Abstract
An effective protocol was developed for in vitro propagation of Psoralea corylifolia via somatic embryogenesis in cell suspension culture. Embryogenic callus was obtained on Murashige and Skoog (MS) medium supplemented with 6 μM naphthaleneacetic acid (NAA) and 30 μM glutamine from transverse TCLs from 10-day-old hypocotyl explants with a 96.4% frequency. Embryogenic callus produced a higher number of somatic embryos (123.7 ± 1.24 per gram fresh weight callus) on MS medium containing 30 g l−1 sucrose, 1 μM NAA, 4 μM benzyladenine (BA), 15 μM glutamine and 2 μM abscisic acid (ABA) after 4 weeks of culture. Somatic embryos successfully germinated (97.6%) on ½ MS medium containing 20 g l−1 sucrose, 8 g l−1 agar and supplemented with 2 μM BA, 1 μM ABA and 2 μM gibberellic acid (GA3) within 2 weeks of culture. Somatic embryos developed into normal plants, which hardened with 100% efficiency in soil in a growth chamber. Plants were successfully transferred to greenhouse and subsequently established in the field. Plant survival percentage in the field differed with seasonal variations. Average psoralen content of 12.9 μg g−1 DW was measured in different stages of somatic embryo development by high-performance liquid chromatography (HPLC). This protocol will be helpful for efficient propagation of elite clones on a mass scale, conservation efforts of this species and for secondary metabolites production studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Psoralea corylifolia L. (Bhavanchi, Fabaceae) is an endangered medicinal plant distributed widely in tropical and sub-tropical regions. It is an attractive annual herb producing with bluish purple flowers. The plant contains psoralen, an important compound which is commercially valuable. Psoralen possesses photosensitizing, photobiological and phototherapeutic properties used for the photochemotherapy of vitiligo and skin diseases such as psoriasis, mycosis fungoides and eczema (Frank et al. 1998). The plant exhibits antitumour, antibacterial, antifungal and antioxidative activities. It is also used in curing stomach ache, antihelmintic, diuretic and diaphoretic in febrile conditions. Many Indian pharmaceutical industries have used P. corylifolia as a raw material in the production of medicines and ayurvedic skin care soap (Baskaran and Jayabalan 2007). This medicinally important plant suffers from high seedling mortality and from very fast declined of wild populations due to indiscriminate, illegal collections and of habitat destruction. Therefore, this species is included in the endangered list (Baskaran and Jayabalan 2008). Hence, there is a need to develop an appropriate protocol for mass propagation and conservation to satisfy the pharmaceutical demand.
In vitro culture techniques provide a means of rapid plant propagation and a tool for crop improvement. Through tTCLs technology, micropropagation could be obtained in recalcitrant (Mulin and Bellio-Spataru 2000; Leguillon et al. 2003) medicinal (Ahn et al. 1996) and other crop plant species (Baskaran et al. 2006; Ben Ghnaya et al. 2008) in rapid multiplication. Moreover, tissue culture technology provides means of mass propagation of a species of interest with profound potential economic spin-offs (Teixeira da Silva 2003). Somatic embryogenesis by cell suspension culture offers an in vitro method for mass propagation of important medicinal plant species. In addition, somatic embryogenesis enables to produce large numbers of plantlets (Martin 2004) and bioactive compounds within a short span of time (Aderkas et al. 2002; Jeong et al. 2005). The technique is also suitable for germplasm conservation, the selection of genetic variants for desirable characters, the generation of somaclonal variants and for performing cellular genetic manipulations (Vasil 1988). Therefore, somatic embryogenesis could play an important role in P. corylifolia including fulfilling the pharmaceutical demands. Although studies on somatic embryogenesis of P. corylifolia have been published previously (Sahrawat and Chand 2001; Chand and Sahrawat 2002, 2007), the present investigation describes for the first time the possibility to obtain rapid and higher numbers of somatic embryos by mass propagation in cell suspension culture.
The objective of the present investigation was aimed at developing efficient plant regeneration systems via indirect (through callus stage) embryogenesis from hypocotyl-derived TCLs explants in cell suspension culture, which can be utilized for conservation to create variation and multiply the P. corylifolia plants in vitro. The present study reports on successful embryogenesis and plant regeneration protocol from tTCLs hypocotyl explants by evaluating various carbon sources, different concentrations and combinations of plant growth regulators for embryo production, and also to investigate the psoralen content in somatic embryos (at different developmental stages) of P. corylifolia.
Materials and methods
Explant preparation and embryogenic callus induction
Ten-day-old hypocotyl explants of P. corylifolia were collected from medicinal plant garden of the Bharathidasan University, Tiruchirappalli, India. Hypocotyl explants were surface sterilized with a solution containing commercial detergent Teepol (Reckitt Benckiser, India) for 2 min and aqueous mercuric chloride (0.1% w/v) for 3 min. The explants were washed five times with sterile distilled water. 10 × 0.5 mm longitudinally thin cell layers (lTCLs) and 5 × 1.0 mm transverse thin cell layers (tTCLs) from hypocotyls were excised under a laminar flow cabinet. For production of embryogenic callus, TCLs were inoculated onto MS (Murashige and Skoog 1962) medium containing 30 g l−1 sucrose and 8 g l−1 agar (Agar Type I, Himedia, India), and supplemented with 2–10 μM 2,4-dichlorophenoxyacetic acid (2,4-D) or 2–10 μM naphthaleneacetic acid (NAA) or 8 μM 2,4-D plus 10–50 μM glutamine, or 6 μM NAA plus 10–50 μM glutamine. MS medium devoid of growth regulators was used as control. The media were adjusted to pH 5.8 ± 0.1 with 0.1 N NaOH or 0.1 N HCl before gelling with 8 g l−1 agar. The cultures were maintained at 25 ± 2°C under a 16/8 h light/dark regime, under a photon flux density of 45–50 μmol m−2 s−1 provided by cool white fluorescent tubes (40 W; Phillips, India). The relative humidity (RH) within culture room was maintained at 55 ± 5%.
Development and germination of somatic embryos from suspension culture
For somatic embryos production, 2-week-old white and friable embryogenic calli derived from tTCLs (approximately, 1.0 g fresh weight) were transferred to 250-ml Erlenmeyer flasks (Borosil, India) containing 50 ml of MS liquid medium supplemented with 1 μM NAA, 4 μM benzyladenine (BA) and 10–50 g l−1glucose or fructose or sucrose or maltose; or 1 μM NAA, 4 μM BA, 30 g l−1 sucrose and 5–25 μM glutamine alone and 1–5 μM abscisic acid (ABA) alone or a combination of glutamine plus ABA. MS medium lacking growth regulators served as control. Cultures were agitated on an orbital shaker (100–110 rpm) under 12 μmol m−2 s−1 cool white fluorescent light with a 16-h photoperiod at 25 ± 2°C. After 10 days of culture, embryogenic cell clumps were filtered using a 200-μm stainless steel sieve to remove the large cell clumps. Aliquots of suspension culture (2–10% [v/v]) were transferred to 50 ml of fresh liquid medium of the same composition for subculture. Further three subcultures were carried out with fresh medium of the same composition at 5–7 days intervals for further proliferation. Growth of suspension cultures was monitored by cell counting using a haemocytometer (after extensive resuspension) and ascertaining the packed cell volume (PCV). PCV was determined by centrifuging 5 ml of the suspension at 200g for 5 min. Different developmental stages of embryos (globular, heart and torpedo) were induced after 4 weeks of culture. The number of embryos per flask of culture was determined. All stages of embryos were photographed under a Magnus Stereo Microscope (MSB type; Grand VCD 2000 plus; SWH 10X; transmitted light-FL). The torpedo embryos were selected from the suspension cultures, and the remaining embryos (globular and heart) were subcultured in the same composition of the medium. For germination and plantlet formation, torpedo embryos were transferred to ½ MS medium containing 20 g l−1 sucrose and 8 g l−1 agar and supplemented with 1–4 μM BA, 0.5–2 μM ABA and 1–4 μM gibberellic acid (GA3) either individually or in combination. The medium lacking growth regulators served as control. The cultures were incubated at 25 ± 2°C under a 16-h photoperiod.
Transplantation
Regenerated plantlets with healthy shoot and root systems were transferred to plastic pots containing soil mixture and vermiculite (150 g/pot) (3:1v/v) and irrigated with 50 ml of tap water used as control, or 50 ml of ½ MS basal salt solution supplemented with 5 μM indole-3-butyric acid (IBA) and 100 mg l−1 Bavistin® (BVN), respectively, used to enhance the root formation and prevent the fungal contamination. The plantlets were covered with transparent polythene bags to prevent desiccation and were maintained in a growth chamber (Sanyo, Japan). The relative humidity was reduced gradually, and after 1 month, plants were transferred to pots filled with 2:1 (v/v) mixture of soil and organic manure and maintained in a greenhouse. After 2 months, the plants were established in the field. The survival percentage was evaluated in summer (March–June) and winter (September–December) seasons.
Estimation of psoralen
After 4 weeks, different developmental stages of somatic embryos (globular, heart and torpedo) of P. corylifolia were evaluated for psoralen content by high-performance liquid chromatography (HPLC). The somatic embryos were oven-dried at 45 ± 2°C for 30 min. The dried tissues (1.0 g) were ground to a powder and soaked overnight in a 100% HPLC grade methanol (10 ml). The mixture was filtered through Whatman No.1 filter paper, and residue was redissolved in methanol (5 ml), filtered through 0.45 μm nylon filter (Millipore Corporation, USA) and centrifuged at 1,200 rpm for 5 min. The extract containing psoralen was analysed by HPLC on a GEMINI (Phenomenex) reverse phase C-18 (5.0 μm particle size) 250 × 4.6 mm column. The mobile phase was methanol/water (50:50 v/v) mixture supplied at the rate of 0.8 ml/min, using a constant temperature of 20°C during the analysis. The psoralen was detected by monitoring absorbance at 254 nm using a UV detector (SHIMADZU-Prominence model). Duplicate injections (20 μl) from sample were compared with the standard for quantification. Standard psoralen was purchased from Sigma Chemical, Mumbai, India. The experiments were repeated thrice. The amount of psoralen was calculated by the external standard method of quantification (Scott 1996).
Data collection and statistical analysis
The data were collected after 2 weeks for frequency of embryogenic callus formation (%), 4 weeks for number of somatic embryos and additional 2 weeks for frequency of germination (%) experiments. All experiments were repeated three times with 50 explants per treatment. The statistical significance of differences among the means was carried out using Duncan’s multiple-range test at 5% level (STATISTICA version 6.0).
Results and discussion
Embryogenic callus induction
Auxins (2,4-D and NAA) and glutamine were used to test their effect on induction of embryogenic callus from hypocotyl TCLs explants (Figs. 1, 2). Whitish friable embryogenic callus was obtained in both TCL explants (Fig. 3a, b). Puigderrajols et al. (2001) documented that callus initially showed highly vacuolated cells and later certain cells of these calli become embryogenic, containing dense cytoplasm, small vacuoles, large nuclei and the presence of large amounts of lipids and starch. It is reported that a single cell divides to form few-celled proembryo structures which are referred to as embryogenic units. Moreover, recurrent systems are especially suited to the continuous production of friable callus, which is a valuable source of single cells with embryogenic capacity (Fransz and Schel 1991). In our studies, concentrations of 2,4-D or NAA alone produced different frequency of embryogenic callus from both lTCL and tTCL explants while control did not produce embryogenic callus (Fig. 1). However, 2,4-D or NAA combination with glutamine considerably increased frequency of somatic embryogenic callus from both explants (Fig. 2). Similar result was also reported in Macrotyloma unifloram by Shamsudeen Varisai et al. (2004). These results suggest that glutamine plays a major role in somatic embryogenesis in P. corylifolia; however, the mechanism needs to be investigated. Lower rate of frequency was observed in lTCL explants on MS medium supplemented with 2 μM 2,4-D (Fig. 1). However, higher rate of frequency of whitish friable embryogenic calli was obtained in tTCL explants on MS medium supplemented with 6 μM NAA and 30 μM glutamine (Fig. 2). Therefore, tTCLs explants possibly influence rate of callus proliferation due to different types of tissue than lTCLs explants. Similar observation was also put forth by Teixeira da Silva (2003).
Influence of 2,4-D or NAA on embryogenic callus formation on TCL hypocotyl explants of P. corylifolia. The results are expressed as the means of three repeat experiments. Different letters indicate significant difference between means (P < 0.05); comparison by DMRT. The data were recorded after 2 weeks of culture
Influence of different glutamine concentrations added to media with 2,4-D or NAA on embryogenic callus formation on TCL hypocotyl explants of P. corylifolia. The results are expressed as the means of three repeat experiments. Different letters indicate significant difference between means (P < 0.05); comparison by DMRT. The data were recorded after 2 weeks of culture
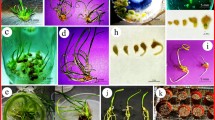
Somatic embryogenesis and plant regeneration from TCLs hypocotyl explants of P. corylifolia L. a Whitish friable embryogenic callus from lTCL hypocotyl explants (bar 5 mm). b Whitish friable embryogenic callus from tTCL hypocotyl explants (bar 5 mm). c Different stages of somatic embryos on MS liquid medium + 1.0 μM NAA + 4.0 μM BA + 10.0 μM glutamine + 2.0 μM ABA (bar 10 mm). d Different stages of somatic embryos on MS liquid medium + 1.0 μM NAA + 4.0 μM BA + 15.0 μM glutamine + 2.0 μM ABA (bar 10 mm). e–l Different developmental stages of somatic embryos from suspension culture (×10). m Germinated embryos (bar 5 mm). n Induction of roots on moist soil mixture and vermiculite (3:1) (bar 5 mm). o Acclimatized plants in growth chamber. p Regenerated plants in greenhouse. q Ex vitro plants of P. corylifolia after 3 months in the field
Development of somatic embryos, germination and psoralen content
MS liquid medium supplemented with 1 μM NAA, 4 μM BA and different carbon sources were used to induce somatic embryos. The medium containing 30 g l−1 sucrose was more effective for promoting somatic embryogenesis than other concentrations of sucrose, glucose, fructose or maltose (Table 1). Similar results were also observed in Cajanus cajan and Macrotyloma unifloram by Patel et al. (1994) and Shamsudeen Varisai et al. (2004). Maltose was also ineffective for somatic embryogenesis in peanut (Eapen and George 1993). Somatic embryos number increased significantly from tTCLs embryogenic callus on MS medium containing 1 μM NAA, 4 μM BA and 30 g l−1 sucrose and different concentrations and combinations of glutamine and ABA. The media containing 15 μM glutamine, and 3 μM ABA or 10 μM glutamine and 2 μM ABA considerably increased the number of somatic embryos (Fig. 3c). Finer and Nagasawa (1988) and Lee et al. (2002) reported that glutamine and ABA were effective in somatic embryogenesis in Glycine max and Aralia cordata. ABA was also documented to regulate normal development of somatic embryos in Daucus carota (Nishiwaki et al. 2000). In the present study, media containing 15 μM glutamine and 2 μM ABA produced the greatest number of embryos (Table 2; Fig. 3d) in different developmental stages (Fig. 3e–l). Increasing the concentrations of glutamine and ABA in the medium decreased the number of somatic embryos (Table 2). The germination of torpedo embryos was achieved in control medium (½ MS + 20 g l−1) as well as in different concentrations and combinations of BA, ABA and GA3. However, the frequency of germination significantly increased in BA, ABA and GA3 combinations (Fig. 4). The maximum frequency of germination (97.6%) was obtained on medium containing 20 g l−1 sucrose and 8 g l−1 agar and supplemented with 2 μM BA, 1 μM ABA, 2 μM GA3 (Fig. 4). Cytokinin alone or in combination with GA3 or ABA developed embryo maturation and subsequent development of embryos into plantlets (Rout and Samantaray 1995). In the present study, higher concentration (2 μM) of ABA reduced the frequency of germination significantly. Attree and Fowke (1993) reported that ABA-enriched medium might prevent precocious germination and accumulation of high level of storage proteins. In our study, the somatic embryos produced green cotyledons, a plumule, and radicular zone without showing secondary callus (Fig. 3m), and subsequently developed into plantlets with shoot and root systems. The present investigation showed an enhancement of germination of somatic embryos within 2 weeks. Thus, we demonstrated that BA, ABA and GA3 combination are essential for higher germination of P.corylifolia.
Influence of BA, ABA and GA3 in ½ MS medium with 20 g l−1 sucrose on the germination of somatic embryos of P. corylifolia. The results are expressed as the means with SE from three replicates per treatment. The data were recorded after 2 weeks of transfer of somatic embryos to media with the indicated plant growth regulator composition
Acclimatization
The plantlets generated further shoot and root systems in moist soil mixture and vermiculite (3:1 v/v) within 1 week. An average number of 4.0 roots per shoot with a 40–50 mm length were observed after 2 weeks of plantation in the water-irrigated control that was infected by fungal contamination. An average number of 16.5 roots per shoot with a 75–90 mm length were obtained in ½-MS basal salt solution supplemented with 5 μM IBA and 100 mg l−1 BVN (Fig. 3n). The addition of BVN in the 1/8-MS basal salt solution prevented fungal contamination of the moist soil mixture and favoured shoot growth. BVN is a systemic fungicide that belongs to benzimidazole family, which also showed a marked cytokinin like activity in Bacopa monnieri and Psorlaea corylifolia (Tiwari et al. 2006; Baskaran and Jayabalan 2008). The plantlets were acclimatized in growth chamber (Fig. 3o). The plants were transferred to mixture of soil and organic manure (2:1, v/v) and maintained in the greenhouse (Fig. 3p). The regenerated plants were successfully established in the field (Fig. 3q). About 200 plants were tested for the survival rate in different seasons. The survival rate during summer was 55–60% whereas in winter it was 100%. High temperatures (36–45°C) could be unfavourable for the establishment of plantlets in the field, whereas relatively low temperatures (25–28°C) during winter could be favourable for establishment. The study therefore suggests that for P. corylifolia hardened plants should be transferred to field only during winter season for the best survival rate.
Psoralen content
Somatic embryos were used to test production of psoralen content. A psoralen content of 12.9 μg g−1 DW was detected in embryos. Supporting this observation, eleutheroside content was investigated by Jeong et al. (2005) in in vitro regenerated embryos of Eleutherococcus chiisanensis. The tissue culture cells typically accumulate large amounts of secondary compounds only under specific conditions. Several products were found to be accumulating in cultured cells at a higher level through optimization of cultural conditions (Mulabagal and Tsay 2004). The present study revealed the psoralen content in different developmental stages of embryos of P. corylifolia. This study should facilitate improvement of psoralen production in future studies.
In the present study, we have provided a protocol for the high frequency of somatic embryogenesis of P. corylifolia from hypocotyl tTCLs explants through cell suspension culture. Higher frequency of somatic embryogenesis was dependent on TCL system, concentration and combination of growth regulators, especially glutamine and ABA. This protocol can be helpful for conservation strategy, phytomedicine production, somaclonal variation and genetic transformation studies. To our knowledge, this is the first report of the successful somatic embryogenesis via suspension culture in P. corylifolia.
References
Aderkas P, Rohr R, Sundberg B, Gutmann M, Be Boux ND, Lelu MA (2002) Abscisic acid and its influence on development of the embryonal root cap, storage product and secondary metabolite accumulation in hybrid larch somatic embryos. Plant Cell Tissue Organ Cult 69:111–120. doi:10.1023/A:1015245627220
Ahn IO, Bui VL, Gendy C, Tran Thanh Van K (1996) Direct somatic embryogenesis through thin cell layer culture in Panax ginseng. Plant Cell Tissue Organ Cult 45:237–243. doi:10.1007/BF00043636
Attree SM, Fowke LC (1993) Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult 35:1–35. doi:10.1007/BF00043936
Baskaran P, Jayabalan N (2007) Rapid micropropagation of Psoralea corylifolia L. using nodal explants cultured in organic additive-supplemented medium. J Hortic Sci Biotechnol 82:908–913
Baskaran P, Jayabalan N (2008) Effect of growth regulators on rapid micropropagation and psoralen production in Psoralea corylifolia L. Acta Physiol Plant 30:345–351. doi:10.1007/s11738-007-0129-z
Baskaran P, Raja Rajeswari B, Jayabalan N (2006) Development of an in vitro regeneration system in Sorghum [Sorghum bicolor (L.) Moench] using root transverse thin cell layers (tTCLs). Turk J Bot 30:1–9
Ben Ghnaya A, Charles G, Branchard M (2008) Rapid shoot regeneration from thin cell layer explants excised from petioles and hypocotyls in four cultivars of Brassica napus L. Plant Cell Tissue Organ Cult 92:25–30. doi:10.1007/s11240-007-9298-0
Chand S, Sahrawat K (2002) Somatic embryogenesis and plant regeneration from root segments of Psoralea corylifolia L., an endangered medicinally important plant. In Vitro Cell Dev Biol 38:33–38
Chand S, Sahrawat AK (2007) Embryogenesis and plant regeneration from unpollinated ovary culture of Psoralea corylifolia. Biol Plant 51(2):223–228. doi:10.1007/s10535-007-0045-5
Eapen S, George L (1993) Somatic embryogenesis in peanut: influence of growth regulators and sugars. Plant Cell Tissue Organ Cult 35:151–156. doi:10.1007/BF00032964
Finer JJ, Nagasawa A (1988) Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tissue Organ Cult 10:202–209
Frank S, Caffieri S, Raffaelli A, Vedaldi D, Dall’Acqua F (1998) Characterization of psoralen-oleic acid cycloadducts and their possible involvement in membrane photo damage. J Photochem Photobiol B 44:39–44. doi:10.1016/S1011-1344(98)00103-1
Fransz PF, Schel JHN (1991) Cytodifferentiation during the development of friable embryogenic callus of maize (Zea mays). Can J Bot 69:26–33. doi:10.1139/b91-111
Jeong JH, Jung SJ, Murthy HN, Yu KW, Paek KY, Moon HK, Choi YE (2005) Production of eleutherosides in in vitro regenerated embryos and plantlets of Eleutherococcus chiisanensis. Biotechnol Lett 27:701–704. doi:10.1007/s10529-005-4693-2
Lee KS, Lee JC, Soh WY (2002) High frequency plant regeneration from Aralia cordata somatic embryos. Plant Cell Tissue Organ Cult 68:241–246. doi:10.1023/A:1013989707725
Leguillon S, Charles G, Branchard M (2003) Plant regeneration from thin cell layers in Spinacia oleracea. Plant Cell Tissue Organ Cult 74:257–265. doi:10.1023/A:1024042522940
Martin KP (2004) Plant regeneration through somatic embryogenesis in medicinally important Centella asiatica L. In Vitro Cell Dev Biol 40:586–591
Mulabagal V, Tsay HS (2004) Plant cell cultures-an alternative and efficient source for the production of the biologically important secondary metabolites. Int J App Sci Eng 2:29–48
Mulin M, Bellio-Spataru A (2000) Organogenesis from hypocotyls thin cell layers of Lupinus mutabilis and Lupinus albus. Plant Growth Regul 30:177–183. doi:10.1023/A:1006345401325
Murashige T, Skoog FA (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759. doi:10.1007/s004250000387
Patel DB, Barne DM, Nagar N, Mehta AR (1994) Regeneration of pigeon pea (Cajanus cajan L.) through somatic embryogenesis. Indian J Exp Biol 32:740–744
Puigderrajols P, Mir G, Molinas M (2001) Ultrastructure of early secondary embryogenesis by multicellular and unicellular pathways in cork oak (Quercus suber L.). Ann Bot (Lond) 87:179–189. doi:10.1006/anbo.2000.1317
Rout GR, Samantaray S (1995) Somatic embryogenesis and plant regeneration from callus culture of Acacia catechu—a multipurpose leguminous tree. Plant Cell Tissue Organ Cult 42:283–285. doi:10.1007/BF00030000
Sahrawat AK, Chand S (2001) Continuous somatic embryogenesis and plant regeneration from hypocotyl segments of Psoralea corylifolia Linn., an endangered and medicinally important Fabaceae plant. Curr Sci 81:1328–1331
Scott RPW (1996) Chromatographic detectors: design, function and operation. Marcel Dekker Inc, New York
Shamsudeen Varisai M, Wang CS, Thiruvengadam M, Jayabalan N (2004) In vitro plant regeneration via somatic embryogenesis through cell suspension cultures of horsegram [Macrotyloma uniflorum (Lam.) Verdc]. In Vitro Cell Dev Biol 40:284–289
Teixeira da Silva JA (2003) Thin cell layer technology in ornamental plant micropropagation and biotechnology. Afr J Biotechnol 2:683–691
Tiwari V, Tiwari KN, Singh BD (2006) Shoot bud regeneration from different explants of Bacopa monnieri (L.) Wettst. By trimethoprim and bavistin. Plant Cell Rep 25:629–635. doi:10.1007/s00299-006-0126-5
Vasil IK (1988) Progress in the regulation and genetic manipulation of cereal crops. Biotechnol 6:397–402. doi:10.1038/nbt0488-397
Acknowledgments
This work was supported by Department of Science and Technology, India in the form of Senior Research Fellow. The authors are indebted to Aaron zercer and Benjamin Steinitz (ARO, Israel) for their critical comments on this manuscript, and are grateful to Dhiraviyadas (Bharathidasan University, India) for his valuable assistance during seed collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Borkowska.
Rights and permissions
About this article
Cite this article
Baskaran, P., Jayabalan, N. In vitro propagation of Psoralea corylifolia L. by somatic embryogenesis in cell suspension culture. Acta Physiol Plant 31, 1119–1127 (2009). https://doi.org/10.1007/s11738-009-0330-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0330-3