Abstract
The efficient plant regeneration system from embryogenic cell suspension cultures of Gynura procumbens (Lour.) Merr. is described. Leaf, stem and petiole explants were cultured on Murashige and Skoog (MS) medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D) in various concentrations (0, 0.1, 0.3, 1.0 and 3.0 mg l−1). Leaf, stem and petiole explants formed pale-yellow nodular callus and off-white calluses at a frequency of 100% when cultured on MS medium supplemented with more than 1 mg l−1 of 2,4-D after 4 weeks incubation. However, only 20% of pale-yellow nodular callus derived from petiole explants developed into white embryonic structures. Upon transfer to MS basal medium without growth regulators, these white embryonic structures differentiated into somatic embryos. Embryogenic cell suspension cultures were initiated from petiole-derived pale-yellow nodular calluses. More than 73.2% of regenerated plantlets via somatic embryogenesis produced roots on MS medium supplemented with 0.1 mg l−1 α-naphthaleneacetic acid and 1 mg l−1 indole-3-butyric acid (IBA), respectively. Rooted plantlets were successfully transplanted to soil mixture of sterile vermiculite and potting soil (1:1) and grown to maturity in a growth chamber, achieving a survival rate of > 95%. The plant regeneration system from embryogenic cell suspension cultures of G. procumbens established in this study could be applied as an alternative for mass proliferation as well as molecular breeding for quality improvement of G. procumbens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gynura procumbens (Lour.) Merr. is a medicinal plant belonging to the Asteraceae family. It has been extensively used as a traditional herbal medicine particularly in Southeast Asia. The species has been used in folk medicines for the treatment of several diseases including skin rash, kidney diseases, eruptive fever, hemorrhoids and skin inflammation (Perry and Metzger 1980; Iskander et al. 2002; Hew et al. 2013). The leaves and aerial parts of G. procumbens are not toxic (Rosidah et al. 2009; Zahra et al. 2011) and have been used to source flavonoids with enhanced antioxidant capacity (Kaewseejan et al. 2015). And, the aerial parts of G. procumbens has also been used to control blood sugar level of diabetic patients (Akowuah et al. 2002), anti-hypertensive (Hoe et al. 2007) and anti-cancer activity (Hew et al. 2013). Recently, ethanolic Gynura extract has been reported to show the presence of alkaloids and volatile oils (Puangpronpitag et al. 2012). However, the exact chemical components in respective medicinal activities need to be further studied.
Despite the great medicinal value, adverse effects have also been reported to be associated with Gynura species due to the presence of pyrrolizidine alkaloids that cause hepatic veno-occlusive disease. However, there is little information available regarding pyrrolizidine alkaloids in G. procumbens as compared to G. segetum containing a number of pyrrolizidine alkaloids such as senecionine, seneciphylline, and seniciphyllinine (Qi et al. 2009). It is presumed that pyrrolizidine alkaloids in Gynura roots might be responsible for the hepatotoxicity (Dai et al. 2007). Additionally, using this plant as herbal product bears the risk of liver failure when the wrong species rich in pyrrolizidine alkaloids are used or when pyrrolizidine alkaloids content is increased due to cultivation or environmental conditions. Therefore, both correct botanical identification and quality control are needed to ensure safe use of the plant material.
Plant cell and organ culture are a promising approach to regenerate the whole plant and to obtain plant-specific bioactive compounds. Moreover, in vitro cell or organ cultures can be optimized for producing the desired metabolites and reducing the production of the toxic compounds. Plant cell suspension culture is the most popular approach since it gives a homogenous fast growing material. It represents a simple technique and is easy to scale up (Jie et al. 2015). Undifferentiated cells in a suspension culture may lack the biosynthetic machinery required for the generation of diverse secondary metabolites of interest, as different cell types may be involved in completing the pathway. Therefore, a plant organ culture is an alternative system instead of plant cell culture.
Although Gynura plant plays an important role in traditional medicine, the in vitro culture system of the Gynura species has not been well-studied. Several studies on the establishment of a micropropagation system for clonal multiplication (Keng et al. 2009) and NMR-based metabolic profiling of adventitious root cultures from leaf explants (Saiman et al. 2012) have been reported. Recently, Alizah and Nurulaishah (2015) reported multiple shoot regeneration from nodal explants of G. procumbens. However, there has been no report on successful somatic embryogenesis of G. procumbens yet.
The present study describes the culture conditions for efficient plant regeneration system from embryogenic cell suspension cultures of G. procumbens. To improve the plant regeneration efficiency, the effects of explant types (leaf, petiole and stem) and plant growth regulators on somatic embryogenesis were examined.
Materials and methods
Plant materials
A wild plant of G. procumbens (approximately 25–30 cm in height) used in this study was acquired from an herbal garden in Korea. The upper leaves and stems of the plant were taken and washed thoroughly under running tap water for 10 min. Under aseptic conditions, leaves and stem explants were surface sterilized with 70% v/v ethanol for 30 s, subsequently soaked in 20% (v/v) solution of commercial sodium hypochlorite (containing 4% w/v active ingredient) for 15 min and rinsed three times with sterile distilled water. After surface sterilization, the explants were transferred into sterile plastic Petri dishes (90 × 15 mm) and the remaining moisture of explants was removed with sterile filter paper.
The culture medium used throughout the experiments consisted of MS (Murashige and Skoog 1962) inorganic salts, 0.4 mg l−1 thiamine∙HCl, 100 mg l−1 myo-inositol, 30 g l−1 sucrose and 4 g l−1 Gelrite. The pH of all media was adjusted to 5.8 with 1 N NaOH before autoclaving. After cooling, 25 ml of medium was dispensed into plastic Petri dishes (90 × 15 mm). The in vitro grown plants of G. procumbens (approximately 2–5 cm in length) were obtained from node cultures of G. procumbens. Multiple shoots of G. procumbens were cultured on MS basal medium and maintained by subcultures at 4-week intervals.
Embryogenic callus induction from leaf, petiole and stem explants of G. procumbens
To examine the effect of 2,4-dichlorophenoxyacetic acid (2,4-D) on embryogenic callus formation, leaf, petiole and stem explants were incubated on MS medium supplemented with different concentrations of 2,4-D (0, 0.1, 0.3, 1 and 3 mg l−1). The leaf, petiole and stem explants were dissected from surface-sterilized explants with a pair of forceps and scalpel in a clean bench. The leaf explants (approximately 5 mm2 in area) were excised from middle region of surface-sterilized leaf sample and placed onto each culture medium. Petiole explants were also excised from the lower part of surface-sterilized leaf sample. Petiole explants (approximately 1–2 mm in diameter and thickness) and stem explants (approximately 2–3 mm in diameter and 1–2 mm in thickness) were dissected from surface-sterilized stem samples. All explants (leaf, petiole and stem) were cultured on MS medium supplemented with different concentrations of 2,4-D. In each treatment, ten explants were placed in a petri dish and replicated three times. All cultures were incubated at 25 °C in the dark. After 6 weeks of culture, the frequency of callus formation was determined.
To compare the difference of embryogenic potentials between in vitro and wild-grown petiole explants of G. procumbens, petiole explants (less than 1 mm in diameter and 1–2 mm in thickness) from in vitro grown shoots were also incubated on MS medium supplemented with different concentrations of 2,4-D. The frequency of callus formation was also examined in the same manner as explants of wild-grown plant.
Establishment of embryogenic cell suspension cultures of G. procumbens
The embryogenic cell suspension cultures of G. procumbens were initiated from petiole-derived pale-yellow nodular calluses. About 1 g of these calluses were transferred into Erlenmeyer flask (250 ml) containing 30 ml of fresh SH liquid medium. The cultures was maintained at gyratory shaker (100 rpm) in the dark. Otherwise mentioned all cultures were maintained at 25 °C. After 1 week of culture, an additional 20 ml of fresh SH liquid medium was added into the culture flask. After initial establishment of cell suspension cultures, 5 ml of cell suspension culture was transferred into a Erlenmeyer flask (250 ml) containing 50 ml of fresh SH liquid medium. The cell suspension culture was sub-cultured at 4 weeks interval.
To check the plant regeneration potential of embryogenic cell suspension cultures of G. procumbens, 4-week-old cell aggregates were transferred to MS basal medium and incubated for 4 weeks in the dark. In each treatment, 20 cell aggregates were placed on MS basal medium. Each treatment consisted of three replicates.
Plant regeneration via somatic embryogenesis from petiole-derived embryogenic cell suspension cultures of G. procumbens
To regenerate whole plants via somatic embryogenesis of G. procumbens, pale-yellow embryogenic cell aggregates were transferred to MS basal medium. After 2 weeks of culture in the light (30 µmol m−2 s−1 from cool-white fluorescent lamps with a 16-h photoperiod), the conversion frequency was determined by counting the number of cell aggregates with developed somatic embryos.
To accelerate root formation of regenerated plantlets from somatic embryos, the effect of α-naphthaleneacetic acid (NAA) and indole-3-butyric acid (IBA) on root development was examined. Individual regenerated plantlets from somatic embryos were carefully dissected and placed onto MS medium containing 0, 0.1, 0.3 and 1 mg l−1 NAA and IBA, respectively. In each treatment, ten explants were placed in a petri dish and replicated three times. After 2 weeks of culture in the dark, the frequency of root development was determined by counting the number of rooted plantlets.
Plantlets developed from somatic embryos were subjected to acclimatization. Regenerated plantlets were carefully removed from Petri dishes and washed under running tap water to eliminate any traces of medium. Rooted plantlets were transplanted to plastic pots containing sterile vermiculite and potting soil (1:1) and maintained in a growth chamber (25 °C day/22 °C night, 80 µmol m−2 s−1 from cool-white fluorescent lamps under a 16-h photoperiod).
Statistical analysis
The results presented are average from two independent experiments carried out in triplicates. The results were expressed as mean ± SD and statistically analyzed using MS Excel 2013 software.
Results and discussion
Establishment of high-frequency plant regeneration system from embryogenic cell suspension cultures of G. procumbens
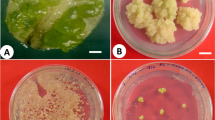
An efficient plant regeneration system via somatic embryogenesis from petiole cultures of Gynura procumbens (Lour.) Merr was established (Fig. 1). After 1 week of culture, petiole explants began to expand and dedifferentiate into calluses when they cultured on MS medium containing 2,4-D (Fig. 1a). After 2 weeks of culture, calluses began to form on the cut edges and surface of petiole explants (Fig. 1b). After 4 weeks of culture, pale-yellow calluses and white friable calluses began to form on the entire surface of petiole explants (Fig. 1c). After subculture of these pale-yellow calluses, numerous white embryonic structures were proliferated on the surface of pale-yellow callus (Fig. 1d). When transferred to MS basal medium, most white embryonic structures were able to develop into multiple somatic embryos after 4 weeks of incubation. These results indicate that the initial pale-yellow calluses and white embryonic structures were embryogenic callus and early stage of somatic embryos of G. procumbens, respectively. The embryogenic cell suspension culture of G. procumbens was established from petiole-derived pale-yellow nodular calluses. Yellow calluses were finely dispersed and proliferated well in SH liquid medium. After two rounds of subculture, cell suspension culture was sub-cultured at 4-week interval. Cell suspension culture consisted of small isodiametric (approximately 30 µm) and compact cell aggregates (Fig. 1e). To check embryogenic potential of cell suspension cultures, 4-week-old cell aggregates (Fig. 1f) were transferred to MS basal medium. Numerous somatic embryos with different developmental stages were developed from cell aggregates after 4 weeks of incubation (Fig. 1g). These somatic embryos were successfully converted into green plantlets in the light incubation (Fig. 1h). After soil transfer for acclimatization, regenerated plantlets from somatic embryos were rooted and grown to normal plants (Fig. 1i).
Plant regeneration from petiole-derived embryogenic cell suspension cultures of G. procumbens. a After 1 week of incubation, small callus began to form on the cut edge of petiole explants. b After 2 weeks of incubation, pale-yellow friable callus began to form on the cut edge of petiole explant when cultured on MS medium containing 2,4-D. c White embryonic structures formed on the surface of pale-yellow callus. d White embryonic structures developed into typical somatic embryos when cultured on MS medium containing 2,4-D. e Establishment of embryogenic cell suspension cultures of G. procumbens. f Globular embryos formation from embryogenic cell aggregates. g Numerous somatic embryos with different developmental stages. h Somatic embryos were successfully converted into plantlets in the light incubation. i Regenerated plants from somatic embryos were successfully grown to normal plant after soil transfer for acclimatization. Scale bars represent 1 mm (a–d, f, g), 0.5 mm (e), 1 cm (h, i)
To our knowledge, this study is the first successful report of high-frequency plant regeneration from embryogenic cell suspension cultures of G. procumbens. The plant regeneration system established in the study could be applied as an alternative for mass proliferation and molecular breeding for quality improvement of G. procumbens.
Effect of 2,4-D concentration on embryogenic callus formation from leaf, petiole and stem explants of G. procumbens
The effect of 2,4-D on callus and adventitious root formation from leaf, petiole and stem explants of G. procumbens was examined (Table 1). After 4 weeks of incubation, leaf, petiole and stem explants were able to form pale-yellow callus when cultured on MS medium containing over than 0.1 mg l−1 of 2,4-D. All explants formed pale-yellow calluses even in low concentration of 2,4-D treatment. In particular, stem explants formed calluses at a frequency of 100% regardless of 2,4-D concentrations. The frequency of pale-yellow callus formation from leaf and petiole explants was 95.8% and 80% when cultured on MS medium supplemented with 0.1 mg l−1 of 2,4-D (Table 1). The frequency of pale-yellow callus formation from leaf and petiole explants slightly increased with increasing concentrations of 2,4-D up to 3 mg l−1, where the frequency reached 100% (Table 1). These results show that all tissues of G. procumbens have good potential for pale-yellow callus formation in low concentration of 2,4-D.
Adventitious root formation was also simultaneously observed from leaf, petiole and stem explants of G. procumbens during callus formation. The highest frequency of adventitious root formation (62.5 ± 25.5%) was observed in leaf explants treated with 1 mg l−1 of 2,4-D (Table 1). However, the frequency of adventitious root formation from leaf explant greatly decreased with increase in 2,4-D concentrations. Both petiole and stem explants also showed the decrease of adventitious root formation at higher concentrations of 2,4-D treatment (Table 1). These results show that a lower concentration of auxin treatment is more suitable for adventitious root formation of G. procumbens.
To check the embryogenic potential of pale-yellow callus derived from leaf, petiole and stem explants of G. procumbens, pale-yellow calluses were transferred to MS basal medium and incubated for 4 weeks in the dark. However, only pale-yellow calluses derived from petiole explants were able to form white embryonic structures at a frequency of 20% when cultured on MS medium supplemented with 3 mg l−1 2,4-D (Fig. 1d). Even though the morphological characteristics of pale-yellow callus derived from leaf, petiole and stem explants of G. procumbens were similar, leaf and stem-derived pale-yellow callus did not show development of embryonic structures from pale-yellow callus.
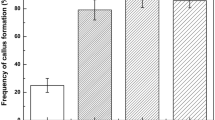
The frequency of pale-yellow callus formation from petiole explants of in vitro grown shoots of G. procumbens. was also examined. Both petiole explants from wild and in vitro grown plants showed similar callus patterns of formation (Fig. 2). The highest frequency of pale-yellow callus formation from both petiole explants was 100% when cultured on MS medium supplemented with 3 mg l−1 of 2,4-D (Fig. 2). However, the frequency greatly decreased to less than 26.7% when cultured on MS medium supplemented with 0.3 mg l−1 of 2,4-D (Fig. 2). Based on these results, it is suggested that higher concentration of 2,4-D is more suitable for embryogenic callus induction from petiole explant of G. procumbens.
In plant tissue culture, 2,4-D is well-known for its promotive role in somatic embryogenesis from several plant species including Ranunculus kazusensis (Min et al. 2007), Brasenia schreberi (Oh et al. 2008) and Houttuynia cordata (Oh et al. 2013). Similar to previous reports, we found that 2,4-D also plays a strong inducer for somatic embryogenesis from petiole explant cultures of G. procumbens. Leaf and stem explants of G. procumbens were also able to form similar pale-yellow calluses compared to petiole explants. However, the conversion of these pale-yellow calluses into embryogenic structures was not observed. These results imply that there is an obvious difference in 2,4-D sensitivity for somatic embryogenesis from different tissue origins of G. procumbens. However, we could not fully examine the exact cause of variation in tissue or organ-dependent embryogenic potential of G. procumbens in this study. To date, only a few studies have been published on somatic embryogenesis from petiole explant with 2,4-D treatment such as Spathiphyllum (Zhao et al. 2012) and African violet (Mithila et al. 2003). Considering these results, petiole explant is more suitable for somatic embryogenesis than other tissue explants of G. procumbens. Recently, epigenetic mechanisms including chromatin modification and DNA methylation pattern have emerged as critical factors during somatic embryogenesis and differentiation of plant meristem (De-la-Peña et al. 2015). Thus, further work is needed to determine the role of epigenetic regulation on somatic embryogenesis.
Effect of auxins on root development from regenerated plantlets of G. procumbens
For the establishment of whole plant regeneration system via somatic embryogenesis, the effect of auxins on root development of regenerated plantlets of G. procumbens was examined (Fig. 3). The frequency of root development was 73.2% and 100% when cultured on MS medium supplemented with 0.1 mg l−1 IBA and NAA, respectively (Fig. 3). In case of IBA treatment, the frequency of root formation from regenerated plantlets was increased with increase of IBA concentrations. However, NAA treatments showed a little different root formation. The frequency of root formation was highest in low concentration of NAA treatment (0.1 mg l−1). Whereas, the frequency of root formation decreased to 75% when plantlets were cultured on MS medium supplemented with 1 mg l−1 NAA (Fig. 3). These results show that low concentrations of auxin was suitable for rooting from regenerated plantlets of G. procumbens regardless of auxin types. The results shown in this study are in agreement with other studies showing that lower concentration of auxin is optimal for rooting of G. procumbens (Chan et al. 2009) and adventitious root production of Centella asiatica (Ling et al. 2009). Recently, Parvin et al. (2014) also reported that 0.5 mg l−1 of NAA was suitable for rooting from shoot tip and nodal segments cultures of G. procumbens. The rooted plantlets were successfully transferred to potting soil and subjected to acclimatization. 100% of the plantlets survived and grew into normal plants (Fig. 1i).
In conclusion, we have established a successful and high-frequency plant regeneration system from embryogenic cell suspension cultures of G. procumbens. The plant regeneration system of G. procumbens established in this study may be useful for mass propagation and long-term preservation of G. procumbens. Furthermore, embryogenic cell lines of G. procumbens could be used for biological resources for the study of metabolic engineering and quality improvements by gene editing technologies.
References
Akowuah GA, Sadikun A, Mariam A (2002) Flavonoid identification and hypoglycaemic studies of the butanol fraction from Gynura procumbens. Pharm Biol 40:405–410
Alizah Z, Nurulaishah Y (2015) Multiple shot regeneration from nodal explants of Gynura procumbens (Lour.) Merr. Annu Res Rev Biol 6:85–88
Chan LK, Lim SY, Pan LP (2009) Micropropagation of Gynura procumbens (Lour.) Merr. an important medicinal plant. J Med Plant Res 3:105–111
Dai N, Yu YC, Ren TH, Wu JG, Jiang Y, Shen LG, Zhang J (2007) Gynura root induces hepatic veno-occlusive disease: a case report and review of the literature. World J Gastroenterol 13:1628–1631
De-la-Peña C, Nic-Can GI, Galaz-Ávalos RM, Avilez-Montalvo R, Loyola-Vargas VM (2015) The role of chromatin modifications in somatic embryogenesis in plants. Front Plant Sci 6:635
Hew CS, Khoo BY, Gam LH (2013) The anti-cancer property of proteins extracted from Gynura procumbens (Lour.) Merr. PLoS One 8:7 e68524
Hoe SZ, Kamaruddin MY, Lam SK (2007) Inhibition of angiotensin-converting enzyme activity by a partially purified fraction of Gynura procumbens in spontaneously hypertensive rats. Med Princ Pract 16:203–208
Iskander MN, Song Y, Coupar IM, Jiratchariyakul W (2002) Anti-inflammatory screening of the medicinal plant Gynura procumbens. Plant Food Hum Nutr 57:233–244
Jie EY, Ryu YB, Choi SA, Ahn MS, Liu JR, Min SR, Kim SW (2015) Mass propagation of microtubers from suspension cultures of Pinellia ternata cells and quantitative analysis of succinic acid in Pinellia tubers. Plant Biotechnol Rep 9:331–338
Kaewseejan N, Sutthikhum V, Siriamornpun S (2015) Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J Funct Foods 12:120–128
Keng CL, Yee LS, Pin PL (2009) Micropropagation of Gynura procumbens (Lour.) Merr. an important medicinal plant. Med Plant Res 3:105–111
Ling APK, Chin MF, Hussein S (2009) Adventitious root production of Centella asiatica in response to plant growth regulator and sucrose concentrations. Med Aromat Plant Sci Biotechnol 3:36–41
Min SR, Liu JR, Kim SW (2007) Plant regeneration from zygotic embryo-derived embryogenic cell suspension cultures of Ranunculus kazusensis. Plant Biotechnol Rep 1:57–60
Mithila J, Hall JC, Victor JMR, Saxena PK (2003) Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl). Plant Cell Rep 21:408–414
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Oh MJ, Na HR, Choi HK, Liu JR, Kim SW (2008) High frequency plant regeneration from zygotic embryo derived embryogenic cell suspension cultures of watershield (Brasenia schreberi). Plant Biotechnol Rep 2:87–92
Oh MJ, Ahn MS, Jie EY, Liu JR, Min BW, Kim SW (2013) High-frequency plant regeneration from immature zygotic embryo cultures of Houttuynia cordata Thunb via somatic embryogenesis. Plant Biotechnol Rep 7:527–534
Parvin F, Md JI, Jahan N, Khan H, Md PES, Md AI, Rahaman MH, Md MR (2014) Efficient in vitro micropropagation of Gynura procumbens—an important rare medicinal plant, through shoot tip and nodal segments explants. J Res Biol 4:1444–1450
Perry LM, Metzger J (1980) Medicinal plants of East and South East Asia: attributed properties and uses. The MIT Press, Cambridge
Puangpronpitag D, Kaewseejan N, Nakornriab M (2012) Evaluation of phytochemical composition and antibacterial property of Gynura procumbens extract. Asian J Plant Sci 11:77–82
Qi X, Wu B, Cheng Y, Qu H (2009) Simultaneous characterization of pyrrolizidine alkaloids and N-oxides in Gynura segetum by liquid chromatography/ion trap mass spectrometry. Rapid Commun Mass Spectrom 23:291–302
Saiman MZ, Mustafa NR, Schulte AE, Verpoorte R, Choi YH (2012) Induction, characterization, and NMR-based metabolic profiling of adventitious root cultures from leaf explants of Gynura procumbens. Plant Cell Tissue Organ Cult 109:465–475
Rosidah, Yam MF, Sadikun A, Ahmad M, Akowuah GA, Asmawi MZ (2009) Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol 123:244–249
Zahra AA, Kadir FA, Mahmood AA, Al hadi AA, Suzy SM, Sabri SZ, Latif II, Ketuly KA (2011) Acute toxicity study and wound healing potential of Gynura procumbens leaf extract in rats. J Med Plant Res 5:2551–2558
Zhao J, Cui J, Liu J, Liao F, Henny RJ, Chen J (2012) Direct somatic embryogenesis from leaf and petiole explants of Spathiphyllum ‘Supreme’ and analysis of regenerants using flow cytometry. Plant Cell Tissue Organ Cult 110(2):239–249
Acknowledgements
This work was supported by a grant from the KRIBB Research Initiative Program (KGM5281711).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jie, E.Y., Atong, N.S., Ahn, W.S. et al. High-frequency plant regeneration from embryogenic cell suspension cultures of Gynura procumbens. Plant Biotechnol Rep 13, 27–33 (2019). https://doi.org/10.1007/s11816-018-0507-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0507-6