Abstract
Arbuscular mycorrhizal (AM) fungi form ubiquitous symbioses with terrestrial plants in different ecosystems and provide a variety of benefits including improved drought tolerance of host plants. However, the difference and contribution of colonized and un-colonized root-system parts within mycorrhizal plants against drought stress is uncertain. A split-root system was used and the root compartments were either non-inoculated or inoculated with Rhizophagus irregularis, and were subjected to either well-watered or drought-stressed conditions. The growth, photosynthesis, reactive oxygen species (ROS) scavenging, and relative gene expression of aquaporins and phosphate transporters of hybrid poplar (Populus × canadensis ‘Neva’) were evaluated. Our results indicated that the inoculation by R. irregularis in either one or both compartments of split-root systems increased poplar biomass accumulation, photosynthesis, and ROS regulation under well-watered and drought-stressed conditions. When inoculum was applied in both compartments of split-root systems, the beneficial effect of R. irregularis was greater than that in treatment where only one compartment received inoculum. The effect of R. irregularis may attribute to improved phosphorus uptake via upregulation of relative expressions of PcPT3, PcPT4, PcPT5, and a possible improvement of water uptake via modulation of aquaporins (PcPIP2-3, PcPIP2-5, PcTIP1-1, and PcTIP1-2) in colonized root-system parts. Our results demonstrated that the benefits of the AM symbiosis depend on the extent of root colonization through which AM fungus may modulate plant phosphate and water uptake to improve tolerance of poplar against drought stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought stress occurs frequently in northwest areas of China, and limits plant growth and productivity (Yan et al. 2017). In response to drought stress, plants induce several physiological and biochemical changes (Liu et al. 2016). One physiological change is the reduction of photosynthesis and stomatal conductance to avoid water loss through leaves (Lawlor and Cornic 2002). Another biochemical change is the build-up of reactive oxygen species (ROS) that due to electron leakage in chloroplast and mitochondria and NADPH oxidization (Ahmed et al. 2009; Gill and Tuteja 2010; Marino et al. 2012). Plants use antioxidative enzymes and small molecular compounds to scavenge ROS (Gill and Tuteja 2010).
Arbuscular mycorrhizal (AM) fungi are widely distributed in different ecosystems and form symbioses with most terrestrial plants (Smith and Read 2008). Plants that form AM symbioses acquire numerus benefits, including nutrient and water uptake through additive mycorrhizal pathway from soil (Smith et al. 2011; Liu et al. 2019b; Wang et al. 2020). In exchange for the benefits, the AM fungi receive photosynthates from host plants (Pfeffer et al. 1999; Jiang et al. 2017), and host plants balance the cost–benefit of the symbiosis by controlling the delivery photosynthates to the AM fungi (Vierheilig 2004; Meixner et al. 2005). Moreover, the AM symbiosis improves plants that under drought stress photosynthesis and stomatal conductance, which has been attributed to the improved water uptake by AM fungi (Liu et al. 2015; Li et al. 2015; He et al. 2017).
Aquaporins form water membrane channels for passive water movement in plants (Maurel et al. 2008). Aquaporins control transcellular water transport and the hydraulic conductance in plants, and limit symplastic water transport to prevent water loss under drought stress. In accompany with the regulation of aquaporin genes expression (He et al. 2016; Quiroga et al. 2017), AM symbiosis improves hydraulic conductance and water status of host plants (Calvo-Polanco et al. 2016; Kilpeläinen et al. 2020). Moreover, aquaporin-mediated long-distance phosphate transport in AM fungal hyphae (Kikuchi et al. 2016) may assist phosphate transport from AM fungi to the plant in conjunction with the phosphate transporters from the PHT1 family (Nussaume et al. 2011; Smith et al. 2011). Under drought stress, mycorrhizal plants additionally have higher activities of antioxidative enzymes, including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), and higher contents of glutathione (GSH) than the non-mycorrhizal plants. These changes have been attributed to the regulation of genes for signal transduction and enzyme synthesis associated with ROS scavenging systems (He et al. 2017, 2020; Huang et al. 2020), that improve scavenging of ROS in mycorrhizal plants and reduce the content of H2O2 and malondialdehyde (MDA), which are products of lipid oxidation (Wu et al. 2006; Liu et al. 2016; He et al. 2017).
Populus spp. are fast-growing woody plants, and have great value for paper industry, biomass production, and ecological conservation (Luo and Polle 2009; Hacke et al. 2010). Populus × canadensis (a hybrid of P. nigra × P. deltoides) ‘Neva’ is widely planted in semi-arid areas of northwest China, and its growth is limited by drought stress. In our previous studies, photosynthesis, antioxidative responses, and aquaporin genes expression of this poplar hybrid under drought stress were improved by inoculation with AM fungi (Liu et al. 2015, 2016). Further, the effect of AM symbiosis on this poplar hybrid was promoted when the colonization rate of root increased (Wu et al. 2017). The results raised a question of whether the improvement of AM symbiosis on this poplar hybrid was depended on only the portion of the AM fungal-colonized root-system parts, or was systemically regulated host physiological responses, or both. To answer this question, a split-root system was used to evaluate the effects of AM fungal colonization and poplar response to drought. The root compartments of the split-root system were either non-inoculated or inoculated with Rhizophagus irregularis, and were subjected to either well-watered or drought-stressed conditions. Plants growth, photosynthesis, ROS scavenging, and relative expression of aquaporin and phosphate transporter genes of this hybrid poplar were evaluated.
Materials and methods
Plant material, growth substrate, and AM fungal inoculum
Hybrid poplar (P. × canadensis ‘Neva’) cuttings were collected and disinfected as described by Li et al. (2021). The cuttings were grown in plastic cups (5 cm in diameter and 7.5 cm in height) containing 0.2 kg growth substrate (without rooting hormones) for 4 months. When there were 6 leaves, the cuttings were used for split-root experiment. The cuttings were watered every other day and fertilized with 10 mL full-strength Hoagland’s solution every 2 weeks (Hoagland and Arnon 1938).
Growth substrate and the Inoculum of R. irregularis were prepared as described by Li et al. (2021).
Experimental design and growth condition
Poplar cuttings were cultivated in split-root systems (Fig. 1) that were made of acrylic plates. Growth substrate (800 g) was added in each root compartment (7 cm × 7 cm × 12 cm, length × width × height). The split-root system was used to evaluate the influence of two factors: (1) AM status, inoculated with R. irregularis inoculum (A) or inoculated with autoclaved inoculum (N); (2) water status, well-watered (W, 70–75% of field capacity) or drought stressed (D, 30–35% of field capacity). When poplar cuttings were transplanted into the split-root systems, 10 g inoculum was applied underneath roots for mycorrhizal treatments while the non-mycorrhizal treatments received autoclaved inoculum with inoculum filtrate (He et al. 2016). The poplar cuttings were divided into 3 groups: (1) cuttings with both root system halves inoculated; (2) cuttings with both root system halves non-inoculated; (3) cuttings with only one root system half inoculated. Four weeks post transplanting, water treatment started and lasted for 8 weeks, where half of the split-root system with different AM status groups were irrigated with limited water amount. The TDR100 system (Campell) was used (once every other day) to monitor soil moisture, of which the 100% soil water-holding capacity of growth substrate corresponds to 20% volumetric soil moisture. At harvest (12 weeks after transplantation), 10 treatments were as follow: (1) AW/AW; (2) AW/AD; (3) AD/AD; (4) NW/NW; (5) NW/ND; (6) ND/ND; (7) AW/NW; (8) AW/ND; (9) AD/NW; (10) AD/ND. One poplar cutting was grown in each split-root system and each treatment had four replicates.
Split-root system established for studies of interaction of R. irregularis and drought stress on poplar. The letter A in root compartment indicates inoculation of R. irregularis and N indicates mock inoculation. The letter W in root compartment indicates well-watered condition (70–75% of field capacity) and D indicates drought-stressed condition (30–35% of field capacity). The shoot treatments were named as the combination of 4 letters in root compartments, and the root system halves were named as the combination of number and letters in root compartments
The split-root systems were arranged in a randomized complete block design and placed in greenhouse as described by Li et al. (2021). Throughout the experiment, the poplar cuttings were irrigated with water to maintain the two treatments, and fertilized by applying 20 mL of full-strength Hoagland’s solution in each root compartment once every 2 weeks.
Biomass accumulation and AM fungal colonization
Harvest was conducted 12 weeks post transplantation, and the shoot and root were separated. Roots from each root compartment were separately collected, thoroughly washed, and dried with paper towels. Leaves from each shoot treatment were evenly divided into two parts: one part was used for enzymatic parameter measurement; one part was used for fresh weight and dry weight ratio calculation. Roots from each compartment were divided into three parts: one part was used for observation of AM fungal colonization; one part was used for assessment of enzymatic activity and gene expression; one part was used for fresh weight and dry weight ratio calculation. Dry weight (DW) of branches, leaves, and root was measured as described by Li et al. (2021) for four cuttings per treatment combination (n = 4).
The observation and measurement of AM fungal colonization used part of fresh root from each root compartment (n = 4). The root was stained (Hu et al. 2016), and over 200 intersections of over 20 stained root segments (5 cm long) from each compartment were observed with × 400 magnification under a light microscope and the gridline intersect method was applied (Giovannetti and Mosse 1980).
Gas exchange measurement and leaf relative water content
One day before harvest, the gas exchange parameters were measured as described by Li et al. (2021). The leaf relative water content was measured as described by Liu et al. (2015). The analysis was done in four independent cuttings per treatment.
Antioxidation enzymes activity, soluble protein concentration, H2O2 concentration, malondialdehyde (MDA) concentration, and phosphorus concentration
Fully expanded, healthy leaves and roots were collected, homogenized in liquid nitrogen, and 0.5 g leaves and roots samples were used to analyze antioxidation enzyme activity, soluble protein concentration, H2O2 concentration, and MDA concentration as described by He et al. (2017). The phosphorus concentration was determined as described by Li et al. (2021). The analysis was done in four independent leaves and root samples per treatment.
RNA extraction and first-strand cDNA synthesis
RNA extraction, RNA quality and quantity assessment, and first-strand cDNA synthesis was conducted as described by Liu et al. (2016).
Quantitative real-time PCR (qRT-PCR) analysis
Full-length cDNA sequences encoding the phosphate transporters in the PHT1 family and aquaporins were obtained from transcriptomic analysis, designated PcPT3, PcPT4, PcPT5, PcPT9, PcPIP1-1, PcPIP1-2, PcPIP1-3, PcPIP2-1, PcPIP2-2, PcPIP2-3, PcPIP2-4, PcPIP2-5, PcTIP1-1, and PcTIP1-2 (supplementary Figure S1 and S2), and deposited in GenBank (MN546004-MN546007, MN546012-MN546014, MN546016–MN546020, MN546022, and MN546023).
qRT-PCR was performed to analyze the transcript accumulation of genes encoding phosphate transporters and aquaporins of roots from each compartment (20 compartments in total) of the split-root system. Primers used for qRT-PCR were listed in Table S1. To ascertain the uniqueness of the product of primers, the products were transformed into 18-T vector and sequenced. qRT-PCR amplifications were performed as described by Liu et al. (2016). All qRT-PCR reactions were performed with 4 biological replications (from each root system half) and 3 technical repetitions. A unique fragment of 18S rRNA gene (guanine-N(7)-methyltransferase) of P. × canadensis ‘Neva’ was used for normalization. The relative expression were calculated as 2−ΔCT (ΔCT = CTgene of interest minus CT18S rRNA) (Zhang and Franken 2014).
Statistical analysis
Statistical analysis was performed using the SPSS 20.0 statistical program (SPSS Inc., Chicago, IL, USA). MANOVA was performed separately to detect the main contributions of R. irregularis and drought stress to the different parameters studied for leaves and root system halves. When MANOVA indicated a significant effect (Wilk’s lambda, P < 0.05), the individual effects of the presence of R. irregularis and drought stress in none, one, or both root compartments were tested. When MANOVA indicated a significant interaction between AM fungus and drought stress, one-way ANOVA and post hoc Tukey’s HSD tests were performed. For root colonization rate, one-way ANOVA and post hoc Tukey’s HSD test were performed. The Shapiro–Wilk’s test was used to test the assumptions of normality, and the Levene’s test was used for the test of equal variance. The heatmap and hierarchical clustering analysis was performed using the MetaboAnalyst 4.0 (Chong et al. 2018).
Results
Plant biomass accumulation and mycorrhizal colonization rate
Drought stress reduced the dry weight (DW) of shoots and roots, and the reduction was intensified when both of the root compartments were subject to the drought-stressed condition (Tables 1, 2, and Supplementary Table S2, S5). Inoculation by R. irregularis in either one or both root compartments increased the DW of shoot and root. The increment of shoot DW in treatment with R. irregularis in both root compartments was higher than that in treatment with R. irregularis in only one root compartment (Fig. 2).
The shoot dry weight and colonization rate of roots in split-root system. The root compartments of the split-root system were either non-inoculated (N) or inoculated with Rhizophagus irregularis (A), and were subjected to either well-watered (W) or drought stressed (D). Data were shown as mean ± SD. The white bars represent the root system halves on the left part of plant root in Fig. 1, and the black represent the root system halves on the right part of plant root in Fig. 1. Means followed by the same letter do not differ significantly at P < 0.05 (Tukey’s HSD-tests, n = 4)
Mycorrhizal colonization was only observed in root compartments that received R. irregularis inoculum (Fig. 2b). The colonization rate was greater in plants growing with only one compartment inoculated than in plants with both compartments inoculated. Drought stress limited the colonization rate only when drought stress was applied in root compartments that received inoculum.
Gas exchange, RWC, phosphorus concentration, enzymatic response, and oxidative damage of poplar leaves
Drought stress reduced net photosynthetic rate (PN), stomatal conductance (gs), transpiration rate (E), intercellular CO2 concentration (Ci), and RWC of poplar leaves (Supplementary Table S2). These reductions occurred when one of the compartments was subjected to drought stress, and intensified when both of the compartments were subjected to drought stress (Table 1). In contrast, the water use efficiency (WUEi) increased with the increment of compartments subjected to drought stress. The presence of R. irregularis in both compartments of the split-root system resulted in the increase of PN, gs, E, and RWC. The presence of R. irregularis in either one or both compartments only reduced the Ci, but not the WUEi (Table 1, Supplementary Tables S2, S4).
The phosphorus concentration of poplar leaves was increased when both compartments received inoculum, but not affected by drought-stress (Supplementary Table S2, Table 1).
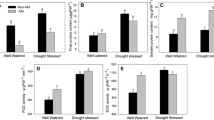
Drought stress in either one or both compartments increased the activity of SOD and APX, increased the concentrations of H2O2, MDA, and GSH, and decreased the activity of POD and CAT and the soluble protein concentration (Table 1, Supplementary Tables S2, S5). The presence of R. irregularis in either one or two compartments increased the activity of APX, CAT, and POD, increased the concentration of soluble protein, and decreased the activity of SOD and the concentration of H2O2 and MDA.
Relative expression of genes, phosphorus concentration, and enzymatic response and oxidative damage in poplar roots from each compartment
The expression of genes encoding phosphate transporters and aquaporins of roots from each compartments of the split-root system were assessed and normalized by the expression of a unique plant 18S rRNA gene (Fig. 3). The relative expression of PcPT3, PcPT4, and PcPT5 were upregulated by fungal inoculation, but not affected by drought stress (Supplementary Table S5). The upregulation of these genes occurred when R. irregularis was inoculated in either one or both of the compartments (Table 2). The relative expression of PcPT9 was downregulated by both fungal inoculation and drought stress. The downregulation occurred when R. irregularis inoculum or drought stress was applied in both compartments. The phosphorus concentration of poplar roots was reduced when both compartments were under drought stress, but not affected by the fungal inoculation (Supplementary Table S5, Table 2).
The relative expressions of genes of PHT1 family and aquaporins in poplar roots. The root compartments of the split-root system were either non-inoculated (N) or inoculated with Rhizophagus irregularis (A), and were subjected to either well-watered (W) or drought stressed (D). The analysis used data including relative expression of PcPT3, PcPT4, PcPT5, PcPT9, PcPIP1-1, PcPIP1-2, PcPIP1-3, PcPIP2-1, PcPIP2-2, PcPIP2-3, PcPIP2-4, PcPIP2-5, PcTIP1-1, and PcTIP1-2 in poplar roots. The genes expression was normalized by the expression of a unique fragment of plant 18S rRNA gene
When drought stress was applied in both of the root compartments, the relative expression of PcPIP1-1, PcPIP2-1, PcPIP2-2, PcPIP2-4, and PcTIP1-2 was downregulated (Table 2, Supplementary Table S5). PcPIP2-2, PcPIP2-4, and PcTIP1-2 were also downregulated when only one compartment of the split-root system was subjected to drought stress (Table 2). The presence of R. irregularis in both of the root compartments upregulated the relative expression of PcPIP2-3, PcPIP2-5, PcTIP1-1, and PcTIP1-2, and downregulated the relative expression of PcPIP1-1 (Table 2). PcPIP2-3 and PcPIP2-5 were also upregulated when R. irregularis inoculum was applied in only one compartment (Table 2, Supplementary Table S6).
The enzymatic response and oxidative damage of root systems were assessed (Fig. 4, Table S7). Drought stress in either one or both compartments increased the activity of APX and POD, increased the concentration of GSH, H2O2, and MDA, and decreased the concentration of soluble protein (Supplementary Table S5, Table 2). When both root compartments were subjected to drought stress, the activity of SOD increased. The presence of R. irregularis in either one or both root compartments increased the activity of APX and the concentration of soluble protein, and decreased the activity of POD. When R. irregularis was present in both compartments, the activity of CAT declined (Supplementary Table S5, Table 2). Drought stress and AM symbiosis showed a significant interaction (P < 0.001) for H2O2 concentration, indicating an involvement of R. irregularis in modulating the H2O2 concentrations in roots.
The activity of enzymes, the concentration of small molecular compounds, and oxidative damage in poplar roots. The root compartments of the split-root system were either non-inoculated (N) or inoculated with Rhizophagus irregularis (A), and were subjected to either well-watered (W) or drought stressed (D). The analysis used data including activity of SOD, POD, CAT, and APX, content of H2O2, glutathione, and MDA in poplar roots
Discussion
Poplars serve economic and ecological purposes, and are widely planted in areas of northwest China where water deficiency is common (Hacke et al. 2010; Yan et al. 2017). In this study, over 50% roots in root compartments that received inoculum were colonized by R. irregularis (Fig. 2). This was similar with other studies of poplars and AM fungi (Cicatelli et al. 2010; Wu et al. 2017; De Oliveira et al. 2020). To control the loss of photosynthates that AM fungi demand, plants may maintain the colonization rate of the whole root system at a certain level (Vierheilig 2004; Meixner et al. 2005), and this might result in the higher colonization rate of poplar roots when only one root compartment received inoculum (Fig. 2) (Wang et al. 2018). Drought stress limits both photosynthesis of plant and development of AM fungal hyphae in soil, and consequently reduces colonization rate (Neumann et al. 2009; Zhang et al. 2018; Leyva-Morales et al. 2019). Another possible reason may be the biosynthesis of strigolactones that differently respond to drought stress and AM symbiosis, and modulates plants interact with AM fungi (Ruiz-Lozano et al. 2016; López-Ráez 2016).
Biomass accumulation is the most distinct index that shows the response of plant to drought stress. The presence of R. irregularis in one root compartment increased the poplar shoot DW that were independent of drought stress, and the effect of R. irregularis was further promoted when both root compartments were inoculated (Table 1). This result is similar with other studies using split-root systems (Neumann et al. 2009; Li et al. 2016), and might be due to the thoroughly exploited water and mineral nutrients in growth substrate by AM fungal hyphae (Bitterlich et al. 2018), which have higher nutrient and water foraging capability than roots (Smith and Read 2008; Liu et al. 2019a). Moreover, the presence of R. irregularis in either one or both root compartments increased root growth (Table 2). This result is similar to previous studies (Li et al. 2016; Wu et al. 2016), and might be explained by the AM symbiosis enhancing carbon sink strength and carbon allocation by the host to cope with drought and nutrient uptake (Hodge 2004; Brunner et al. 2015). More than that, AM fungi also modifies root architecture to promote uptake of nutrient and water (Zhang et al. 2016, 2021; Zheng et al. 2020). However, there are also reports that the AM symbiosis did not improve root growth in split-root systems (Hao et al. 2012; Bárzana et al. 2015), and the different results might be due to the different experimental protocols.
Carbon assimilation of plants depends on photosynthesis of leaves (Lawlor and Cornic 2002). When R. irregularis was present in both root compartments, the photosynthetic parameters were improved (Table 1, Supplementary Table S3). This was in accordance with previous reports that AM fungi improve poplar photosynthesis when the poplar root was colonized (Liu et al. 2015; Li et al. 2015) and matched the biomass accumulation results. When plant photosynthesis is limited by drought stress, the electron leakage results in the reactive oxygen species (ROS) accumulation, and the scavenging of ROS depends on antioxidative enzymes and small molecular compounds (Ahmed et al. 2009; Gill and Tuteja 2010). The presence of R. irregularis in either one or two root compartments increased the activity of enzymes (except for SOD) and the concentrations of soluble protein, and decreased the concentrations of H2O2 and MDA (Table 1). This result is in accordance with the AM symbiosis improving photosynthetic parameters and increasing leaves RWC, and might be due to the AM symbiosis regulating plant genes involved in signal transduction and enzymes synthesis (He et al. 2017, 2020; Huang et al. 2020).
The absorption of phosphate from soils by roots requires participation of phosphate transporters from the PHT1 family (Nussaume et al. 2011). The presence of R. irregularis in root compartment upregulated the relative expressions of PcPT3, PcPT4, and PcPT5 (Supplementary Table S5, Table 2). The expressions of PcPT3, PcPT4, and PcPT5 orthologues in Populus tremula x P. alba were also upregulated by inoculation with Glomus intraradices or G. mosseae (Loth-Pereda et al. 2011). However, PtPT3, PtPT4, and PtPT5 were not classified in the mycorrhizal inducible subfamilies, and their upregulation of expression might be involved in the transport of extra phosphate that uptake by the mycorrhizal pathway (Smith et al. 2011). Although the phosphorus concentration of poplar root was not affected by the presence of R. irregularis in neither one nor both root compartments, the phosphorus content of poplar root (data not shown) was increased due to the mycorrhizal effect on root DW accumulation. The improved phosphorus content also supported the transport of phosphate by the mycorrhizal pathway. Moreover, the expressions of PcPT3, PcPT4, and PcPT5 were not affected by drought stress, and the concentration phosphorus was reduced by drought stress (Supplementary Table S5). This result resembles the expression of mycorrhizal-induced phosphate transporter genes and phosphorus uptake of Lycium barbarum under drought stress, and suggests the efficiency and independence of mycorrhizal Pi uptake regardless water status (Hu et al. 2016). The relative expression of PcPT9 was downregulated by inoculation of R. irregularis and drought stress (Supplementary Table S5, Table 2). This might be due to the improved plant phosphate status of the AM symbiosis and the drought stress lowered plant phosphate demand by limiting growth, as its orthologues PtPT9 in Populus tremula × P. alba and OsPT9 in rice were induced by the low phosphate conditions (Loth-Pereda et al. 2011; Wang et al. 2014; Gan et al. 2015).
Aquaporins participate in water homeostasis in plants (Maurel et al. 2008). Drought stress downregulated the expressions of PcPIP1-1, PcPIP2-1, PcPIP2-2, and PcPIP2-4 (Table 2), and this result indicated the responding of poplar to water deficiency by limiting water permeability in roots (Bárzana et al. 2014; Calvo-Polanco et al. 2016; Quiroga et al. 2017; Kilpeläinen et al. 2020). The presence of R. irregularis upregulated the relative expressions of PcPIP2-3 and PcPIP2-5, and downregulated the relative expression of PcPIP1-1 (Table 2). This result suggested an improved water permeability of poplar roots by R. irregularis as the PIP2s usually have higher water permeability than PIP1s (Chaumont et al. 2000; Bárzana et al. 2014; He et al. 2016), water transport by PIP2-5 has important contribution to poplar under water stress (Ranganathan et al. 2017) and the mycorrhizal-promoted transpiration. The relative expressions of TIPs were upregulated by the presence of R. irregularis in both root compartments (Table 2). The result suggests improved cellular osmotic balance in AM fungal-colonized roots (Ruiz-Lozano 2003), and a possible involvement of TIPs in nutrient and H2O2 transport (Bienert et al. 2007; Bárzana et al. 2014; Quiroga et al. 2020).
In plant roots, ROS is generated through electron leakage and NADPH oxidization (Gill and Tuteja 2010; Marino et al. 2012). Drought stress increased the activity of antioxidative enzymes and concentrations of low molecular compounds independent of R. irregularis inoculation (Supplementary Table 7). This resembles previous studies, and fits the response of plants to balance ROS generation and scavenging (Wu et al. 2006; Bárzana et al. 2015; He et al. 2017). On the contrary, inoculation by R. irregularis decreased the activity of POD and CAT, but increased the activity of APX and concentration of soluble protein (Table 2). A possible explanation might be that the AM symbiosis improved plant water uptake as discussed above, and shifted plant roots from using enzymes to low molecular compounds that including soluble protein and ascorbates to scavenge ROS (Bárzana et al. 2015). Although the process of arbuscule development produce limited H2O2 (Belmondo et al. 2016), the presence of R. irregularis did not influence the concentration of H2O2 in roots (Table 2). This might be due to the limited colonization rate under drought stress (Fig. 2), the influence of drought stress on the antioxidative response of plant roots, and the influence of AM symbiosis on antioxidative responses in colonized root-system part (Bárzana et al. 2014; He et al. 2017).
In conclusion, our results indicated that inoculation by R. irregularis in either one or both compartments of the split-root system increased poplar biomass accumulation, photosynthesis, and ROS regulation under both well-watered and drought-stressed conditions. When inoculum was applied in both root compartments, the effect of R. irregularis was higher than that in treatment when only one compartment received inoculum. The effect of R. irregularis may be attribute to the improved phosphorus uptake via upregulation of relative expressions of PcPT3, PcPT4, PcPT5, and a possible improvement of water uptake via modulation of aquaporins in colonized root-system part. Further study directly compares the transcriptomic differences of AM fungal colonized and non-colonized root-system parts in the same poplar will find more AM fungi-regulated plant genes.
Author contribution statement
HZ, LL, MT, and HC conceived and designed the study. HZ, LL, WREN, WZ conducted the experiments. HZ, LL, and WR analyzed the experimental data. HZ, LL, MT, and HC prepared and revised the manuscript.
References
Ahmed CB, Rouina BB, Sensoy S, Boukhris M, Abdallah FB (2009) Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ Exp Bot 67:345–352. https://doi.org/10.1016/j.envexpbot.2009.07.006
Bárzana G, Aroca R, Bienert GP, Chaumont F, Ruiz-Lozano JM (2014) New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol Plant Microbe 27:349–363. https://doi.org/10.1094/MPMI-09-13-0268-R
Bárzana G, Aroca R, Ruiz-Lozano JM (2015) Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial root drying. Plant Cell Environ 38(8):1613–1627. https://doi.org/10.1111/pce.12507
Belmondo S, Calcagno C, Genre A, Puppo A, Pauly N, Lanfranco L (2016) The Medicago truncatula MtRbohE gene is activated in arbusculated cells and is involved in root cortex colonization. Planta 243(1):251–262. https://doi.org/10.1007/s00425-015-2407-0
Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn T (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282(2):1183–1192. https://doi.org/10.1074/jbc.M603761200
Bitterlich M, Sandmann M, Graefe J (2018) Arbuscular mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front Plant Sci 9:154. https://doi.org/10.3389/fpls.2018.00154
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci 6:547. https://doi.org/10.3389/fpls.2015.00547
Calvo-Polanco M, Sánchez-Castro I, Cantos M, García JL, Azcón R, Ruiz-Lozano JM, Beuzón CR, Aroca R (2016) Effects of different arbuscular mycorrhizal fungal backgrounds and soils on olive plants growth and water relation properties under well-watered and drought conditions. Plant Cell Environ 39(11):2498–2514. https://doi.org/10.1111/pce.12807
Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Physiol Plant 122:1025–1034. https://doi.org/10.1104/pp.122.4.1025
Chong J, Soufan O, Li C, Caraus I, Li SZ, Bourque G, Wishart DS, Xia JG (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46(W1):W486–W494. https://doi.org/10.1093/nar/gky310
Cicatelli A, Lingua G, Todeschini V, Biondi S, Torrigiani P, Castiglione S (2010) Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann Bot 106(5):791–802. https://doi.org/10.1093/aob/mcq170
De Oliveira VH, Ullah I, Dunwell JM, Tibbett M (2020) Mycorrhizal symbiosis induces divergent patterns of transport and partitioning of Cd and Zn in Populus trichocarpa. Environ Exp Bot 171:103925. https://doi.org/10.1016/j.envexpbot.2019
Gan H, Jiao Y, Jia J, Wang X, Li H, Shi W, Peng C, Polle A, Luo Z (2015) Phosphorus and nitrogen physiology of two contrasting poplar genotypes when exposed to phosphorus and/or nitrogen starvation. Tree Physiol 36(1):22–38. https://doi.org/10.1093/treephys/tpv093
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500
Hacke UG, Plavcová L, Almeida-Rodriguez A, King-Jones S, Zhou W, Cooke JEK (2010) Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol 30:1016–1025. https://doi.org/10.1093/treephys/tpq058
Hao Z, Fayolle L, Van Tuinen D, Chatagnier O, Li X, Gianinazzi S, Gianinazzi-Pearson V (2012) Local and systemic mycorrhiza-induced protection against the ectoparasitic nematode Xiphinema index involves priming of defence gene responses in grapevine. J Exp Bot 63(10):3657–3672. https://doi.org/10.1093/jxb/ers046
He F, Zhang HQ, Tang M (2016) Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 26:311–323. https://doi.org/10.1007/s00572-015-0670-3
He F, Sheng M, Tang M (2017) Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front Plant Sci 8:183. https://doi.org/10.3389/fpls.2017.00183
He J, Zou Y, Wu Q, Kuca K (2020) Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci Hortic 262:108745. https://doi.org/10.1016/j.scienta.2019.108745
Hoagland DR, Arnon DI (1938) The water-culture method for growing plants without soil. Univ Calif Agric Exp Stn (berkeley) Circ 347:1–39
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162(1):9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Hu W, Zhang H, Zhang X, Chen H, Tang M (2016) Characterization of six PHT1 members in Lycium barbarum and their response to arbuscular mycorrhiza and water stress. Tree Physiol 37(3):351–366. https://doi.org/10.1093/treephys/tpw125
Huang D, Ma M, Wang Q, Zhang M, Jing G, Li C, Ma F (2020) Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol Biochem 149:245–255. https://doi.org/10.1016/j.plaphy.2020.02.020
Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D et al (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356:1172–1175. https://doi.org/10.1126/science.aam9970
Kikuchi Y, Hijikata N, Ohtomo R, Handa Y, Kawaguchi M, Saito K, Masuta C, Ezawa T (2016) Aquaporin-mediated long-distance polyphosphate translocation directed towards the host in arbuscular mycorrhizal symbiosis: application of virus-induced gene silencing. New Phytol 211(4):1202–1208. https://doi.org/10.1111/nph.14016
Kilpeläinen J, Aphalo PJ, Barbero-López A, Adamczyk B, Nipu SA, Lehto T (2020) Are arbuscular-mycorrhizal Alnus incana seedlings more resistant to drought than ectomycorrhizal and nonmycorrhizal ones? Tree Physiol 40(6):782–795. https://doi.org/10.1093/treephys/tpaa035
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25(2):275–294
Leyva-Morales R, Gavito ME, Carrillo-Saucedo SM (2019) Morphological and physiological responses of the external mycelium of Rhizophagus intraradices to water stress. Mycorrhiza 29:141–147. https://doi.org/10.1007/s00572-019-00880-8
Li Z, Wu N, Liu T, Chen H, Tang M (2015) Effect of arbuscular mycorrhizal inoculation on water status and photosynthesis of Populu cathayana males and females under water stress. Physiol Plant 155(2):192–204. https://doi.org/10.1111/ppl.12336
Li T, Sun Y, Ruan Y, Xu L, Hu Y, Hao Z, Zhang X, Li H, Wang Y, Yang L, Chen B (2016) Potential role of D-myo-inositol-3-phosphate synthase and 14-3-3 genes in the crosstalk between Zea mays and Rhizophagus intraradices under drought stress. Mycorrhiza 26:879–893. https://doi.org/10.1007/s00572-016-0723-2
Li L, Zhang H, Tang M, Chen H (2021) Nutrient uptake of distribution in mycorrhizal cuttings of Populus × canadensis ’Neva’ under drought stress. J Soil Sci Plant Nut 21:2310–2324. https://doi.org/10.1007/s42729-021-00523-y
Liu T, Sheng M, Wang C, Chen H, Li Z, Tang M (2015) Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 53(2):250–258. https://doi.org/10.1007/s11099-015-0100-y
Liu T, Li Z, Hui C, Tang M, Zhang H (2016) Effect of Rhizophagus irregularis on osmotic adjustment, antioxidation and aquaporin PIP genes expression of Populus× canadensis ‘Neva’ under drought stress. Acta Physiol Plant 38(8):191. https://doi.org/10.1007/s11738-016-2207-6
Liu B, Li L, Rengel Z, Tian J, Li H, Lu M (2019a) Roots and arbuscular mycorrhizal fungi are independent in nutrient foraging across subtropical tree species. Plant Soil 442:97–112. https://doi.org/10.1007/s11104-019-04161-3
Liu JJ, Liu JL, Liu JH, Cui MM, Huang YJ, Tian Y, Chen A, Xu GH (2019b) The potassium transporter SIHAK10 is involved in mycorrhizal potassium uptake. Plant Physiol 180(1):465–479. https://doi.org/10.1104/pp.18.01533
López-Ráez JA (2016) How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis. Planta 243:1375–1385. https://doi.org/10.1007/s00425-015-2435-9
Loth-Pereda V, Orsini E, Courty P, Lota F, Kohler A, Diss L, Blaudez D, Chalot M, Nehls U, Bucher M, Martin F (2011) Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal Populus trichocarpa. Plant Physiol 156(4):2141–2154. https://doi.org/10.1104/pp.111.180646
Luo ZB, Polle A (2009) Wood composition and energy content in a poplar short rotation plantation on fertilized agricultural land in a future CO2 atmosphere. Glob Change Biol 15:38–47. https://doi.org/10.1111/j.1365-2486.2008.01768.x
Marino D, Dunand C, Puppo A, Pauly N (2012) A burst of plant NADPH oxidases. Trends Plant Sci 17(1):9–15. https://doi.org/10.1016/j.tplants.2011.10.001
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624. https://doi.org/10.1146/annurev.arplant.59.032607.092734
Meixner C, Ludwig-Muller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H (2005) Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 222:709–715. https://doi.org/10.1007/s00425-005-0003-4
Neumann E, Schmid B, Romheld V, George E (2009) Extraradical development and contribution to plant performance of an arbuscular mycorrhizal symbiosis exposed to complete or partial rootzone drying. Mycorrhiza 20(1):13–23. https://doi.org/10.1007/s00572-009-0259-9
Nussaume L, Kanno S, Javot H, Marin E, Pochon N, Ayadi A, Nakanishi TM, Thibaud M (2011) Phosphate import in plants: focus on the PHT1 transporters. Front Plant Sci 2:83. https://doi.org/10.3389/fpls.2011.00083
Pfeffer PE, Douds DD, Bécard G, Shachar-Hill Y (1999) Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhizal. Plant Physiol 120:587–598
Quiroga G, Erice G, Aroca R, Chaumont F, Ruiz-Lozano JM (2017) Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front Plant Sci 8:1056. https://doi.org/10.3389/fpls.2017.01056
Quiroga G, Erice G, Araca R, Delgado-Huertas A, Ruiz-Lozano JM (2020) Elucidating the possible involvement of maize aquaporins and arbuscular mycorrhizal symbiosis in the plant ammonium and urea transport under drought stress conditions. Plants 9:148. https://doi.org/10.3390/plants9020148
Ranganathan K, Cooke JE, El Kayal W, Equiza MA, Vaziriyeganeh M, Zwiazek JJ (2017) Over-expression of PIP2;5 aquaporin alleviates gas exchange and growth inhibition in poplars exposed to mild osmotic stress with polyethylene glycol. Acta Physiol Plant 39:187. https://doi.org/10.1007/s11738-017-2486-6
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New Perspecti Mol Stud Mycorrhiza 13:309–317. https://doi.org/10.1007/s00572-003-0237-6
Ruiz-Lozano JM, Aroca R, Zamarreño ÁM, Molina S, Andreo-Jiménez B, Porcel R, García-Mina JM, Ruyter-Spira C, López-Ráez JA (2016) Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ 39:441–452. https://doi.org/10.1111/pce.12631
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York
Smith SE, Jakobsen I, Gronlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156(3):1050–1057. https://doi.org/10.1104/pp.111.174581
Vierheilig H (2004) Regulatory mechanisms during the plant-arbuscular mycorrhizal fungus interaction. Can J Bot 82:1166–1176. https://doi.org/10.1139/B04-015
Wang X, Wang Y, Pineros MA, Wang Z, Wang W, Li C, Wu Z, Kochian LV, Wu P (2014) Phosphate transporters OsPHT1,9 and OsPHT1,10 are involved in phosphate uptake in rice. Plant Cell Environ 37(5):1159–1170. https://doi.org/10.1111/pce.12224
Wang C, Reid JB, Foo E (2018) The art of self-control—autoregulation of plant–microbe symbioses. Front Plant Sci 9:998. https://doi.org/10.3389/fpls.2018.00988
Wang SS, Chen AQ, Xie K, Yang XF, Luo ZZ, Chen JD et al (2020) Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2000926117
Wu QS, Xia RX, Zou YN (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163(11):1101–1110. https://doi.org/10.1016/j.jplph.2005.09.001
Wu QS, Cao MQ, Zou YN, Wu C, He XH (2016) Mycorrhizal colonization represents functional equilibrium on root morphology and carbon distribution of trifoliate orange grown in a split-root system. Sci Hortic 199:95–102. https://doi.org/10.1016/j.scienta.2015.12.039
Wu F, Zhang H, Fang F, Wu N, Zhang Y, Tang M (2017) Effects of nitrogen and exogenous Rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus × canadensis ‘Neva.’ J Plant Growth Regul 36(4):824–835. https://doi.org/10.1007/s00344-017-9686-6
Yan M, Wang L, Ren H, Zhang X (2017) Biomass production and carbon sequestration of a short-rotation forest with different poplar clones in northwest China. Sci Total Environ 586:1135–1140. https://doi.org/10.1016/j.scitotenv.2017.02.103
Zhang H, Franken P (2014) Comparison of systemic and local interactions between the arbuscular mycorrhizal fungus Funneliformis mosseae and the root pathogen Aphanomyces euteiches in Medicago truncatula. Mycorrhiza 24(6):419–430. https://doi.org/10.1007/s00572-013-0553-4
Zhang H, Liu Z, Chen H, Tang M (2016) Symbiosis of arbuscular mycorrhizal fungi and Robinia pseudoacacia L. improves root tensile strength and soil aggregate stability. PLoS ONE 11(4):e0153378. https://doi.org/10.1371/journal.pone.0153378
Zhang F, Zou Y, Wu Q (2018) Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci Hortic 229:132–136. https://doi.org/10.1016/j.scienta.2017.10.038
Zhang J, Bi YL, Song ZH, Xiao L, Christie P (2021) Arbuscular mycorrhizal fungi alter root and foliar responses to fissure-induced root damage. Ecol Indic 127:107800. https://doi.org/10.1016/j.ecolind.2021.107800
Zheng FL, Liang SM, Chu XN, Yang YL, Wu QS (2020) Mycorrhizal fungi enhance flooding tolerance of peace through inducing proline accumulation and improving root architecture. Plant Soil Environ 66(12):624–631. https://doi.org/10.17221/520/2020-PSE
Acknowledgements
This research was funded by the National Natural Science Foundation of China (31700530, 32071639), the National Key Research and Development Program of China (2018YFD0600203), and the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources (SKLCUSA-b202007). We also thank the anonymous reviewers for reviewing the manuscript and offering helpful suggestions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by M. J. Reigosa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11738_2022_3393_MOESM1_ESM.tif
Supplementary file1 Figure S1 Circular tree of the 4 full-length phosphate transporters sequences from Populus × canadensis ‘Neva’, along with the NCBI PHT1 protein sequences from Populus trichocarpa, Populus euphratica, Glycine max, Oryza sativa L., Zea mays L., Lycium barbarum, Arabidopsis thaliana, Nicotiana tabacum, Solanum melongena, Medicago truncatula, Lycopersicon esculentum, and Solanum tuberosum (TIF 13981 KB)
11738_2022_3393_MOESM2_ESM.tif
Supplementary file2 Figure S2 Circular tree of the 10 full-length aquaporin sequences from Populus × canadensis ‘Neva’, along with the NCBI aquaporin sequences from Populus trichocarpa, Populus euphratica, Glycine max, Oryza sativa L., Zea mays L., Lycium barbarum, Arabidopsis thaliana, Nicotiana tabacum, Solanum melongena, Medicago truncatula, Lycopersicon esculentum, and Solanum tuberosum (TIF 76573 KB)
Rights and permissions
About this article
Cite this article
Zhang, H., Li, L., Ren, W. et al. Arbuscular mycorrhizal fungal colonization improves growth, photosynthesis, and ROS regulation of split-root poplar under drought stress. Acta Physiol Plant 44, 62 (2022). https://doi.org/10.1007/s11738-022-03393-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03393-8