Abstract
Background
Laparoscopic greater curvature plication (LGCP) is an emerging bariatric procedure that reduces the gastric volume without implantable devices or gastrectomy. The aim of this study was to explore changes in glucose homeostasis, postprandial triglyceridemia, and meal-stimulated secretion of selected gut hormones [glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), ghrelin, and obestatin] in patients with type 2 diabetes mellitus (T2DM) at 1 and 6 months after the procedure.
Methods
Thirteen morbidly obese T2DM women (mean age, 53.2 ± 8.76 years; body mass index, 40.1 ± 4.59 kg/m2) were prospectively investigated before the LGCP and at 1- and 6-month follow-up. At these time points, all study patients underwent a standardized liquid mixed-meal test, and blood was sampled for assessment of plasma levels of glucose, insulin, C-peptide, triglycerides, GIP, GLP-1, ghrelin, and obestatin.
Results
All patients had significant weight loss both at 1 and 6 months after the LGCP (p ≤ 0.002), with mean percent excess weight loss (%EWL) reaching 29.7 ± 2.9 % at the 6-month follow-up. Fasting hyperglycemia and hyperinsulinemia improved significantly at 6 months after the LGCP (p < 0.05), with parallel improvement in insulin sensitivity and HbA1c levels (p < 0.0001). Meal-induced glucose plasma levels were significantly lower at 6 months after the LGCP (p < 0.0001), and postprandial triglyceridemia was also ameliorated at the 6-month follow-up (p < 0.001). Postprandial GIP plasma levels were significantly increased both at 1 and 6 months after the LGCP (p < 0.0001), whereas the overall meal-induced GLP-1 response was not significantly changed after the procedure (p > 0.05). Postprandial ghrelin plasma levels decreased at 1 and 6 months after the LGCP (p < 0.0001) with no significant changes in circulating obestatin levels.
Conclusion
During the initial 6-month postoperative period, LGCP induces significant weight loss and improves the metabolic profile of morbidly obese T2DM patients, while it also decreases circulating postprandial ghrelin levels and increases the meal-induced GIP response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity plays an important role in the pathogenesis of insulin resistance and type 2 diabetes mellitus (T2DM). Morbidly obese patients are at higher risk of T2DM, hypertension, hyperlipidemia, and cardiovascular disease. Bariatric surgery is currently the most effective treatment for weight loss and is also shown to lead to rapid and prolonged improvement in insulin resistance and hyperglycemia in the majority of morbidly obese T2DM patients [1].
Laparoscopic greater curvature plication (LGCP; also referred to as laparoscopic gastric plication, total gastric vertical plication, or gastric imbrication) is an emerging bariatric procedure, introduced as a relatively less invasive restrictive technique compared to sleeve gastrectomy since no gastric or intestinal resection is required [2–8]. LGCP reduces the stomach volume by laparoscopic plication/infolding of the greater gastric curvature through placement of one or two rows of nonabsorbable sutures or staples. Thus, LGCP results in elimination of the greater curvature and formation of a gastric tube, achieving a restrictive effect without utilizing implantable devices (e.g., band), gastrectomy, or intestinal bypass. In addition, LGCP is potentially reversible, if required.
Current reports have shown that LGCP results in effective weight loss in the short and medium term [2–8], with percent excess weight loss (%EWL) reaching up to 32 % at 6 months and almost 50 % at 18 months postoperatively in a recent series of 244 patients [2]. Notably, in this study, 96.9 % of patients with T2DM exhibited significant improvement or resolution of their diabetic status at 6 months after the procedure [2]. Furthermore, LGCP is also shown to have a good safety profile without major safety concerns in the short- and medium-term, as low complication rates have been reported so far; however more evidence is required in order to establish the long-term efficacy and safety of this novel bariatric procedure [2–8].
To date, there is a lack of studies focusing on the effects of LGCP in T2DM patients. Particularly, there are no reports regarding the metabolic and hormonal changes that are associated with improvement of T2DM following LGCP. The aim of this study was to explore changes in glucose homeostasis, postprandial triglyceridemia, and meal-stimulated secretion of selected gastrointestinal hormones [namely, glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), ghrelin, and obestatin) in T2DM patients at 1 and 6 months after LGCP.
Methods and Materials
Study Cohort and Protocol
The study cohort included Caucasian adult women with morbid obesity [body mass index (BMI) ≥35 kg/m2] and T2DM who were operated at the OB Clinic, Prague, Czech Republic, between 2009 and 2012. The study was approved by the Ethics Committee of the Institute of Endocrinology, Prague, Czech Republic, and informed consent was obtained from all study participants, in accordance with the Declaration of Helsinki. For the purposes of this study, all patients were prospectively investigated before the LGCP (baseline) and at 1 and 6 months following the procedure. Women on incretin mimetics and/or insulin for T2DM treatment were excluded from this study.
Anthropometric measurements were performed for all patients at the three study time points (baseline, 1 month post-op, and 6 months post-op), as per protocol. Body weight was measured to the nearest 0.5 kg and height to the nearest 1 cm. BMI was calculated as body weight in kilograms divided by the square of the height in meters. Percent excess weight loss (%EWL) and percent of excess BMI lost (%EBL) were calculated according to the following equations: (1) %EWL = [(preoperative weight − follow-up weight)/preoperative weight − ideal body weight] × 100; and (2) %EBL = [(preoperative BMI − follow-up BMI)/(preoperative BMI − 25)] × 100; as previously described [2, 9–11]. Body fat mass was also assessed using a standardized calibrated bioimpedance instrument (Tanita TBF-300; Tanita Corporation).
For the purposes of this study, all patients underwent a standardized liquid mixed-meal test [300 ml; 375 kcal; 1,581 kJ; 30 % (28.2 g) protein, 25 % (10.5 g) fat, 45 % (42 g) carbohydrate] at each of the three time points of the study, namely, at baseline (exam 1), at 1 month (exam 2), and at 6 months (exam 3) after the LGCP. All patients were examined in the morning after an overnight fast, and venous blood was sampled for assessing GIP, GLP-1, ghrelin, and obestatin levels at 0, 30, 60, 90, 120, and 180 min after the liquid meal ingestion. These blood samples were collected into chilled ethylenediaminetetraacetic acid (EDTA) containing tubes with aprotinin. Dipeptidyl-peptidase-4 inhibitor (Millipore, Billerica, MA, USA) was also added immediately after blood sampling. Blood samples were also collected into chilled EDTA containing tubes without aprotinin for assessment of glucose, insulin, and C-peptide levels at −15, −10, 0, 15, 30, 45, 60, 90, 120, 150, and 180 min. All samples were immediately cooled, and plasma was prepared, aliquoted, and stored at −80 °C until assayed. Plasma levels of blood glucose, insulin, C-peptide, HbA1c, and triglycerides were measured using the Cobas 6000 analyzer. Plasma concentrations of GIP, GLP-1, and total ghrelin were assessed using a multiplex assay (Bio-Plex ProTM Human Diabetes Assay Panel, BioPlex 200 System, Bio-Rad Laboratories). Obestatin levels were also determined by a commercially available RIA kit (Phoenix Pharmaceuticals, Inc., California, USA) according to the manufacturer's protocol. Finally, the homeostatic model assessment (HOMA) method was used to assess insulin resistance (HOMA-IR), as previously described [12], according to the following equation: HOMA-IR = [fasting glucose (mmol/L) × fasting insulin (mIU/L)]/22.5.
Surgical Technique
All study participants underwent a LGCP procedure for weight loss, as previously described [2]. Briefly, our technique consisted of dissection of the greater gastric curvature; beginning 3–5 cm proximally from the pylorus, proceeding towards the angle of Hiss, and stopping approximately 2 cm below the angle of Hiss, thus preserving the first one to two short gastric vessels. Through this dissection, the fundus and gastric body were mobilized, allowing the greater curvature to be infolded and secured with continuous polypropylene sutures into two plication rows, one on top of the other. A 36-F bougie was used for calibration during suturing in order to maintain a standardized stomach lumen under its guidance. None of the patients in our study cohort exhibited any major intraoperative and/or postoperative complications.
Statistical Analysis
Results are presented as median (lower confidence limit and upper confidence limit) or mean ± standard error, unless otherwise stated. Due to skewed distribution in most variables, these were transformed by a power transformation in order to stabilize the variance and obtain normal distribution, while the homogeneity of the transformed data was verified using residual analysis, as previously described [13, 14]. Values for the area under the curve (AUC) were calculated using the trapezoidal rule. For statistical analyses, end points were compared by repeated measures analysis of variance (ANOVA) consisting of subject factor and within-subject factor tests (StatGraphix Centurion XVI, 2009), or by the Wilcoxon test (NCSS, 2004), as appropriate. Statistical significance was set at p < 0.05.
Results
Weight Loss Outcomes
Thirteen morbidly obese T2DM women were recruited into this study [age,55 (47, 60) years]. The duration of T2DM for the study cohort was 3 (1.8, 10) years. All study participants had statistically significant weight loss both at 1 and 6 months following the LGCP compared to baseline, exhibiting significant reductions in body weight, BMI, %EWL, and %EBL (p ≤ 0.002). Table 1 presents the weight loss outcomes for our study cohort, including the significant reductions noted in body fat mass and in waist and hip circumference.
Glucose Homeostasis and Triglycerides
Figure 1 presents the statistically significant improvement in fasting hyperglycemia and hyperinsulinemia at 6 months after the LGCP (p < 0.0001), and the parallel improvement in insulin resistance as expressed by the significant reduction in HOMA-IR (p < 0.003). Of note, HbA1c significantly declined in the period after the operation (p < 0.0001) (Fig. 1), with only one of the 13 patients not achieving statistically significant reduction in HbA1c during the study follow-up.
Improvement in fasting hyperglycemia, hyperinsulinemia, homeostatic model assessment-insulin resistance (HOMA-IR), and glycated hemoglobin (HbA1c) levels at 1 month (exam 2) and 6 months (exam 3) after laparoscopic greater curvature plication (LGCP), compared to baseline (exam 1) [Full, gray, and empty circles represent retransformed mean values with their 95 % confidence intervals at baseline, 1 month, and 6 months, respectively. Comparison performed by repeated-measures analysis of variance (ANOVA); exam, influence of time after LGCP; Subj, subject factor. *p < 0.05]
Furthermore, both at 1 and 6 months after the LGCP a left shift of the postprandial glucose curve was noted in response to the liquid mixed-meal test, exhibiting an earlier peak (at 45 min) compared to the postprandial glucose curve at baseline (peak at 60 min) (Fig. 2). Overall, the total AUC for plasma glucose levels in response to the liquid mixed-meal test was also significantly reduced at 6 months after the LGCP (p < 0.05). In addition, at 1 month after the LGCP, higher postprandial levels of insulin at 30 min and C-peptide at 30, 45, and 60 min were noted (p < 0.05), but not at 6 months (Fig. 2). Moreover, although the total AUC for C-peptide significantly increased at 1 month after the LGCP (p < 0.05), the total AUC for insulin postprandial levels was not significantly changed both at 1 and 6 months after the operation compared to baseline (p > 0.05) (Fig. 2).
Plasma glucose, insulin, and C-peptide levels in response to a standardized liquid mixed-meal test at baseline (exam 1), 1 month (exam 2), and 6 months (exam 3) after laparoscopic greater curvature plication (LGCP) [Full, gray, and empty circles represent retransformed mean values with their 95 % confidence intervals at baseline, 1 month, and 6 months, respectively. Comparison performed by repeated-measures analysis of variance (ANOVA); exam, influence of time after LGCP; time, influence of time during the meal test; exam × time, interaction of time after LGCP and time after meal ingestion; Subj, subject factor. AUC area under the curve. *p < 0.05]
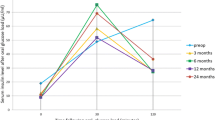
Finally, significant improvement in postprandial triglyceridemia was also noted at 6 months after the LGCP, with significantly lower plasma triglycerides in response to the liquid mixed-meal test compared to baseline levels (p ≤ 0.001) (Fig. 3).
Plasma triglyceride levels in response to a standardized liquid mixed-meal test at baseline (exam 1), 1 month (exam 2), and 6 months (exam 3) after laparoscopic greater curvature plication (LGCP) [Full, gray, and empty circles represent retransformed mean values with their 95 % confidence intervals at baseline, 1 month, and 6 months, respectively. Comparison performed by repeated-measures analysis of variance (ANOVA); exam, influence of time after LGCP; time, influence of time during the meal test; exam × time, interaction of time after LGCP and time after meal ingestion; Subj, subject factor. AUC area under the curve. *p < 0.05]
Gastrointestinal Hormones
Higher meal-stimulated GIP levels were noted after the LGCP (p < 0.0001), with (1) the total AUC for GIP at 1 month (exam 2) being significantly higher compared to baseline (exam 1); and (2) the total AUC for GIP at 6 months (exam 3) being significantly higher compared to both baseline and 1 month values (Fig. 4a). Of note, postprandial GIP levels at 1 and at 6 months after the operation exhibited an earlier peak (at 30 min) compared to the meal-stimulated GIP response at baseline, which peaked at 60 min. Furthermore, the drop of postprandial GIP levels following the corresponding peak was more rapid at baseline (exam 1) and at 1 month (exam 2) compared to 6 months (exam 3) after the LGCP (Fig. 4a).
Plasma levels of a glucose-dependent insulinotropic polypeptide (GIP) and b glucagon-like peptide-1 (GLP-1) in response to a standardized liquid mixed-meal test at baseline (exam 1), 1 month (exam 2), and 6 months (exam 3) after laparoscopic greater curvature plication (LGCP) [Full, gray, and empty circles represent retransformed mean values with their 95 % confidence intervals at baseline, 1 month, and 6 months, respectively. Comparison performed by repeated-measures analysis of variance (ANOVA); exam, influence of time after LGCP; time, influence of time during the meal test; exam × time, interaction of time after LGCP and time after meal ingestion; Subj, subject factor. AUC area under the curve. *p < 0.05]
GLP-1 plasma levels peaked at 60 min after meal ingestion during the 1 month meal-test, whereas at 6 months, the postprandial GLP-1 levels reached a peak at 30 min, as was also noted for the postprandial GLP-1 response at baseline (Fig. 4b). However, contrary to GIP, the total AUC for the meal-stimulated GLP-1 response at 1 and 6 months after the LGCP did not significantly change compared to the baseline value (p > 0.05) (Fig. 4b).
In addition, significantly decreased postprandial ghrelin plasma levels were observed during the mixed-meal test at 1 month after the LGCP, with more pronounced reduction at 6 months compared to the preoperative baseline levels (p < 0.0001) (Fig. 5). Overall, the total AUC for the postprandial ghrelin response tended to decrease at the 1 month follow-up and was significantly lower at 6 months compared to the baseline value (p < 0.05) (Fig. 5). Contrary to ghrelin, there were no significant changes in circulating obestatin levels in response to the ingested meal preoperatively at baseline, and this lack of postprandial obestatin response remained both at 1 and 6 months after the LGCP (figure not shown).
Plasma levels of ghrelin in response to a standardized liquid mixed-meal test at baseline (exam 1), 1 month (exam 2), and 6 months (exam 3) after laparoscopic greater curvature plication (LGCP) [Full, gray, and empty circles represent retransformed mean values with their 95 % confidence intervals at baseline, 1 month, and 6 months, respectively. Comparison performed by repeated-measures analysis of variance (ANOVA); exam, influence of time after LGCP; time, influence of time during the meal test; exam × time, interaction of time after LGCP and time after meal ingestion; Subj, subject factor. AUC area under the curve. *p < 0.05]
Discussion
The objective of this study was to report novel data regarding the effects of LGCP in T2DM patients, focusing on changes in fasting and postprandial plasma levels of glucose, insulin, triglycerides, and selected gastrointestinal hormones at 1 and 6 months after the procedure. In accord with the existing literature, our results showed that LGCP induces significant weight loss in morbidly obese T2DM patients during the initial 6-month postoperative period. Based on published data, including series with nondiabetic and T2DM obese patients, the mean %EWL following LGCP is reported to reach 32–66 %, 50–79 %, and 60–74 % at 6, 12, and 24 months, respectively [2–8]. Indeed, in our cohort of T2DM women, the %EWL reached 30.2 % (22.6, 34.2) at 6 months after the LGCP, approaching the reported range of 6-month %EWL outcomes.
Weight loss following LGCP is considered to be mainly the result of restricted food intake, due to reduced volume capacity of the plicated stomach. Thus, LGCP can be classified as an alternative restrictive bariatric procedure, which, to a certain extent, simulates the effects of laparoscopic sleeve gastrectomy (LSG) [2–8]. Importantly, mounting evidence indicates that LSG is not merely a restrictive procedure but also affects the secretion of several orexigenic (e.g., ghrelin) and anorexigenic (e.g., PYY) gut hormones inducing appetite suppression and other metabolic/endocrine effects [15–17]. In LSG, the resection of the gastric fundus, where most of the ghrelin-secreting cells of the gastric mucosa are located, leads to marked and sustained reductions in circulating ghrelin levels [15–17]. Furthermore, LSG is also shown to increase the secretion of certain gastrointestinal hormones that exert potent satiety effects (e.g., PYY), presumably due to faster gastric emptying and/or less well-digested chyme entering the duodenum [15–17].
Contrary to LSG, LGCP preserves the gastric mucosa since the greater gastric curvature is imbricated/infolded into the body of the stomach. However, it may be hypothesized that the devascularization and infolding of the highly vascular gastric fundus as part of the LGCP may result in decreased vascular perfusion of the mucosal cells, potentially affecting their endocrine function and leading to ghrelin hyposecretion. Accordingly, in our study, we documented decreased postprandial plasma levels of ghrelin during the initial 6-month postoperative period after the LGCP compared to preoperative levels (Fig. 5). In contrast, we observed no significant changes in circulating obestatin levels, either basal or postprandial, before and after the LGCP. Obestatin, which shares a common precursor with ghrelin, i.e., preproghrelin, was initially described as an anorexigenic peptide opposing the effects of ghrelin [18]. Although the secretion pattern of obestatin and its physiological role in appetite regulation remains controversial, it has been proposed that the circulating ghrelin/obestatin ratio may be implicated in the regulation of feeding behavior and body weight [18]. Thus, the marked decrease in circulating ghrelin levels, and the corresponding decrease in the ghrelin/obestatin ratio, noted in our cohort after the LGCP, may also account for the postoperative weight loss through enhanced appetite suppression. Notably, ghrelin has also been shown to exert various diabetogenic effects [15, 19, 20], and so decreased circulating ghrelin levels may additionally contribute to improved glucose homeostasis after the LGCP.
In parallel with weight loss, all but one patient in our study (92.3 %) achieved significant reductions in HbA1c during the 6-month postoperative follow-up. This agrees with published data by Fried et al. [2] reporting that 96.9 % of T2DM patients exhibited at least improvement of their preoperative diabetic status at 6 months after LGCP. In our cohort, the amelioration of glucose homeostasis was characterized by markedly decreased fasting glucose and insulin plasma levels and increased insulin sensitivity as assessed by HOMA-IR (Fig. 1). In addition, our results show that the postprandial plasma levels of glucose also peaked earlier (peak at 45 min) at 1 and 6 months after the LGCP, with the AUC for the meal-induced glucose response being significantly lower at 6 months compared to baseline (Fig. 2).
Changes of the incretin response in T2DM patients after the LGCP may also improve glucose homeostasis. GIP is secreted postprandially from enteroendocrine cells of the small intestine (K cells; located mainly in the duodenum and upper jejunum) and rapidly stimulates insulin secretion from the pancreatic beta-cells, while it also exerts multiple effects in other organs (e.g., adipose tissue, brain) promoting glucose and lipid storage [21, 22]. Importantly, the incretin effect of GIP is deficient in T2DM due to relative beta-cell GIP resistance [22]. Our results document a significant rise in postprandial GIP levels at 1 month after the LGCP, which was even more pronounced at 6 months (Fig. 4). Interestingly, Romero et al. [23] have reported increased postprandial GIP levels in morbidly obese T2DM patients at 6 weeks after LSG. It is also noteworthy that, although reports on fasting and postprandial GIP levels after Roux-en-Y gastric bypass (RYGB) are also inconsistent, there are data suggesting that meal-induced GIP levels in T2DM patients may be increased early (1 month) following RYGB and decreased in the longer-term [15]. Regarding the mechanism that could induce the higher meal-induced GIP levels after LGCP noted in our study, it can be hypothesized that this GIP response may reflect over-stimulation of the K cells in the duodenum due to either accelerated gastric emptying or/and contact with less well-digested chyme, as it has also been hypothesized for LSG. However, this hypothesis remains to be elucidated, and additional long-term studies will be required in order to explore whether the rise in postprandial GIP levels that we noted during the initial 6-month period following the LGCP is sustained in the longer term.
GLP-1 is secreted from the enteroendocrine L cells (located mainly in the distal small bowel) in response to the presence of nutrients in the lumen of the small intestine, particularly of carbohydrates and fat [21]. In addition to its insulinotropic actions, GLP-1 also suppresses glucagon secretion, delays gastric emptying, increases satiety, and may improve insulin sensitivity. GLP-1 postprandial secretion is markedly decreased in T2DM; however, its insulinotropic effect is preserved [21, 22]. In our study, postprandial GLP-1 levels exhibited a higher peak at 60 min after meal ingestion at 1-month following the LGCP, but this finding was not preserved at the 6-month follow-up (Fig. 4b). Moreover, we found no change in the total AUC of the meal-stimulated GLP-1 response both at 1 and 6 months following the procedure. Contrary, several reports have shown increased meal-stimulated GLP-1 secretion early after LSG, which is also sustained in the longer term [16, 23–27]. However, this effect after LSG may be less pronounced than the well-documented rise in postprandial GLP-1 levels after RYGB [15]. Recently, it has been suggested that additional mechanisms other than faster gastric emptying may account for the changes in GLP-1 levels after LSG (e.g., increased secretion of foregut hormones such as cholecystokinin) [16, 28, 29]. Whether such additional mechanisms play a role in the meal-stimulated GLP-1 response after LGCP needs to be further studied.
Finally, the improved metabolic profile of our T2DM patients after the LGCP was also characterized by ameliorated postprandial hypertriglyceridemia, which is recognized as an independent risk factor for early atherosclerosis, ischemic heart disease, myocardial infarction, and death [30–32]. Indeed, we noted a decrease in postprandial triglyceride levels that was apparent at 1 month and achieved significance at 6 months after the LGCP (Fig. 5). This is in accord with the available evidence showing that LSG results in improved circulating triglyceride levels in T2DM patients [27, 33, 34]. Notably, Stefater et al. [35] have demonstrated that sleeve gastrectomy in rats improves postprandial lipidemia in a weight-independent manner due to reduced intestinal secretion of triglycerides following ingestion of a lipid meal.
In conclusion, our results show that LGCP in morbidly obese T2DM women leads to significant weight loss during the initial 6-month postoperative period. During this follow-up period, our study patients also achieved significant improvement in glucose homeostasis and postprandial hypertriglyceridemia, reflecting primarily the result of the significant weight loss due to the restrictive effect of the operation on food intake. Importantly, we further demonstrated that postprandial plasma levels of ghrelin are decreased at 1 and 6 months after the LGCP, a finding that has also been noted after other bariatric operations such as sleeve gastrectomy, vertical banded gastroplasty and biliopancreatic diversion with duodenal switch [15, 36], whereas increased circulating ghrelin levels have been shown following gastric banding and with diet alone [15, 36, 37]. Finally, it is of note that meal-induced GIP secretion was also increased after the LGCP in our study, a change that has also been reported after certain bariatric operations such as sleeve gastrectomy and gastric bypass and cannot be attributed to caloric restriction and weight loss alone [15, 38].
Bearing in mind the limitations of our study, such as the relatively limited size of our cohort, the lack of a controlled comparison group and the relatively short duration of follow-up, our study suggests that LGCP can offer an effective addition to the arsenal of bariatric surgery with effects that may extend beyond gastric volume restriction. Larger and long-term studies are required to further explore the spectrum of metabolic/hormonal effects of LGCP and establish the role of this bariatric technique in the treatment of obesity and T2DM.
References
Dixon JB, le Roux CW, Rubino F, et al. Bariatric surgery for type 2 diabetes. Lancet. 2012;379(9833):2300–11. PMID: 22683132.
Fried M, Dolezalova K, Buchwald JN, et al. Laparoscopic greater curvature plication (LGCP) for treatment of morbid obesity in a series of 244 patients. Obes Surg. 2012;22(8):1298–307. PMID: 22648797.
Mui WL, Lee DW, Lam KK, et al. Laparoscopic greater curve plication in Asia: initial experience. Obes Surg. 2013;23(2):179–83. PMID: 22968832.
Niazi M, Maleki AR, Talebpour M. Short-term outcomes of laparoscopic gastric plication in morbidly obese patients: importance of postoperative follow-up. Obes Surg. 2013;23(1):87–92. PMID: 23007660.
Skrekas G, Antiochos K, Stafyla VK. Laparoscopic gastric greater curvature plication: results and complications in a series of 135 patients. Obes Surg. 2011;21(11):1657–63. PMID: 21898042.
Ramos A, Galvao Neto M, Galvao M, et al. Laparoscopic greater curvature plication: initial results of an alternative restrictive bariatric procedure. Obes Surg. 2010;20(7):913–8. PMID: 20407932.
Brethauer SA, Harris JL, Kroh M, et al. Laparoscopic gastric plication for treatment of severe obesity. Surg Obes Relat Dis. 2011;7(1):15–22. PMID: 21144804.
Abdelbaki TN, Huang CK, Ramos A, et al. Gastric plication for morbid obesity: a systematic review. Obes Surg. 2012;22(10):1633–9. PMID: 22960951.
Lee DY, Guend H, Park K, et al. Outcomes of laparoscopic Roux-en-Y gastric bypass versus laparoscopic adjustable gastric banding in adolescents. Obes Surg. 2012;22(12):1859–64. PMID: 22923312.
Montero PN, Stefanidis D, Norton HJ, et al. Reported excess weight loss after bariatric surgery could vary significantly depending on calculation method: a plea for standardization. Surg Obes Relat Dis. 2011;7(4):531–4. PMID: 21159563.
Deitel M, Gawdat K, Melissas J. Reporting weight loss 2007. Obes Surg. 2007;17(5):565–8. PMID: 17658011.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. PMID: 3899825.
Meloun M, Hill M, Militký J, et al. Transformation in the PC-aided biochemical data analysis. Clin Chem Lab Med. 2000;38(6):553–9. PMID: 10987205.
Meloun M, Hill M, Militký J, et al. New methodology of influential point detection in regression model building for the prediction of metabolic clearance rate of glucose. Clin Chem Lab Med. 2004;42(3):311–22. PMID: 15080566.
Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R15–27. Review. PMID: 21474429.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8. PMID: 22354457.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7. PMID: 18376181.
Zizzari P, Hassouna R, Grouselle D, et al. Physiological roles of preproghrelin-derived peptides in GH secretion and feeding. Peptides. 2011;32(11):2274–82. PMID: 21530598.
Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083–6. PMID: 11600590.
Sun Y, Asnicar M, Saha PK, et al. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3(5):379–86. PMID: 16679295.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. Review. PMID: 17928588.
Paschetta E, Hvalryg M, Musso G. Glucose-dependent insulinotropic polypeptide: from pathophysiology to therapeutic opportunities in obesity-associated disorders. Obes Rev. 2011;12(10):813–28. Review. PMID: 21815989.
Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–9. PMID: 22302537.
Dimitriadis E, Daskalakis M, Kampa M, et al. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257(4):647–54. PMID: 23108120.
Papamargaritis D, le Roux CW, Sioka E, et al. Changes in gut hormone profile and glucose homeostasis after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2013;9(2):192–201. PMID: 23183113.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236–42. PMID: 15273546.
Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F, Alexandrides TK. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis. 2013;9:667–77
Melissas J, Daskalakis M, Koukouraki S, et al. Sleeve gastrectomy—a “food limiting” operation. Obes Surg. 2008;18(10):1251–6. PMID: 18663545.
Gaylinn BD, Thorner MO. Luminal influences to orchestrate gastroenterological hormone secretion: the fat, long-chain fatty acid, cholecystokinin, glucagon-like peptide 1 axis. J Clin Endocrinol Metab. 2010;95(2):503–4. PMID: 20133469.
Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. PMID: 17635890.
Bansal S, Buring JE, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–16. PMID: 17635891.
Teno S, Uto Y, Nagashima H, et al. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care. 2000;23(9):1401–6. PMID: 10977041.
Leonetti F, Capoccia D, Coccia F, et al. Obesity, type 2 diabetes mellitus, and other comorbidities: a prospective cohort study of laparoscopic sleeve gastrectomy vs medical treatment. Arch Surg. 2012;147(8):694–700. PMID: 22508671.
Moustarah F, Gilbert A, Després JP, et al. Impact of gastrointestinal surgery on cardiometabolic risk. Curr Atheroscler Rep. 2012;14(6):588–96. PMID: 23054664.
Stefater MA, Sandoval DA, Chambers AP, Wilson-Pérez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011 Sep;141(3):939-949.e1-4. PMID: 21699773.
Kotidis EV, Koliakos GG, Baltzopoulos VG, et al. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—a prospective study. Obes Surg. 2006;16(11):1425–32. PMID: 17132406.
Chandarana K, Gelegen C, Karra E, et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes. 2011;60(3):810–8. PMID: 21292870.
Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–85. PubMed PMID: 18430778.
Acknowledgments
The EFSD funded this study through the New Horizons Collaborative Research Initiative (EFSD New Horizons research grant no. 1113 09).
Conflict of Interest
Professor S. Kumar declares support from Novo Nordisk for research and honoraria for lectures and research consultancy. Dr. O. Bradnova, Dr. I. Kyrou, Dr. V. Hainer, Dr. J. Vcelak, Dr. T. Halkova, Dr. P. Sramkova, Dr. K. Dolezalova, Dr. Fried, Dr. P. McTernan, Dr. M. Hill, Dr. M. Kunesova, Dr. B. Bendlova, and Dr. J. Vrbikova have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
O. Bradnova and I. Kyrou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bradnova, O., Kyrou, I., Hainer, V. et al. Laparoscopic Greater Curvature Plication in Morbidly Obese Women with Type 2 Diabetes: Effects on Glucose Homeostasis, Postprandial Triglyceridemia and Selected Gut Hormones. OBES SURG 24, 718–726 (2014). https://doi.org/10.1007/s11695-013-1143-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-1143-4