007/s00572-015-0670-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0670-3