Abstract
Background/Aim
Laparoscopic sleeve gastrectomy (SG) is an increasingly used bariatric surgery, which is reported to be effective for nonalcoholic fatty liver disease (NAFLD). Recently, activation of farnesoid X receptor (FXR), which is a nuclear receptor of bile acid (BA), was reported to contribute to the resolution of NAFLD. However, it is unclear whether SG has an effect on expression of FXR in the liver. We aimed to investigate the expression of FXR and its related factors in the liver after SG and to clarify the relationship between changes in FXR expression and NAFLD in an obese rat model.
Methods
Thirty male Zucker fatty rats were divided into three groups: sham-operated (SO) control, pair-fed (PF) control, and SG. Eight weeks after the surgery, metabolic parameters, plasma levels of total BA and liver enzymes, liver triglyceride (TG) content, and mRNA expression of FXR and its related factors, such as small heterodimer partner (SHP) and peroxisome proliferator-activated receptor α (PPARα), were measured.
Results
Metabolic parameters in the SG group were significantly improved compared with the SO group. Liver enzymes and TG were significantly lower in the SG group than in the SO group. Plasma levels of BA were significantly higher in the SG group than in the SO and PF groups. mRNA expression of FXR, SHP, and PPARα in the liver was significantly higher in the SG group than in the SO group.
Conclusions
These results suggest that the effects of SG on NAFLD should be associated with the expression of the FXR pathway in the liver in a Zucker fatty rat model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity has increased globally in recent decades [1]. Obesity is reported to be associated with significantly higher all-cause mortality compared with normal weight and is now one of the greatest public health problems on a worldwide scale [2]. Although bariatric surgery, as well as nonsurgical treatments, is available for obesity, bariatric surgery is more effective in losing body weight and resolving obesity-related comorbidities, such as diabetes and hyperlipidemia [3, 4]. Recently, sleeve gastrectomy (SG) has become a markedly increased bariatric procedure used worldwide [5, 6]. We previously reported improvements in glucose and lipid metabolisms in an obese diabetic rat model [7, 8]. In addition, we also reported changes in the hypothalamic feeding center following SG in a diet-induced obese rat model [9]. Recently, SG was reported to improve nonalcoholic fatty liver disease (NAFLD) in a diet-induced NAFLD rat model [10].
NAFLD is defined by the presence of hepatic steatosis either by imaging or by histology and exclusion of secondary hepatic fat accumulation such as alcohol consumption, use of medication, or hereditary disorders [11]. Risk factors associated with NAFLD include obesity, diabetes mellitus, dyslipidemia, and hypertension [12, 13]. Recently, the prevalence of NAFLD has increased with the rise of obesity and metabolic syndrome. In addition, NAFLD increases overall mortality deriving from cardiovascular disease and liver-related diseases, such as liver cirrhosis and hepatocellular carcinoma, and has become a serious public health issue [14]. Treatment of NAFLD includes intensive lifestyle intervention, such as dietary restrictions, exercise, and drug therapy. Johnson et al. reported that regular aerobic exercise reduced hepatic lipids in obesity even in the absence of body weight reduction [15]. Promrat et al. reported that weight reduction achieved through lifestyle intervention led to improvement of liver histology in nonalcoholic steatohepatitis (NASH) [16]. Thiazolidinediones and vitamin E have been reported to significantly resolve NAFLD [17, 18]. Recently, a farnesoid X receptor (FXR) agonist, obeticholic acid, significantly resolved NAFLD and insulin sensitivity in a randomized controlled clinical trial in patients with type 2 diabetes [19, 20]. It was also demonstrated that bariatric surgery is effective in patients with NAFLD and morbid obesity [21, 22].
FXR, first cloned in 1995 from rat liver cDNA, belongs to a family of nuclear hormone receptors and is highly expressed in the liver, intestines, and kidney [23]. Bile acid (BA) is a physiological ligand of FXR [24]. It was demonstrated that expression of FXR and activation of FXR by BA in the liver were associated with resolution of hepatic steatosis [25, 26]. Serum BA was reported to increase after SG, and BA may have the potential to contribute to resolution of morbid obesity and its comorbidities in animals and humans [27, 28]. However, it is unclear how SG influences the expression of FXR and its related factors in the liver and resolves NAFLD. Therefore, the aims of this study were to evaluate the expression of FXR and its related factors after SG and to clarify the relationship between changes in the expression and NAFLD in a Zucker fatty rat model.
Materials and Methods
Animals
Thirty male Zucker fatty rats (8 weeks old) were obtained from Charles River Japan, Inc. (Saga, Japan) and housed in individual cages under controlled temperature (24 ± 2 °C), at 50 ± 10% humidity, and a 12 h-light cycle (7:00 am–7:00 pm) with ad libitum access to standard rat chow (CE-2, Clea Japan, Tokyo, Japan) and tap water. Fourteen days before surgery, the rats were acclimated to their local facilities. This study was approved by the Animal Committee of Oita University (Oita, Japan) and conformed to the Guidelines for Animal Experimentation of Oita University. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Surgical Procedure
The rats were divided into three groups—sham-operated (SO) control group (n = 10), pair-fed (PF) control group (n = 10), and SG group (n = 10)—and were fasted for 24 h before surgery, which was performed under anesthesia (4% sevoflurane; Maruishi Pharmaceutical Co., Osaka, Japan). SG was performed as described previously [7, 29]. Briefly, the greater curvature from the antrum to the fundus across the forestomach and glandular stomach was incised, and approximately 90% of the forestomach and 70% of the glandular stomach were removed. The incision line in the stomach was then closed using 5-0 PDS® in three layers, to create the gastric sleeve. The SO and PF control rats underwent laparotomy, and their stomachs were elevated and returned to the abdominal cavity. The SO and SG groups had free access to standard rat chow for 8 weeks after surgery. The amount of food in the PF group was yoked ad lib to the weekly intake observed in the SG group as previously described [9]. Body weight and food intake were measured (Animal Scale; Clare, Tokyo, Japan) weekly, in all the groups (at 10:00 am). Livers and blood samples were collected 8 weeks after surgery.
Biochemical Tests
Blood glucose, total cholesterol (TC), triglyceride (TG), free fatty acid (FFA), aspartate transaminase (AST), and alanine transaminase (ALT) levels were estimated using an H7180 automatic biochemical analyzer (Hitachi, Tokyo, Japan). Enzyme-linked immunosorbent assay (ELISA) kits were used to evaluate plasma insulin level (rat insulin ELISA kit; Shibayagi, Gunma, Japan), high molecular weight adiponectin (mouse/rat high molecular weight adiponectin ELISA kit; Shibayagi, Gunma, Japan), glucagon-like peptide-1 (GLP-1) (YK160 GLP-1 EIA; Yanaihara Institute Inc., Shizuoka, Japan), ghrelin (YK251 Rat GIP (Active) ELISA kit; Yanaihara Institute Inc.), and total BA (total bile acids assay half kit; Cosmobio, Tokyo, Japan). To evaluate GLP-1, total blood was treated with dipeptidyl dipeptidase (DDP)-IV inhibitor at the moment of extraction. DDP-IV is a peptide in the blood that causes the degradation of GLP-1. To evaluate insulin resistance, the homeostasis model assessment ratio (HOMA-R) was calculated by the formula: HOMA-R = fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5 [30].
Liver TG Content
Liver TG was measured as described previously [8]. In brief, liver samples (200 mg) were homogenized using a tissue homogenizer and then centrifuged at 10,000×g for 10 min at 4 °C. The TG content of the samples was then determined using a commercial kit (Triglyceride E-test kit; Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Liver Histological Analysis
Histological changes in the liver were evaluated using hematoxylin and eosin (H&E) and oil red O staining under a light microscope. After freezing at − 80 °C, the liver was stained with oil red O (oil red O for microscopy Certistain, Merck, Germany) to reveal intracellular lipids. A pathologist who was blinded to the study evaluated all histological sections at × 200 magnification.
Quantitative Real-Time PCR for mRNA Quantification of FXR, SHP, PPARα, LXRα, and SREBP1c
Total RNA isolation was performed as described previously [9]. Quantitative real-time polymerase chain reaction (PCR) was performed as described previously with a Light Cycler system (Roche Diagnostics, Lewes, East Sussex, UK) [9]. The sequences of the primers used are listed in Table 1. Data were analyzed using the LightCycler analysis software (Roche Diagnostics), and a standard curve correlating cycle number with the amount of products formed was plotted for each sequence of interest. mRNA expression of FXR, small heterodimer partner (SHP), peroxisome proliferation-activated receptor α (PPARα), liver X receptor α (LXRα), and sterol regulatory element-binding protein 1c (SREBP1c) was then normalized to rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical Analysis
All data are expressed as mean ± standard deviation. All data were evaluated using one-way analysis of variance with Bonferroni correction for multiple comparisons. A P value < 0.05 was considered to be statistically significant. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) II software (SPSS, Inc., Chicago, IL, USA).
Results
Changes in Body Weight, Food Intake, and Liver Weight
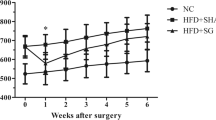
All animals survived the operations. Body weight after surgery was significantly lower in the PF and SG groups than the SO group; however, there was no significant difference in weight between the PF and SG groups (Fig. 1a). Weekly food intake except 8 weeks after surgery was significantly decreased in the PF and SG groups compared with the SO group (Fig. 1b). Liver weight 8 weeks after surgery was significantly lower in the PF and SG groups than the SO group, and there was no significant difference between the PF and SG groups (Fig. 1c).
Changes in body weight and food intake, and liver weight. a Changes in body weight after surgery in the sham-operated (SO) control, pair-fed (PF) control, and sleeve gastrectomy (SG) groups. *P < 0.05 versus the SO group. b Weekly food intake after surgery in the SO, PF, and SG groups. *P < 0.05 versus the SO group. c Liver weight after surgery in the SO, PF, and SG groups. *P < 0.05
Changes in Metabolic Parameters and Hormones in the Plasma
The mean plasma levels of glucose, TC, TG, FFA, insulin, HOMA-R, adiponectin, GLP-1, ghrelin, AST, ALT, and total BA 8 weeks after surgery are shown in Table 2. Levels of TG, HOMA-R, AST, and ALT in the SG group were significantly lower than the SO group; however, there were no significant differences between the SO and PF groups. Significant differences in TC and FFA were only seen between the SO and PF groups. Levels of insulin and ghrelin were significantly lower in the SG group than the SO and PF groups. Conversely, levels of adiponectin, GLP-1, and total BA were significantly higher in the SG group than the SO and PF groups.
Tissue TG Content and Histological Changes in the Liver
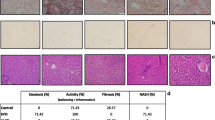
Liver TG content 8 weeks after surgery was significantly lower in the SG group than the SO group, and there were no significant differences between SO and PF groups (Fig. 2). Oil red O staining revealed that intracellular lipid in the liver was markedly decreased in the SG group compared with the SO group and slightly decreased compared with the PF group (Fig. 3).
mRNA Expression of FXR, SHP, PPARα, LXRα, and SREBP1c in the Liver
mRNA expression of FXR, SHP, and PPARα in the liver was significantly higher in the SG group than the SO group (Fig. 4a–c). Conversely, mRNA expression of LXRα and SREBP1c were significantly lower in the SG group than the SO group (Fig. 4d, e). There were no significant differences in mRNA expression of FXR and its related factors between the SG and PF groups, but the SG group had better lipid metabolism in the liver than the PF group.
mRNA expression of farnesoid X receptor (FXR) (a), small heterodimer partner (SHP) (b), peroxisome proliferator-activated receptor α (PPARα) (c), liver X receptor α (LXRα) (d), and sterol regulatory-binding protein 1c (SREBP1c) (e) in the liver were quantified by real-time PCR and expressed as a ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). SO, sham-operated; PF, pair-fed; and SG, sleeve gastrectomy. *P < 0.05
Discussion
Liver steatosis results from disruption of hepatic TG metabolism and is influenced by various factors, such as insulin, GLP-1, and adiponectin. Insulin induces enhanced expression of SREBP1c in the hepatocyte and hyperinsulinemia with the insulin resistance enhanced hepatic steatosis [31]. GLP-1 agonists significantly reduce hepatic expression of SREBP-1c and increase expression of PPARα [32]. Hepatic steatosis is enhanced in adiponectin knockout mice compared with wild-type mice [33]. FXR is also associated with hepatic TG metabolism, and FXR activation suppresses liver steatosis by downregulating lipogenesis and promoting TG oxidation. FXR induces SHP, which in turn suppresses the expression of SREBP1c. SREBP1c is a critical transcription factor that regulates hepatic TG synthesis by inducing key enzymes involved in lipogenesis. Watanabe et al. reported that activation of SHP by FXR suppresses LXRα, which is a promoter of SREBP1c [26]. In addition to suppressing lipogenesis, FXR promotes TG oxidation through the activation of PPARα [34].
Losing weight is the gold standard treatment of NAFLD. Bariatric surgery, which can achieve weight loss, has been reported to resolve NAFLD in animals and humans. We previously demonstrated that SG significantly resolved hepatic steatosis and increased hepatic expression of PPARα in an obese diabetic rat model [8]. Myronovych et al. compared SG with SO and PF controls in high-fat diet-induced obese mice. SG and PF mice lost equal weight after surgery, but SG mice had the lowest hepatic TG content [28]. Recently, Talavera-Urquijo et al. reported that SG was superior to a very low-calorie diet in point of insulin resistance and cardiovascular risk markers related to NAFLD [10]. Meta-analyses have reported the effects of bariatric surgery on NAFLD in clinical setting. Bower et al. reported that bariatric surgery was associated with a significant resolution in not only histological features of NAFLD, including steatosis, fibrosis, hepatocyte, ballooning, and lobular inflammation, but also biochemical markers including AST and ALT [21]. Mummadi et al. reported that pooled proportions of patients with resolution of steatosis, steatohepatitis, and fibrosis were 91.6, 81.3, and 65.5%, respectively, and with complete resolution of NASH was 69.5% [22]. As for the effects of SG on NAFLD, Algooneh et al. demonstrated that the rate of complete resolution of NAFLD was 56% postoperatively, and a significant resolution was related to achievement of more than 50% excess weight loss [35]. Although the effects of bariatric surgery on NAFLD have been reported, the mechanism remains unclear.
Some reports have demonstrated that resolution of NAFLD is associated with FXR activation by BA. In animals, FXR-deficient mice were reported to exhibit hepatosteatosis and hyperlipidemia compared with the wild type [36]. In addition, it was also shown that mice with obesity or aging exhibited hepatosteatosis and lipidemia and decreased expression of FXR [26, 37]. Aguilar-Olivos et al. reported that protein expression of FXR in the liver was decreased in patients with NASH compared with those with simple steatosis, and FXR expression was correlated with progression of NAFLD [38]. The effects of endogenous and synthetic FXR agonists on NAFLD were evaluated in animals and humans. Watanabe et al. reported that the activation of FXR by BA suppressed the expression of SREBP1c and inhibited hepatosteatosis [26]. Pineda et al. reported that BA increased expression of PPARα through the FXR activation [34]. Furthermore, Neuschwander-Tetri et al. evaluated the efficiency of obeticholic acid on NAFLD in a randomized controlled trial and reported that the histological features of NASH in the obeticholic acid group showed more resolution than in the placebo group [20].
There have been some reports about the relationship between SG, FXR, and BA. Ryan et al. demonstrated reduced body weight, altered feeding behavior, improved glucose tolerance, and altered composition of cecal microbial communities after SG in wild-type mice but not FXR knockout mice [39]. Myronovych et al. reported that elevated serum BA after SG in mice changed serum BA composition, which included elevated cholic and tauroursodeoxycholic acids, might be associated with a reduction of hepatic steatosis [28]. Belgaumkar et al. also showed that SG changed serum BA composition and these changes of BA were associated with a reduction of insulin resistance, proinflammatory cytokines, and cytokeratin-18, a marker of hepatocyte apoptosis [40].
In this study, we used Zucker fatty rats as a NAFLD model. Zucker fatty rats are widely used not only as an animal model of genetic obesity and metabolic syndrome but also NAFLD [41]. Liver weight and TG content were significantly lower in the SG group than the SO group but not in the PF group. Similarly, mRNA expression of FXR and its related factors was significantly changed in the SG group compared with the SO group but not the PF group. Therefore, this study demonstrates that SG activated expression of FXR and its related factors in the liver and resolved NAFLD through changed mRNA expression. Until now, it has been unclear whether expression of FXR is independent of weight loss. However, weight loss by bariatric surgery and diet therapy influences changes of the microbiota, which can affect BA and BA composition [42, 43]. In fact, the BA composition in SG and PF groups was clearly altered compared with that in high-fat native and SO groups [28]. Since the change in BA composition can affect FXR expression [44], it seems that the expression may be partially dependent on weight loss. This study also showed that SG can influence insulin, adiponectin, GLP-1, ghrelin, and BA, independent of weight. However, this study has limitations. First, our results were obtained from a rodent model, which was different from humans. Second, although NAFLD and expression of FXR and its related factors were similarly changed after SG, this study did not functionally evaluate the FXR pathway using the antagonists. Therefore, further experimental and clinical studies are necessary to investigate the association between SG and activation of FXR pathway.
In conclusion, these results suggest that the effects of SG on NAFLD should be associated with the expression of the FXR pathway in the liver in a Zucker fatty rat model.
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. https://doi.org/10.1016/S0140-6736(14)60460-8.
Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. https://doi.org/10.1001/jama.2012.113905.
Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013; 22:347:f5934.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13. https://doi.org/10.1056/NEJMoa1401329.
Ohta M, Kai S, Iwashita Y, et al. Initial experience in laparoscopic sleeve gastrectomy for Japanese morbid obesity. Asian J Endosc Surg. 2009;2(3):68–72. https://doi.org/10.1111/j.1758-5910.2009.00007.x.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–32. https://doi.org/10.1007/s11695-015-1657-z.
Masuda T, Ohta M, Hirashita T, et al. A comparative study of gastric banding and sleeve gastrectomy in an obese diabetic rat model. Obes Surg. 2011;21(11):1774–80. https://doi.org/10.1007/s11695-011-0512-0.
Kawano Y, Ohta M, Hirashita T, et al. Effects of sleeve gastrectomy on lipid metabolism in an obese diabetic rat model. Obes Surg. 2013;23(12):1947–56. https://doi.org/10.1007/s11695-013-1035-7.
Kawasaki T, Ohta M, Kawano Y, et al. Effects of sleeve gastrectomy and gastric banding on the hypothalamic feeding center in an obese rat model. Surg Today. 2015;45(12):1560–6. https://doi.org/10.1007/s00595-015-1135-1.
Talavera-Urquijo E, Rodríguez-Navarro S, Beisani M, et al. Morphofunctional changes after sleeve gastrectomy and very low calorie diet in an animal model of non-alcoholic fatty liver disease. Obes Surg. 2017 Jul 15. [Epub ahead of print]; https://doi.org/10.1007/s11695-017-2805-4.
Chalasani N, Younossi Z, Lavine JE, et al. American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107(6):811–26. https://doi.org/10.1038/ajg.2012.128.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. https://doi.org/10.1111/j.1365-2036.2011.04724.x.
Fan JG, Saibara T, Chitturi S, et al. Asia-Pacific Working Party for NAFLD. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22(6):794–800. https://doi.org/10.1111/j.1440-1746.2007.04952.x.
Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–49. https://doi.org/10.3109/07853890.2010.518623.
Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105–12. https://doi.org/10.1002/hep.23129.
Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–9. https://doi.org/10.1002/hep.23276.
Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. https://doi.org/10.1111/j.1365-2036.2011.04912.x.
Sato K, Gosho M, Yamamoto T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31(7-8):923–30. https://doi.org/10.1016/j.nut.2014.11.018.
Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–82. https://doi.org/10.1053/j.gastro.2013.05.042.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. https://doi.org/10.1016/S0140-6736(14)61933-4.
Bower G, Toma T, Harling L, et al. Bariatric surgery and non-alcoholic fatty liver disease: a systematic review of liver biochemistry and histology. Obes Surg. 2015;25(12):2280–9. https://doi.org/10.1007/s11695-015-1691-x.
Mummadi RR, Kasturi KS, Chennareddygari S, et al. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6(12):1396–402. https://doi.org/10.1016/j.cgh.2008.08.012.
Forman BM, Goode E, Chen J, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–93. https://doi.org/10.1016/0092-8674(95)90530-8.
Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53. https://doi.org/10.1016/S1097-2765(00)80348-2.
Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4(4):741–8. https://doi.org/10.1007/s12072-010-9202-6.
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–18. https://doi.org/10.1172/JCI21025.
Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400–7. https://doi.org/10.1016/j.metabol.2009.05.006.
Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 2014;22(2):390–400. https://doi.org/10.1002/oby.20548.
Lopez PP, Nicholson SE, Burkhardt GE, et al. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. J Surg Res. 2009;157(2):243–50. https://doi.org/10.1016/j.jss.2008.10.025.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. https://doi.org/10.1007/BF00280883.
Matsumoto M, Ogawa W, Teshigawara K, et al. Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes. 2002;51(6):1672–80. https://doi.org/10.2337/diabetes.51.6.1672.
Ding X, Saxena NK, Lin S, et al. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43(1):173–81. https://doi.org/10.1002/hep.21006.
Kamada Y, Matsumoto H, Tamura S, et al. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47(4):556–64. https://doi.org/10.1016/j.jhep.2007.03.020.
Pineda Torra I, Claudel T, Duval C, et al. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17(2):259–72. https://doi.org/10.1210/me.2002-0120.
Algooneh A, Almazeedi S, Al-Sabah S, et al. Non-alcoholic fatty liver disease resolution following sleeve gastrectomy. Surg Endosc. 2016;30(5):1983–7. https://doi.org/10.1007/s00464-015-4426-0.
DUMMY
Xiong X, Wang X, Lu Y, et al. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J Hepatol. 2014;60(4):847–54. https://doi.org/10.1016/j.jhep.2013.12.003.
Aguilar-Olivos NE, Carrillo-Córdova D, Oria-Hernández J, et al. The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in non-alcoholic fatty liver disease. Ann Hepatol. 2015;14(4):487–93.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;8(509):183–8.
Belgaumkar AP, Vincent RP, Carswell KA, et al. Changes in bile acid profile after laparoscopic sleeve gastrectomy are associated with improvements in metabolic profile and fatty liver disease. Obes Surg. 2016;26(6):1195–202. https://doi.org/10.1007/s11695-015-1878-1.
Kucera O, Cervinkova Z. Experimental models of non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2014;20(26):8364–76. https://doi.org/10.3748/wjg.v20.i26.8364.
Medina DA, Pedreros JP, Turiel D, Quezada N, Pimentel F, Escalona A, Garrido D. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ. 2017; 20;5:e3443.
Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;5(17):225–35.
Zhang HM, Wang X, ZH W, et al. Beneficial effect of farnesoid X receptor activation on metabolism in a diabetic rat model. Mol Med Rep. 2016;13(3):2135–42. https://doi.org/10.3892/mmr.2016.4761.
Acknowledgments
We thank Ms. Mayumi Takeda and Yuiko Aso for the technical assistance.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Informed Consent
Does not apply.
Rights and permissions
About this article
Cite this article
Watanabe, K., Ohta, M., Takayama, H. et al. Effects of Sleeve Gastrectomy on Nonalcoholic Fatty Liver Disease in an Obese Rat Model. OBES SURG 28, 1532–1539 (2018). https://doi.org/10.1007/s11695-017-3052-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-3052-4