Abstract
Objective
This study aimed to evaluate the cost-effectiveness of bariatric surgery (BS) compared to non-surgical treatment (NST) in Korean people with morbid obesity according to comorbidities and body mass index (BMI) severity.
Methods
The target cohort was people with morbid obesity, defined as BMI of ≥ 35 kg/m2, or obese people with BMI of 30–34.9 kg/m2 having obesity-related comorbidities. A decision-tree model for 1-year obesity treatment and Markov model for the rest of life were used. In the decision-tree model, the comorbidity remission rate and BMI change after 1-year treatment were decided based on a prospective clinical trial. In the Markov model, the transition probabilities were calculated considering the BMI level and age. The starting age of 20 years, a cycle length of 1 year, a time horizon of 80 years, and a 5% discount rate were applied for the base case from the healthcare system perspective.
Results
In the base case, BS improved quality-adjusted life years (QALYs) and was the cost-effective option in total cohort (incremental cost-effectiveness ratio of BS vs. NST was 674 USD/QALY). It was shown to be cost-effective in all subgroup analyses based on BMI level. In particular, BS was a dominant alternative for the subgroup with basal BMI of 35.0–37.4 kg/m2. Various sensitivity analyses showed the robustness of results indicating the cost-effectiveness of BS.
Conclusion
BS at BMI of > 30 kg/m2 was more effective than NST for a reduction in BMI and remission of obesity-related comorbidities and was cost-effective in Korea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the number of obese people increases worldwide, obesity-related health problems are soaring. Obesity is a risk factor for chronic diseases, such as hypertension, diabetes, and cardiovascular diseases, and it was also reported to increase the risk of mortality independently [1,2,3,4]. Therefore, obesity is now classified as a disease to be managed with high priority from the perspective of global public health.
Asians tend to have low body mass index (BMI) and a lower prevalence of obesity compared to Westerners. However, they have a higher proportion of body fat [5, 6] and higher prevalence and mortality rate of obesity-related chronic diseases than Westerners [7]. Therefore, Asian countries set the BMI threshold for obesity at a lower BMI than Western countries (BMI cut-off point for obesity is 30 kg/m2 for West, 25 kg/m2 for Asia) and have tried to strengthen the management of obesity [8].
Although the overall increase in obesity prevalence is stagnant in Asia, morbid obesity, defined by BMI > 35 kg/m2, is increasing, particularly among young people due to westernized diets and the lack of outdoor activities [9, 10]. In a recent research in a Korean population, the mortality risk was reportedly increased by 1.18–1.71 times in morbid obesity cases than in non-obese controls [11]. Prevention and proper treatment of obesity are crucial considering the severity of obesity-related chronic diseases, and morbid obesity should be treated more urgently. In addition, social support for the treatment of obesity should be provided more adequately to the people in unstable socioeconomic conditions, given the high incidence rate of obesity in the group [12, 13].
Bariatric surgery (BS) is an effective treatment for morbid obesity, whereas non-surgical treatment (NST) with altered diet, exercise, or medications showed insufficient efficacy with short-term effects [14, 15]. BS has already been established as a general treatment for obesity in the West [16, 17] and its benefits in terms of cost-effectiveness have been evaluated in many researches [18, 19]. In comparison, there is a lack of clinical and economic assessment data for BS in Asia [20]. Interests and discussions on the need of BS to treat obesity have been growing in Asia [21], and research on and evaluation of the clinical usefulness and economic feasibility of BS are urgently required in the region.
The cost-effectiveness of BS has been evaluated in 2013 based on a retrospective trial in a Korean population, and the results showed acceptable cost-effectiveness in morbidly obese individuals. However, the study presented only the results of total subjects and did not assess the cost-effectiveness in the context of specific comorbidities and BMI statuses, which directly impact mortality and medical expenditure [22]. This study was conducted to evaluate the cost-effectiveness of BS and compare it with that of NST in Korean people with morbid obesity or obesity at BMI 30–34.9 kg/m2 with comorbidity according to their comorbidities and BMI severity.
Methods
Target Analysis Cohort and Setting
This study used individual patient data from a prospective clinical trial [23]. The target cohort included morbidly obese people with basal BMI ≥ 35 kg/m2. People with BMI of 30–34.9 kg/m2 were also included if they had obesity-related comorbidities, such as hypertension, diabetes, and hyperlipidemia. BS comprised Roux-en-Y gastric bypass and sleeve gastrectomy, and NST included regular hospital outpatient visits for obesity treatment via medications, diet, or exercise support. A cycle length of 1 year, a time horizon of 80 years, and a discount rate of 5% were applied for base case analysis from the healthcare system perspective.
Model Structure and Estimation
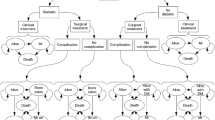
The decision-tree model for 1-year obesity treatment (BS vs. NST) and the Markov model for the rest of the lifetime were used (Fig. 1). The decision-tree model reflected the changes in the BMI level and comorbidity remission rates for 1 year of obesity treatment. The analysis was moved to the Markov model according to the changed BMI level and comorbidity status of the individuals after 1 year of treatment. In the Markov model, five health statuses—no comorbidity, mild/moderate comorbidity (hypertension and/or diabetes and/or hyperlipidemia), severe comorbidity (coronary artery disease and/or stroke), death due to cardiovascular disease (CVD), and death due to any other reasons—were assumed to reflect changes in the comorbidity status according to the changed BMI level and aging over the time horizon.
Clinical Data and Utility
The clinical data and utility weight used in this study were collected through a prospective multicenter clinical trial conducted in 13 university hospitals in Korea from August 2016 to October 2018 [23]. The initial proportion of comorbidity before obesity treatment, the comorbidity remission rate, and the changed BMI level after 1 year of obesity treatment were obtained from the clinical trial results. Quality of life was investigated using patient questionnaires of the EuroQol-5 dimension (EQ-5D) 3 level in the clinical trial. The utility weight according to the BMI level was calculated by applying Korean tariff and was adjusted for age, sex, treatment group (BS or NST), and comorbidity status by the generalized estimation equation. The utility weight of severe comorbidity was obtained by multiplying the utility weights of mild/moderate comorbidity by 0.854, the ratio obtained from the Korea National Health and Nutrition Examination Survey (KNHNES, 2007–2015) because people with severe comorbidity were not included in the clinical data. The KNHNES is a national survey implemented by the Korea Centers for Disease Control (KCDC) to evaluate the health and nutritional status of Koreans [24].
Model Structure and Transition Probability
It was assumed that the BMI level at 1 year after obesity treatment was maintained lifetime according to a previous article which reported the long-term clinical results of BS vs. NST in Swedish people with morbid obesity [25]. The transition of comorbidity only allowed movement to a more severe status in the Markov model. For example, people with severe comorbidity could not move to no comorbidity and mild/moderate comorbidity statuses. The transition probabilities among health statuses by the BMI level were assumed using the incidence rates of comorbidity in the reference group (18.5–24.0 kg/m2 BMI) in each age group. Hazard ratio for transition probability according to BMI of ≥ 25.0 kg/m2 were assumed based on a re-analysis of the database used for the research by Song et al. [25] and by Park et al. [11]. The incidence rate of comorbidity in the reference group (BMI of 18.5–24.9 kg/m2) was obtained from the representative Korean data based on the National Health Insurance Service-Health Screening cohort (NHIS-HEALS, 2002–2013) [26] and KNHAES data. The NHIS-HEALS comprises a random sample of 10% of subjects aged 40–79 years who underwent health screening between 2002 and 2003, and the subjects were followed up for 10 years [27]. The hazard ratio of moderate or severe comorbidity by the BMI level was drawn from the NHIS-HEALS database [11]. The transition probability of death due to other causes was obtained from Korean Death Statistics (2016) [28] excluding the people with cardiovascular disease (CVD). The transition probability of death due to CVD was obtained by multiplying death due to other causes by 1.81 based on a previous research [29]. The hazard ratio of death according to the BMI level was assumed based on the analysis of the NHIS-HEALS cohort data, and it was applied to the transition probability to reflect the difference of death according to the BML level.
Costs
The costs in the first and following years were the medical expenses associated with the obesity treatment and obesity-related comorbidity treatment, respectively. The first-year cost was assumed by micro-costing simulations. The costs for resource use were assumed based on unit cost provided by the Health Insurance Review and Assessment Service (HIRA) [30] and frequency assumption based on clinical trial data and expert advices. In the Markov model, the cost of health status was calculated based on the unit cost and proportion of each comorbidity (e.g., hypertension, diabetes, and hyperlipidemia), which was derived from data analysis of the Korean HIRA-National Patients Sample database (HIRA-NPS, 2010–2014). HIRA-NPS was composed of an annual random sample of 3% (n = ~ 1,400,000) of the entire population that visited clinics or hospitals [31].
Statistics and Sensitivity Analysis
The results of this study were presented by the incremental cost-effectiveness ratio (ICER) of BS compared with NST in people with morbid obesity or obesity at BMI 30–34.9 kg/m2 with comorbidity. The uncertainty was evaluated through deterministic sensitivity analysis and probabilistic sensitivity analysis. In the deterministic sensitivity analysis, change of input values for various variables, such as the comorbidity remission rate at 1 year after obesity treatment, utility weight, surgery cost, starting age, time horizon, and discount rate, was considered. In the probabilistic sensitivity analysis, 1000 micro-simulations were performed based on the assumptions of parameter distribution for utility, transition probabilities, and cost.
Results
Characteristics of Target Analysis Cohort, Effectiveness, and Cost
Characteristics of the target cohort are presented in Table 1. The proportions of BMI 30–34.9 kg/m2 and ≥ 35 kg/m2 were 50% and 50%, respectively. People with mild/moderate comorbidity accounted for 81.4% of included individuals. At 1 year after the obesity treatment, 65.6% of people who underwent BS and 14.5% people who underwent NST had a BMI of < 30 kg/m2. The remission rate (change from mild/moderate comorbidity status to no comorbidity status) was 43.9% with BS and 9.5% with NST.
The input data, such as utility weights, cost, and transition probabilities for this model, are summarized in Table 2. The utility weight tended to significantly decrease as BMI levels increased. However, the utility weights for no comorbidity and mild/moderate comorbidity were not statistically significant, and the same utility weight was applied. The first-year cost for BS was approximately 6.7 times higher than that of NST (BS, 7780 USD; NST, 1160 USD). The costs after the first year were approximately 1400 USD for mild/moderate comorbidity and approximately 2000 USD for severe comorbidity.
Base Case Analysis
In the base case analysis, people who underwent BS spent 235 USD more and gained 0.348 quality-adjusted life years (QALY) more for a lifetime. BS was a cost-effective alternative with an ICER of 674 USD/QALY to NST (Table 3).
Sensitivity Analysis
In the results of the deterministic sensitivity analysis, the ICER was affected by the discount rate, comorbidity remission in the first year after obesity treatment, surgery cost, starting age, time horizon, and utility weight, in that order. When the discount rate was set at 7.5%, the ICER was the highest at 9012 USD/QALY. A discount rate of 0–3%, 25% increase in the remission rate of mild/moderate comorbidity in BS, and 25% decrease in the surgery cost showed cost-saving results with higher QALYs (Fig. 2).
The BMI level before obesity treatment was divided into five groups: 30.0–32.4 kg/m2, 32.5–34.9 kg/m2, 35.0–37.4 kg/m2, 37.5–39.9 kg/m2, and ≥ 40.0 kg/m2, and then, sub-group analyses were performed (Table 3). Consequently, BS was a cost-effective alternative in all subgroups. In particular, BS was a dominant alternative at basal BMI of 35.0–37.4 kg/m2.
Probabilistic sensitivity analysis revealed that BS was a cost-effective alternative under the willingness to pay threshold of 32,000 USD/QALY [32] with higher than 90% probability (Fig. 3).
Discussion
This study evaluated the cost-effectiveness of BS in people with morbid obesity or obesity at BMI 30–34.9 kg/m2 with comorbidity using a prospective clinical trial conducted in South Korea. The ICERs in base and deterministic sensitivity analyses were all less than 10,000 USD/QALY, confirming that BS was a cost-effective alternative to NST for the target people. In particular, this study presented more elaborated ICERs by applying different incidence rates of comorbidity and mortality rate according to the level of BMI.
ICERs were mostly affected by the efficacy of BMI reduction and comorbidity remission after 1 year of obesity treatment in the prospective clinical study. The proportion of people with reduced BMI of < 30 kg/m2 after 1 year was 65.6% in BS, which was much higher than 14.5% reported for NST. The results in terms of BMI reduction with BS in this study were in agreement with those of previous studies on Westerners [33, 34] or Asians [35, 36]. BS was also effective in reducing BMI and increasing remission rate associated with comorbidities, and the rate of remission was 34.4% higher in BS than in NST (43.9% for BS, 9.5% for NST). In a British study, which involved 33,718 highly obese people who underwent an operation for obesity treatment, the rates of comorbidity remission were 23–96% after BS; the remission rates were 78% for diabetes, 69% for hypertension, and 60% for hyperlipidemia when people received Roux-en-Y gastric bypass [37]. In a meta-analysis, the remission rates were reported to be 66.7% for diabetes, 38.2% for hypertension, and 60.4% for hyperlipidemia [38]. Compared with these studies, the remission rates assumed for this study were lower, and this may be attributed to the short observational period of 1 year to normalize chronic diseases in the clinical trial used for the source data in this study. The subgroup analysis conducted by dividing BMI levels by 2.5 kg/m2 also showed that BMI reduction and remission rates had a very crucial impact on the outcome of cost-effectiveness. BS was the most effective option in the subgroup of 35.0–37.4 kg/m2 at the basal BMI level. In this subgroup, the differences in terms of the proportion of patients with post-treatment BMI < 30 kg/m2 and the remission rate between BS and non-surgical groups were 97.5% and 46.2%, respectively, which were the highest differences among subgroups. The high surgery cost was thought to be compensated by the savings of future costs that could have incurred in comorbidity management.
The utility weights did not significantly affect ICERs in the deterministic sensitivity analysis; however, the utility values assumed in this study could be meaningful because they were analyzed based on real patient data from the clinical trial. These data were derived from patient responses to the 3-level EQ-5D questionnaire that was acquired at the baseline and post-treatment visits with 12-week intervals until 48 weeks. The utility values decreased with increasing BMI levels, and a similar trend was observed in several previous studies [39,40,41]. In a research on the relation between the quality of life and BMI level in the general population aged 45 or more in the UK [39], the absolute values of utility weights tended to be lower than those reported in this study; 0.803 for BMI 18.5–< 25.0 kg/m2, 0.780 for BMI 25.0–< 30.0 kg/m2, 0.704 for BMI 30.0–< 35.0 kg/m2, 0.682 for BMI 35.0–< 40.0 kg/m2, and 0.621 for BMI > 40.0 kg/m2. In general, utility weights are affected by the social and economic factors of the subject, and the absolute values may vary by country. However, the differences in utilities between subgroups were similar in the two studies. The dropped utility weights in this study were − 0.051, − 0.111, and − 0.184 in the increasing order of BMI subgroup with reference of BMI 18.5–< 25.0 kg/m2 group, and they were − 0.023, − 0.099, − 0.121, and − 0.182 in the UK study. Thus, the utility weights used in this study appeared to be valid in comparing the tendency of the decrease of utility values with increasing BMI level.
The cost-effectiveness of BS in morbidly obese people in Korea has been published by Song et al. based on retrospective data in 2013 [22], and this study is the second evaluation based on prospective clinical trial. The base ICER in this study was slightly lower than 1771 USD/QALY of the first evaluation. The difference might be caused by the following reasons; First, changes in comorbidities could not be applied to the model in the previous study due to the limitation of data because the source data was a retrospective chart review study, but this study overcame these factors and reflected changes in comorbidities in the assessment. Second, the previous study included only the individuals with BMI 30–40 kg/m2 and assumed a fixed post-treatment BMI level after 1 year of treatment as 25–30 kg/m2 for BS and 30–40 kg/m2 for NST. This study included people with BMI ≥ 30 kg/m2 without any upper limits and assumed post-treatment BMI distribution as analyzed as the source data of clinical trial. In addition, the transition probability and mortality were also assumed differently according to the BMI level in this study. Lastly, BS is now covered by insurance in Korea, which has led to a slight cost reduction for BS.
BS has been considered cost-effective in the West as the ICERs of BS were about 1506–36,570 USD/QALY, which were all lower than their accepted willingness to pay threshold [18, 19]. ICERs inevitably differ according to the country as socio-economical and public health environments are different, and therefore, the threshold value of willingness to pay can be a basis for determining cost-effectiveness. In Asia, cost-effectiveness of BS has rarely been assessed for individuals with morbid obesity. Tang et al. (2016) presented the cost-effectiveness ratio by treatment options for individuals with type 2 diabetes and BMI ≥ 28 kg/m2. The medical treatment costs per QALY were 1589 USD for medical treatment, 1028 USD for laparoscopic sleeve gastrectomy, and 1198 USD for laparoscopic Roux-en-Y gastric bypass. Thus, surgery showed the lowest medical cost per QALY, but ICERs were not presented [42].
On the basis of the deterministic sensitivity analyses, discount rate, remission rate of comorbidity, surgery cost, and starting age at the time of entering the model affected ICERs relatively more than other factors. However, all ICERs from the deterministic sensitivity analysis were less than 10,000 USD/QALY, which is lower than the threshold of willingness to pay in South Korea (approximately 32,000 USD/QALY [32]). The results of probabilistic sensitivity analysis also showed > 90% cost-effectiveness at the willingness to pay threshold, and these results supported the cost-effectiveness of BS.
This study had limitations as follows. First, the follow-up period of the clinical trial which was the main reference for clinical efficacy assumption in this study was limited to 1 year. Thus, the severity of comorbidity and mortality data were not sourced from the trial and were assumed based on the real-world data instead. Second, it was assumed that the BMI level at the end of the first-year treatment was maintained for a lifetime. However, this weakness may not affect the results significantly as the results of deterministic sensitivity showed stable ICERs on this assumption. Third, the same probabilities of transferring to severe comorbidity were applied regardless of previous comorbidity status. A patient in mild comorbidity status is more likely to move to severe comorbidity than a patient in non-comorbidity status, and therefore, this assumption of the same probability was considered to make the base ICER conservative. Fourth, even though the treatment costs for the non-surgical group generally last more than a year, they were assumed to last only a year in this study. This is also thought to be a conservative assumption as ICERs may be more favored for BS if the non-surgical costs of beyond 1 year were added.
Despite these limitations, this study effectively showed the cost-effectiveness of BS over non-surgery in both total subjects and subgroups according to the base BMI level using real-world data, such as base distribution of comorbidity, remission rate of comorbidity and detailed BMI distribution at the end of 1 year of treatment, and utility weights derived from clinical study participants. In addition, individuals with a basal BMI of 30–34.9 kg/m2 who would not be considered morbidly obese in the West were included if they had obesity-related comorbidities, such as hypertension, diabetes, and hyperlipidemia, like Asian countries, including Korea, have stricter criteria for defining morbid obesity. NST also has a marginal effect on resolving obesity in the subgroup of BMI of 30–35 kg/m2, and the efficacy difference between BS and NST could be lower than estimated. However, BS showed significantly better effect in individuals in the clinical trial, and the ICERs for the subgroup having basal BMI of 30–35 kg/m2 were also shown to be cost-effective. These results could be useful in establishing standards and guidelines for future studies on BS in other countries.
Conclusions
To our knowledge, this study is the first study in Korea to evaluate the cost-effectiveness of BS in morbid obesity or obesity at BMI 30–34.9 kg/m2 with comorbidity using data from a prospective clinical trial. BS was more effective than NST for people with BMI of ≥ 30 kg/m2 as it offered a better reduction in BMI and remission of obesity-related comorbidities and was cost-effective considering the lifetime. In particular, surgical treatment was a dominant alternative for people with basal BMI of 35.0–37.5 kg/m2.
References
Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks: World Health Organization; 2009.
Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9.
Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66(Suppl 2):7–12.
Xu H, Cupples LA, Stokes A, et al. Association of Obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Netw Open. 2018;1(7):e184587.
Wang J, Thornton JC, Russell M, et al. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60(1):23–8.
Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701.
Deurenberg-Yap M, Yian TB, Kai CS, et al. Manifestation of cardiovascular risk factors at low levels of body mass index and waist-to-hip ratio in Singaporean Chinese. Asia Pac J Clin Nutr. 1999;8(3):177–83.
Pacific WHOROftW. The Asia-pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000.
Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42.
Shin HY, Kang HT. Recent trends in the prevalence of underweight, overweight, and obesity in Korean adults: the Korean National Health and Nutrition Examination Survey from 1998 to 2014. J Epidemiol. 2017;27(9):413–9.
Park S, Pi S, Hwang J, et al. Effects of initial body mass index and weight change on all-cause mortality: a 10-year cohort study in Korea. Asia Pac J Public Health. 2018;30(3):217–26.
Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–40.
Chung W, Lim SJ, Lee S, et al. Gender-specific interactions between education and income in relation to obesity: a cross-sectional analysis of the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V). BMJ Open. 2017;7(12):e014276.
Fried M, Hainer V, Basdevant A, et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes Facts. 2008;1(1):52–9.
Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184(6B):9S–16S.
Cheng J, Gao J, Shuai X, et al. The comprehensive summary of surgical versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomized controlled trials. Oncotarget. 2016;7(26):39216–30.
Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275–87.
Avenell A, Robertson C, Skea Z, et al. Bariatric surgery, lifestyle interventions and orlistat for severe obesity: the REBALANCE mixed-methods systematic review and economic evaluation. Health Technol Assess. 2018;22(68):1–246.
Alsumali A, Al-Hawag A, Samnaliev M, et al. Systematic assessment of decision analytic models for the cost-effectiveness of bariatric surgery for morbid obesity. Surg Obes Relat Dis. 2018;14(7):1041–59.
Ohta M, Seki Y, Wong SK, et al. Bariatric/metabolic surgery in the Asia-Pacific region: APMBSS 2018 survey. Obes Surg. 2019;29(2):534–41.
Gaeun Kim SJ, Choi Y-B, Hur Y, et al. Is surgery necessary for morbid obesity patient? NECA round-table conference consensus statement. Korean J Obes. 2013;22(1):7–12.
Song HJ, Kwon JW, Kim YJ, et al. Bariatric surgery for the treatment of severely obese patients in South Korea--is it cost-effective? Obes Surg. 2013;23(12):2058–67.
Comparative study in morbidity obese patient with surgical and medical treatments: effectiveness, safety and cost-effectiveness R&D Report. Korean Ministry of Health and Welfare Affairs. Korea Health Industry Development Institute.; Available from: https://www.khidi.or.kr/kps/researchInfo/list?menuId=MENU02230&searchContinuStYear=&searchSprcRsrhInttNm=&searchFlnmKrn=&searchGwrd=&searchPjtMngmNo=HC15C1322&searchPjtNm=#.
Korean Ministry of Health and Welfare Affairs. Korea Centers for Disease Control. Korean National Health and Nutritional Examination Survey (2007-2015). Available from: https://knhanes.cdc.go.kr/knhanes/main.do.
Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34.
Song HJ, Hwang J, Pi S, et al. The impact of obesity and overweight on medical expenditures and disease incidence in Korea from 2002 to 2013. PLoS One. 2018;13(5):e0197057.
Seong SC, Kim YY, Park SK, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7(9):e016640.
Statistics Korea (2016). Available from: http://kostat.go.kr. Accessed in 3 Dec 2018.
Sung KC, Ryu S, Cheong ES, et al. All-cause and cardiovascular mortality among Koreans: effects of obesity and metabolic health. Am J Prev Med. 2015;49(1):62–71.
Health Insurance Review and Assessment Service. Available from: http://www.hira.or.kr/main.do. Accessed in 3 Dec 2018.
Healthcare Bigdata Hub. Available from: https://opendata.hira.or.kr.
Song HJ, Lee EK. Evaluation of willingness to pay per quality-adjusted life year for a cure: a contingent valuation method using a scenario-based survey. Medicine (Baltimore). 2018;97(38):e12453.
Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Sierzantowicz R, Lewko J, Hady HR, et al. Effect of BMI on quality of life and depression levels after bariatric surgery. Adv Clin Exp Med. 2017;26(3):491–6.
Zachariah SK, Chang PC, Ooi AS, et al. Laparoscopic sleeve gastrectomy for morbid obesity: 5 years experience from an Asian center of excellence. Obes Surg. 2013;23(7):939–46.
Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg. 2014;24(10):1617–24.
Hatoum IJ, Blackstone R, Hunter TD, et al. Clinical factors associated with remission of obesity-related comorbidities after bariatric surgery. JAMA Surg. 2016;151(2):130–7.
Puzziferri N, Roshek 3rd TB, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42.
Sach TH, Barton GR, Doherty M, et al. The relationship between body mass index and health-related quality of life: comparing the EQ-5D, EuroQol VAS and SF-6D. Int J Obes. 2007;31(1):189–96.
Doring N, de Munter J, Rasmussen F. The associations between overweight, weight change and health related quality of life: longitudinal data from the Stockholm Public Health Cohort 2002-2010. Prev Med. 2015;75:12–7.
Song HJ, Lee EK, Kwon JW. Gender differences in the impact of obesity on health-related quality of life. Asia Pac J Public Health. 2016;28(2):146–56.
Tang Q, Sun Z, Zhang N, et al. Cost-effectiveness of bariatric surgery for type 2 diabetes mellitus: a randomized controlled trial in China. Medicine (Baltimore). 2016;95(20):e3522.
Acknowledgments
We thank the Health Insurance Review and Assessment Service (HIRA-NPS) for providing data for this study.
Funding
This study was funded by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number HC15C1322) and the Korea National Research Foundation (grant number NRF-2018R1D1A3B07047356).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the IRB of the Kyungpook National University (KNU 2016–0007). In addition, prospective clinical trials for the source data in this study were approved by the Institutional Review Board (IRB) of their respective centers (the approval number of IRB at principal investigator center: INHAUH 2016-06-015).
Informed Consent
The NHIS-HEALS database was retrospectively established in an anonymous format, and the informed consent requirement for this study was waived, and informed consent was obtained from all individual participants included in the prospective clinical trial used for source data in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
An, S., Park, HY., Oh, SH. et al. Cost-effectiveness of Bariatric Surgery for People with Morbid Obesity in South Korea. OBES SURG 30, 256–266 (2020). https://doi.org/10.1007/s11695-019-04122-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04122-w