Abstract
Objective
To examine the relationship between Edmonton Obesity Staging System (EOSS) and perioperative complications as well as surgical procedure.
Background
The application of EOSS for the selection of patients with obesity is a more comprehensive measure of obesity-related diseases and a predictor of mortality than body mass index (BMI).
Methods
This was a nationwide cohort study using prospectively inserted data from the German register for obesity and metabolic surgery StuDoQ|MBE. All patients undergoing sleeve gastrectomy (SG), Roux-en Y gastric bypass (RYGB), and one-anastomosis gastric bypass (OAGB) between February 2015 and July 2017 as a primary treatment for severe obesity were included. Data included gender, age, BMI, ASA score, EOSS, early postoperative complications next to the Clavien-Dindo grading system, readmission, and 30-day mortality.
Results
A total of 9437 patients were included. The mean BMI was 49.5 kg/m2 ± 7.8 (range 35–103.5). The total postoperative complication rate was 5.3%, with the highest rate in EOSS 3 (7.8%) and 4 (6.8%). Thirty-day mortality was 0.2% with the highest mortality after SG in EOSS 3 (1.16%) and EOSS 4 (0.92%) (p = 0.0068). Crosstabs showed a prevalence of Clavien-Dindo III and IV complications of 3.4% (SG), 3.6% (RYGB), and 1.6% (OAGB) in EOSS 2 (p = 0.0032) and 3.5% (SG), 5.1% (RYGB), and 5.6% (OAGB) in EOSS 3.
Conclusion
The highest postoperative complications and mortality occurred in patients with EOSS ≥ 3. SG and OAGB could be the procedure of choice to reduce perioperative morbidity; nevertheless, it has to be in mind that in EOSS ≥ 3, SG has the highest mortality.
Trial Registration
ClinicalTrials.gov Identifier NCT03556059.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is highly efficacious in treating obesity and its comorbidities. For this reason, in 2016 the term “bariatric surgery” was replaced by “surgery for obesity and weight-related diseases” [1]. Additionally, it has been shown that body mass index (BMI) and anthropometric measures demonstrate limitations for classifying obesity and do not provide information about the presence or extent of weight-related diseases or functional limitations. In 2009, Sharma et al. [2] provided a novel classification system for obesity, the so-called Edmonton Obesity Staging System (EOSS). EOSS classifies obesity considering the patient’s medical, mental, and functional symptoms and allows the clinician to describe the morbidity and functional limitations associated with excess weight [2]. Clinicians are able to identify individuals with obesity at elevated risk for mortality [3]. Furthermore, it offers improved clinical utility for assessing obesity-related risk and prioritizing treatment [4]. Surgery for obesity and metabolic disorders is therefore strongly recommended in EOSS ≥ 2. Indeed, patients with obesity-related chronic disease, especially type 2 diabetes mellitus (T2DM), profit from the amelioration and remission of metabolic disease [5, 6]. The most commonly performed surgical procedures are laparoscopic sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and gastric banding in America and SG, RYGB, and one-anastomosis gastric bypass (OAGB) in Europe [7].
In a previous study, we have demonstrated that EOSS may be useful for presurgical stratification and risk assessment in clinical practice, since patients with EOSS ≥ 3 have a higher risk of postoperative complications [8]. The aim of this study was to determine whether EOSS can be applied for procedure selection and be used as a predictive tool to minimize perioperative complications analyzing the German nationwide register-based cohort study (StuDoQ|MBE).
Methods
This was a nationwide cohort study using prospectively inserted data from the German register for obesity and metabolic surgery StuDoQ|Metabolische und bariatrische Erkrankungen (StuDoQ|MBE). Ninety-eight hospitals were included. Data export was performed on April 22, 2018. All patients undergoing SG, RYGB, and OAGB between February 2015 and July 2017 as a primary treatment for severe obesity were included. The eligibility criteria were a BMI ≥ 35 kg/m2, and at least one metabolic disease or a BMI ≥ 40 kg/m2. The exclusion criteria were bariatric surgery in medical history, missing signed consent for data sharing, and patients with incomplete data. After exclusion next to the abovementioned criteria, from 33,584 existing datasets 9437 patients were included. Data included gender, age, BMI, American Society of Anesthesiologists (ASA) class, comorbidities, functional status, laboratory investigations, early postoperative complications according to the Clavien-Dindo grading system [9], length of hospital stay, readmissions, and 30-day mortality. The patients were followed up for 1 month after surgery. The EOSS score was retrospectively assigned to each patient using the method of Sharma and Padwal [2, 4]. Each patient was independently reviewed by two evaluators, who assigned an EOSS stage (SC, CS). Prospective inserted parameters from StuDoQ|MBE were obtained to assign EOSS: presence and pharmacological treatment of T2DM, arterial hypertension and hyperlipidemia, presence of osteoarthritis, obstructive sleep apnea syndrome (OSAS), cerebrovascular events, heart insufficiency, coronary heart disease, liver cirrhosis, gastroesophageal reflux disease (GERD), patient’s mobility, and need of home care. Preoperative laboratory investigations included fasting blood glucose, total, LDL and HDL cholesterol, triglycerides, and creatinine.
Patients without any functional, mental, or medical symptoms were given an EOSS stage of 0 (EOSS 0). Patients with obesity-related subclinical risk factors and not receiving pharmacological treatment (e.g., borderline hypertension, impaired fasting glucose level) were classified as EOSS 1. Patients with obesity-related chronic disease (e.g., arterial hypertension, T2DM, OSAS, osteoarthritis, GERD) and receiving pharmacological therapy were considered EOSS 2. Patients with end-organ damage caused by a related chronic disease (e.g., myocardial infarction, heart failure, stroke, renal insufficiency) or significant psychopathology and functional limitations and/or impairment of well-being were classified as EOSS 3. Patients with severe disabilities from obesity-related chronic disease, severe disabling psychopathology, severe functional limitations, and/or severe impairment of well-being were classified as EOSS 4 [4, 8]. Using this scheme, the two evaluators assigned all patients an EOSS stage independently and in the same way. The surgical techniques for SG, RYGB, and OAGB have been described elsewhere [8].
Statistical analysis was performed using SPSS 21.0 statistical software for Microsoft Windows (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as the mean ± standard deviation (SD) and range. For quantitative variables that followed a normal distribution, differences between groups were analyzed using Student’s t test, and differences between qualitative variables were analyzed using the chi-square test. The correlation between EOSS and age, BMI, ASA, readmission, length of hospital stay, reoperation, death, and Clavien-Dindo complication and the correlation between Clavien-Dindo complication and age, ASA, and BMI were calculated using the Spearman correlation coefficient. A coefficient between 0 and 0.3 means a weak correlation, between 0.3 and 0.5 a medium correlation, and coefficient of > 0.5 a strong correlation. The correlation between gender and EOSS and gender and Clavien-Dindo complication was tested with the Mann-Whitney U test, and the correlation between surgical procedure and EOSS was tested using the Kruskal-Wallis test. The correlation of EOSS, surgical procedure, and Clavien-Dindo III and IV complications and EOSS, surgical procedure, and death were analyzed with cross-tables. A p value < 0.05 was considered significant. Informed consent was obtained from all individual participants included in StuDoQ|MBE and in the study. The ethics committee of the Landesärztekammer Hessen, Germany (Institutional Review Board) approved the clinical investigations (FF 83_2/2015), and the study is registered on ClinicalTrials.gov (NCT03556059).
Results
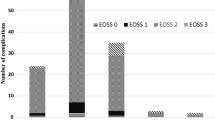
Between February 2015 and July 2017, 9437 patients underwent SG, RYGB, or OAGB. Mean age was 43.9 ± 11.5 years (18–85). Mean preoperative BMI was 49.5 ± 7.8 kg/m2 (35–103.1), and mean preoperative weight was 142.7 ± 27.5 kg (60–287). Three thousand nine hundred and sixty-one patients (42%) had a BMI ≥ 50 kg/m2. The study group included 2632 men (27.9%) and 6805 women (72.1%): 4456 patients underwent SG (47.2%), 3850 underwent RYGB (40.8%), and 1131 underwent OAGB (12%). Most patients were classified as EOSS 2 (76.1%). The next most frequent EOSS stages were 3 (13.4%), 4 (7.1%) 0 (3.1%), and 1 (0.3%). Figure 1 shows the distribution of EOSS in the different surgical groups. Notably, BMI levels were virtually identical (~ 50 kg/m2) across all EOSS groups.
Distribution of EOSS in the different surgical groups SG, RYGB, and OAGB (SG: EOSS 0: 3.1%, EOSS 1: 0.3%, EOSS 2: 73.9%, EOSS 3: 15.4%, EOSS 4: 7.3%; RYGB: EOSS 0: 3.5%, EOSS 1: 0.3%, EOSS 2: 79.8%, EOSS 3: 11.2%, EOSS 4: 5.2%; OAGB: EOSS 0: 1.9%, EOSS 1: 0.2%, EOSS 2: 72.1%, EOSS 3: 12.8%, EOSS 4: 13%)
Patients were preoperatively classified as ASA I (n = 279, 3%), ASA II (n = 4136, 42.8%), ASA III (n = 4903, 52%), ASA IV (n = 117, 1.2%), and ASA V (n = 2, 0.02%).
Postoperative complications occurred in 5.3% (n = 503) with 3.7% (n = 348) having a postoperative complication > II next to the Clavien-Dindo grading system (0 = 8934, 1 = 84, 2 = 71, 3a = 82, 3b = 203, 4a = 35, 4b = 7, and 5 = 21). Postoperative complications included bleeding (n = 136, 1.4% (n = 51 after RYGB (1.3%), n = 18 after OAGB (1.6%), n = 67 after SG (1.5%))), n = 74 extraluminal bleeding (n = 17 after RYGB (0.4%), n = 9 after OAGB (0.8%), n = 48 after SG (1.1%)), n = 62 intraluminal bleeding (n = 34 after RYGB (0.9%), n = 19 after SG (0.4%), n = 9 after OAGB (0.8%)), n = 50 required erythrocyte transfusions (n = 21 after RYGB (0.5%), n = 3 after OAGB (0.3%), n = 26 after SG (0.6%)), leakage (n = 174, 1.8%; n = 93 after RYGB (2.4%), n = 12 after OAGB (1.1%), n = 69 after SG (1.5%)), renal failure (n = 19, 0.2%; n = 5 after RYGB (0.1%), n = 2 after OAGB (0.2%), n = 12 after SG (0.3%), pneumonia (n = 40, 0.4%; n = 17 after RYGB (0.4%), n = 6 after OAGB (0.5%), n = 17 after SG (0.4%)), heart attack (n = 5, 0.05%; n = 3 after RYGB (0.08%), n = 2 after SG (0.04%), respiratory insufficiency > 48 h (n = 37, 0.4%; n = 14 after RYGB (0.4%), n = 5 after OAGB (0.4%), n = 18 after SG (0.4%), thrombosis (n = 16, 0.2%; n = 2 after RYGB (0.05%), n = 2 after OAGB (0.2%), n = 12 after SG (0.3%)), lung embolism (n = 17, 0.2%; n = 4 after RYGB (0.1%), n = 4 after OAGB (0.4%), n = 9 after SG (0.2%)), stroke (n = 1, 0.01%; n = 1 after RYGB (0.03%)), urinary infection (n = 35, 0.4%; n = 7 after RYGB (0.2%), n = 1 after OAGB (0.09%); n = 27 after SG (0.05%)), and trocar hernia (n = 13, 0.1%; n = 5 after RYGB (0.1%), n = 2 after OAGB (0.2%), n = 6 after SG (0.1%) and other (n = 10, 0.12%).
Reoperation was performed in 3% (n = 279) of the patients. Postoperative mortality was 0.2% (n = 21). Mean postoperative stay was 5.1 ± 6 days (1–246). The 30-day readmission rate was 3.3% (n = 314) (Table 1).
BMI did not differ significantly between patients with (n = 8934) and without complications (n = 503). The non-complication group had a BMI of 49.5 ± 7.8 kg/m2 (35–103.5), and the complication group had a BMI of 50 ± 7.8 kg/m2 (35.7–84.8) (p = 0.1619).
Baseline demographic (BMI, age, gender, ASA) data and postoperative data including Clavien-Dindo complications and length of hospital stay in relation to EOSS and in relation to the surgical procedures are listed in Tables 1 and 2. Notably, mean BMI levels had a range of 49–50.6 kg/m2 across EOSS groups 0, 1, 2, and 3.
Complications occurred in 4.1% of patients in EOSS 0, 3.4% of the patients in EOSS 1, 4.8% of the patients in EOSS 2, 7.8% of the patients in EOSS 3, and 6.8% of the patients in EOSS 4. Complications next to the Clavien-Dindo grading system in the different EOSS scores are listed in Table 2. Complications occurred in 5.1% patients after RYGB, 5.9% after SG, and 4.1% after OAGB. Complications next to the Clavien-Dindo grading system in the different surgical groups are listed in Table 1. Comparing the complications in EOSS 0, 1, 2 (n = 358/7502, 4.77%) and the complications in EOSS 3 and 4 (n = 145/1935, 7.49%), a statistical difference was seen in the chi-square test (p < 0.0001). Comparing the complications Clavien-Dindo > II in EOSS 0, 1, 2 (n = 254/7502, 3.39%) and the complications Clavien-Dindo > II in EOSS 3 and 4 (n = 94/1935, 4.86%), a statistical difference was seen in the chi-square test (p < 0.0036). Comparing the reoperations in EOSS 0, 1, 2 (n = 207/7502, 2.76%) and the reoperations in EOSS 3 and 4 (n = 72/1935, 3.7%), a statistical difference was seen in the chi-square test (p < 0.0290). Comparing the readmissions in EOSS 0, 1, 2 (n = 247/7502, 3.29%) and EOSS 3 and 4 (n = 68/1935, 3.51%), no statistical difference was seen in the chi-square test (p = 0.6196). Comparing the death in EOSS 0, 1, 2 (n = 9/7502, 0.12%) and EOSS 3 and 4 (n = 12/1935, 0.62%), a statistical difference was seen in the chi-square test (p = 0.0002).
In comparison of EOSS with age, BMI, ASA, Clavien-Dindo complications, length of hospital stay and readmission, reoperation, and death, a significant positive correlation was found between EOSS and age (Spearman 0.314, p < 0.0001), EOSS and BMI (Spearman 0.115, p < 0.0001), and EOSS and ASA (Spearman 0.191, p < 0.0001).
A statistically significant positive correlation was found in the Mann-Whitney U test between EOSS and gender with a higher mean correlation rank of 4976.48 for men and 4619.41 for women (p < 0.0001).
A statistically significant positive correlation was found in the Kruskal-Wallis test (p < 0.0001, χ2 79.5) between EOSS and surgical procedure with a mean correlation rank of 5048.9 for OAGB, 4814.29 for SG, and 4511.79 for RYGB.
In comparison of Clavien-Dindo complications with age, BMI, ASA, and EOSS, a significant positive correlation was found between Clavien-Dindo complications and age (Spearman 0.145, p = 0.009) and Clavien-Dindo complications and EOSS (Spearman 0.170, p = 0.002). No statistical correlation was found between Clavien-Dindo complications and ASA (p = 0.193) and Clavien-Dindo complications and BMI (p = 0.134).
No statistically significant correlation was found in the Mann-Whitney U test between Clavien-Dindo complications and gender (p = 0.369), but men had a higher complication rate with a higher mean correlation rank of 169.85 for men and 161.19 for women.
A statistically significant positive correlation was found in the Kruskal-Wallis test (p = 0.001, χ2 14.685) between surgical procedure and Clavien-Dindo complications with a mean correlation rank of 203.67 for OAGB, 172.10 for SG, and 147.30 for RYGB.
The contingency tables (Tables 3 and 4) display the multivariate frequency distribution of the variables Clavien-Dindo III and IV, EOSS, and surgical procedure in Table 3 and death (Clavien-Dindo V), EOSS and surgical procedure in Table 4.No statistical difference was seen regarding the occurrence of Clavien-Dindo III and IV complications between the different surgical procedures in EOSS 0 and 1. The lowest complication rate (Clavien-Dindo III and IV) was seen for OAGB (1.6%) in EOSS 2 (p = 0.0032). Complication rate (Clavien-Dindo III and IV) was lowest for SG in EOSS 3 (3.5%) and 4 (3.7%). No statistical difference was seen regarding the occurrence of Clavien-Dindo III and IV complications between the different surgical procedures in EOSS 3 and 4. No statistical difference was seen regarding mortality rate between the different procedures in EOSS 2. A statistical difference was seen regarding mortality rate in EOSS 3 and 4 with the highest mortality rate after SG (p = 0.0068).
Discussion
In this German nationwide register-based cohort study (StuDoQ|MBE), we observed that having a higher EOSS stage (EOSS ≥ 3) predicts postoperative complications and mortality. Importantly, this effect appears independent of BMI, which was virtually identical between EOSS groups. Rather unexpectedly, we also found that EOSS stage appeared to be a predictor of the type of surgical procedure. Thus, our findings not only support the notion that EOSS may be of clinical utility for determining the perioperative risk but may also help in selecting the appropriate procedure for a given patient.
Our finding that EOSS is a better measure of perioperative risk than BMI alone is well in line with our previous report of higher risk in higher EOSS stages in metabolic surgery patients [8]. The idea that additional parameters beyond BMI are required to assess risk is not new. Thus, for example DeMaria et al. developed a scoring system to stratify the mortality risk for patients undergoing gastric bypass with the five independent variables including male gender, BMI ≥ 50 kg/m2, age ≥ 45 years, hypertension, and pulmonary embolus risk [10]. Blackstone et al. developed a metabolic acuity score (MAS) for identifying specific patient acuity characteristics [11]. Independent risk factors in the MAS include age, BMI, weight, history of deep vein thrombosis/pulmonary embolism, OSAS, T2DM, hypertension, immobility, heart disease, and psychological classification. Lak et al. recently analyzed 59,404 patients from the National Surgical Quality Improvement Program dataset undergoing bariatric surgery and the effects of metabolic syndrome on morbidity and mortality and found that morbidity was greater in patients with metabolic syndrome (7.5% versus 5%, p < 0.0001) and patients with metabolic syndrome had a 3.2-fold risk of increased mortality (p < 0.0001). EOSS includes T2DM and metabolic syndrome and is a more comprehensive measure of obesity-related diseases. As noted above, in our study, BMI levels were similar across patients with and without complications supporting our hypothesis that preoperative risk stratification should be focused on EOSS rather than BMI alone.
Notably, EOSS was also a statistically significant parameter for postoperative complications when used in conjunction with the Clavien-Dindo grading system. This may be because EOSS considers a wide range of risk factors, including metabolic, mental, and functional status of the patient in one parameter.
Interestingly, male patients in our study presented with higher EOSS scores than female participants. This is in line with our previous observation from a multicenter cohort study on patient expectations by Fischer et al. showing that male patients undergoing obesity surgery are often older and suffer from more comorbidities [12]. Given their higher EOSS scores, it is therefore perhaps not surprising that male patients had more postoperative complications than female patients.
Since EOSS is now increasingly being integrated into patient selection, its application as a predictor for procedure selection might be a further useful aspect of using EOSS in obesity and metabolic surgery centers.
Interestingly, in our cohort of 9437 patients, there was a significant difference between the surgical procedures performed across the different EOSS stages. Thus, patients with the highest EOSS underwent more often OAGB, and with lowest EOSS more often than RYGB. Why patients with higher EOSS undergo more likely OAGB might be due to the higher reported metabolic impact and the less perioperative complications in relation to SG and RYGB [13,14,15]. Additional, it is plausible that the speed and ease of OAGB with its low complication rate may make it the preferred approach for more complex high-risk patients [16]. Thus, our observation of a statistically significant correlation between surgical procedure and Clavien-Dindo complications with the highest mean correlation rank for OAGB and lowest for RYGB may explain the differences in preoperative selection of these patients. On the other hand, the higher choice of OAGB in EOSS 3 and 4 might create a bias in relation to the complications of this procedure. Benchmarks for the most performed bariatric procedures are necessary, to compare the procedures in patients with and without comorbidities.
Our observation of significantly different outcomes and choice of procedures across EOSS stages raises the issue of the individualized approach to obesity and metabolic surgery. Thus, as in cancer surgery [17], the concept of individualized surgery is gaining more acceptance in metabolic surgery. In 2017, Aminian et al. categorized T2DM into three validated stages of severity and analyzed 900 patients undergoing SG and RYGB for selection of metabolic surgery in relation to diabetes remission with a follow-up of 7 years. The study group concluded in relation to the risk-benefit ratio that patients with mild and moderate T2DM would profit more from RYGB and patients with severe T2DM should undergo SG as the metabolic procedure of choice, since both procedures in severe T2DM have similarly low efficacy for diabetes remission since clinical features suggest limited functional β-cell reserve, and SG is associated with a lower perioperative risk [18]. Our findings suggest that EOSS may further help in selecting the appropriate procedure for each patient, and our results contradict the fact that SG is associated with lower perioperative risk, since it had the highest mortality of all procedures in EOSS 3 and 4.
The total postoperative complication rate in our study was 5.3%, with 5.1% after RYGB, 5.9% after SG, and 4.1% after OAGB. The highest rate of postoperative complications occurred in EOSS 3 (7.8%) and 4 (6.8%). Thirty-day mortality was 0.2%, having the same mortality in SG 0.3% and OAGB 0.3% and the lowest one after RYGB 0.1%. Highest mortality was observed after SG in EOSS 3 (1.16%) and 4 (0.92%). Different studies have shown the security of SG with a fallen rate of complications over time [19]. Still, when leakage occurs, management is difficult [20], and this might be the cause for the high mortality rate in patients with end-organ damage (EOSS 3 and 4). Even the management of leakage after OAGB might be a challenge due to the bile flow, which often let the surgeon convert the OAGB in a RYGB reconstruction to treat the leakage sufficiently. The lower leakage rate in the bypass group might also be explained by the decreased intragastric pressure caused by pylorus exclusion [21]. From our own experience, insufficiencies after RYGB are the easiest way to treat; even so, RYGB has the lowest mortality in the surgical group (0.1%). Current literature contradicts our results. In the study of Peterli et al., mortality after SG was 0% and after RYGB it was 1.9% [22]. It has to be in mind that only 217 patients were included and mean BMI was 43.9 kg/m2.
Crosstabs analyzing Clavien-Dindo III and IV showed the lowest incidence of postoperative complications after SG in patients with EOSS 3 (3.5%) and lowest incidence of postoperative complications after OAGB in patients with EOSS 2 (1.6%). Crosstabs analyzing Clavien-Dindo V (death) showed the highest mortality after SG in EOSS 3 (1.16%) and EOSS 4 (0.92%).
Postoperative complications are declining after obesity and metabolic surgery due to implementation of accreditation systems including institutional requirements, national and international guidelines, and surgeon’s credentials [23, 24]. The German data has equivalent results in terms of perioperative complications in relation to the current literature. Daigle et al. published the frequency of 135.431 bariatric surgery patients from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database undergoing SG (67%), RYGB (29%), gastric banding (3%), and duodenal switch (1%) and reported a complication rate of < 1% for eight index complications with bleeding (0.7%), surgical site infection (0.5%), and urinary tract infection (0.3%) being the most common complications [25]. ASA classification was similar with our study having most patients classified ASA III (72.3% versus 52% in our cohort). In our study, cohort bleeding was higher (1.4%), but equivalent with the MBSAQIP analyzation of postoperative bleeding after laparoscopic RYGB published by Zafar et al. with an incidence of 1.5% [26]. Overall readmission and reoperation rates were 4.2% and 1.3%, respectively, and 3.3% (n = 314) and 3% (n = 279) in our cohort.
EOSS combines all risk factors in one parameter, since there is a statistically significant positive correlation between EOSS with age, BMI, and ASA. Patients with higher BMI, higher ASA class, and older age might have more obesity-related diseases, and the statistically significant correlation of the Clavien-Dindo grading system with age and EOSS underline again the higher postoperative complications in older patients and higher EOSS scores [8]. Age has always been interpreted as a risk factor and is indeed an independent variable in the risk scores from Blackstone et al. [11] and DeMaria et al. [10]. Even if obesity and metabolic surgery is safe in the elderly [27], older patients are known to have higher perioperative risks and higher ICU admissions, with ASA scores and OSAS being possible predictive factors [28].
The study has some limitations. First, the study does not analyze its impact on long-term complications. Nevertheless, long-term complications such as GERD, Barrett and weight regain after SG [29, 30], bile reflux and malnutrition after OAGB [31] and dumping syndrome, internal hernia, and weight regain after RYGB [22, 32] have always to be in mind in the long-term follow-up.
Second, the non-homogeneity of the sample might provide a bias. On the other hand, the three different procedures SG, RYGB, and OAGB are somehow similar and are the most performed ones in Germany and Europe. Further studies are necessary to include other well-recognized procedures such as gastric banding or biliopancreatic diversion.
Third, the data of these studies includes the whole German registry. Obviously, high-volume and low-volume centers are included and mixed. There might be a procedure selection bias due to different surgical skills.
Fourth, whether EOSS has parallels to the TNM staging system in oncologic surgery and whether it predicts long-term survival and reduction of end-organ damage after obesity and metabolic surgery should be addressed in further studies.
Conclusion
These findings support the use of EOSS for risk stratification in the clinical setting, and could be adapted as a potential tool for procedure selection to reduce perioperative morbidity in obesity and metabolic surgery. This large German cohort with 9437 patients showed highest postoperative complications and highest mortality in patients with EOSS ≥ 3. To achieve lowest perioperative complication rates, it appears that OAGB should be the procedure of choice in EOSS 2. Although SG has the lowest perioperative morbidity regarding Clavien-Dindo complications III and IV in EOSS 3 and 4, it must be noted that SG has the highest mortality of all procedures in EOSS 3 and 4.
Abbreviations
- BMI:
-
Body mass index
- EOSS:
-
Edmonton Obesity Staging System
- T2DM:
-
Type 2 diabetes mellitus
- SG:
-
Sleeve gastrectomy
- RYGB:
-
Roux-en-Y gastric bypass
- OAGB:
-
One-anastomosis gastric bypass
- SD:
-
Standard deviation
- GERD:
-
Gastroesophageal reflux disease
- ASA:
-
American Society of Anesthesiologists
- MAS:
-
Metabolic acuity score
- OSAS:
-
Obstructive sleep apnea syndrome
References
De Luca M, Angrisani L, Himpens J, et al. Indications for surgery for obesity and weight-related diseases: position statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg. 2016;26(8):1659–96.
Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes. 2009;33(3):289–95.
Kuk JL, Ardern CI, Church TS, et al. Edmonton Obesity Staging System: association with weight history and mortality risk. Appl Physiol Nutr Metab. 2011;36(4):570–6.
Padwal RS, Pajewski NM, Allison DB, et al. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183(14):E1059–66.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73.
Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery or intensive medical therapy for diabetes after 5 years. N Engl J Med. 2017;376(20):1997.
Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–94.
Chiappetta S, Stier C, Squillante S, et al. The importance of the Edmonton Obesity Staging System in predicting postoperative outcome and 30-day mortality after metabolic surgery. Surg Obes Relat Dis. 2016;12(10):1847–55.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
DeMaria EJ, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246(4):578–82. discussion 83-4
Blackstone RP, Cortes MC. Metabolic acuity score: effect on major complications after bariatric surgery. Surg Obes Relat Dis. 2010;6(3):267–73.
Fischer L, Nickel F, Sander J, et al. Patient expectations of bariatric surgery are gender specific--a prospective, multicenter cohort study. Surg Obes Relat Dis. 2014;10(3):516–23.
Seetharamaiah S, Tantia O, Goyal G, et al. LSG vs OAGB-1 year follow-up data-a randomized control trial. Obes Surg. 2017;27(4):948–54.
Quan Y, Huang A, Ye M, et al. Efficacy of laparoscopic mini gastric bypass for obesity and type 2 diabetes mellitus: a systematic review and meta-analysis. Gastroenterol Res Pract. 2015;2015:152852.
Lee WJ, Chong K, Lin YH, et al. Laparoscopic sleeve gastrectomy versus single anastomosis (mini-) gastric bypass for the treatment of type 2 diabetes mellitus: 5-year results of a randomized trial and study of incretin effect. Obes Surg. 2014;24(9):1552–62.
Taha O, Abdelaal M, Abozeid M, et al. Outcomes of omega loop gastric bypass, 6-years experience of 1520 cases. Obes Surg. 2017;27(8):1952–60.
Zhu M, Wang Q, Luo Z, et al. Development and validation of a prognostic signature for preoperative prediction of overall survival in gastric cancer patients. Oncol Targets Ther. 2018;11:8711–22.
Aminian A, Brethauer SA, Andalib A, et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266(4):650–7.
Stroh C, Kockerling F, Volker L, et al. Results of more than 11,800 sleeve gastrectomies: data analysis of the German bariatric surgery registry. Ann Surg. 2016;263(5):949–55.
El-Sayes IA, Frenken M, Weiner RA. Management of leakage and stenosis after sleeve gastrectomy. Surgery. 2017;162(3):652–61.
Tolone S, Cristiano S, Savarino E, et al. Effects of omega-loop bypass on esophagogastric junction function. Surg Obes Relat Dis. 2016;12(1):62–9.
Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–65.
Nguyen NT, Nguyen B, Nguyen VQ, et al. Outcomes of bariatric surgery performed at accredited vs nonaccredited centers. J Am Coll Surg. 2012;215(4):467–74.
Nguyen NT, Paya M, Stevens CM, et al. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg. 2004;240(4):586–93. discussion 93-4
Daigle CR, Brethauer SA, Tu C, et al. Which postoperative complications matter most after bariatric surgery? Prioritizing quality improvement efforts to improve national outcomes. Surg Obes Relat Dis. 2018;14(5):652–7.
Zafar SN, Miller K, Felton J, et al. Postoperative bleeding after laparoscopic Roux en Y gastric bypass: predictors and consequences. Surg Endosc. 2019;33(1):272–80.
Quirante FP, Montorfano L, Rammohan R, et al. Is bariatric surgery safe in the elderly population? Surg Endosc. 2017;31(4):1538–43.
Khidir N, El-Matbouly M, Al Kuwari M, et al. Incidence, indications, and predictive factors for ICU admission in elderly, high-risk patients undergoing laparoscopic sleeve gastrectomy. Obes Surg. 2018;28(9):2603–8.
Felsenreich DM, Ladinig LM, Beckerhinn P, et al. Update: 10 years of sleeve gastrectomy-the first 103 patients. Obes Surg. 2018;28(11):3586–94.
Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of Barrett’s esophagus: results of a multicenter study. Obes Surg. 2019;29(5):1462–9.
Musella M, Susa A, Manno E, et al. Complications following the mini/one anastomosis gastric bypass (MGB/OAGB): a multi-institutional survey on 2678 patients with a mid-term (5 years) follow-up. Obes Surg. 2017;27(11):2956–67.
Baig SJ, Priya P, Mahawar KK, et al. Indian bariatric surgery outcome reporting G. weight regain after bariatric surgery-a multicentre study of 9617 patients from Indian bariatric surgery outcome reporting group. Obes Surg. 2019;29(5):1583–92.
Acknowledgments
This work has been conducted using the StuDoQ|MBE registry provided by the Study, Documentation and Quality Center (Studien-, Dokumentations- und Qualitätszentrum, StuDoQ) of the German Society for General Surgery (Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie, DGAV) with the ID StuDoQ-2017-0019.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Statement of Human and Animal Rights
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiappetta, S., Stier, C., Weiner, R.A. et al. The Edmonton Obesity Staging System Predicts Perioperative Complications and Procedure Choice in Obesity and Metabolic Surgery—a German Nationwide Register-Based Cohort Study (StuDoQ|MBE). OBES SURG 29, 3791–3799 (2019). https://doi.org/10.1007/s11695-019-04015-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04015-y