Abstract
Background
The study was conducted to evaluate the impact of laparoscopic sleeve gastrectomy (LSG) on type 2 diabetes mellitus (T2DM) in patients with a body mass index (BMI) of 30.0–35.0 kg/m2. Possible mechanisms, including alterations in gastric emptying time (GET), glucagon-like peptide 1 (GLP-1), ghrelin and leptin, were evaluated.
Methods
Twenty obese patients with T2DM and with a BMI of 30.0–35.0 kg/m2 underwent LSG during March 2012 to February 2015. Glycosylated haemoglobin (HbA1c), fasting plasma glucose (FPG) and GET were measured at baseline, 3 months, 6 months, 12 months and 24 months after surgery. Fasting and post-prandial levels of serum GLP-1, ghrelin and leptin were measured pre-operatively and after 3 and 6 months.
Results
The average duration of follow-up was 17.6 months, and 10 patients had completed 2 years of follow-up. After 2 years, the average BMI decreased from 33.4 ± 1.2 to 26.7 ± 1.8 kg/m2. The mean HbA1c decreased from 8.7 ± 1.6 to 6.7 ± 1.5 %, respectively. Ten patients achieved complete remission. Insulin could be stopped in all six patients who were on it pre-operatively. Meal-stimulated GLP-1 response and serum insulin at 30 min showed a significant increase following surgery. There was a significant decrease in GET.
Conclusions
This prospective study confirms the positive impact of LSG on diabetic status of non-morbidly obese patients. The possible mechanisms include the rise in post-prandial GLP-1 level induced by accelerated gastric emptying, leading to an increase in insulin secretion. LSG also leads to decreased ghrelin and leptin levels which may have a role in improving glucose homeostasis after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is currently the most common non-communicable disease worldwide. According to the International Diabetes Federation, 6.4 % of 20–79-year age groups are diabetic which is translated to roughly 285 million people and is expected to reach 7.7 % by 2030 [1]. Type 2 diabetes mellitus (T2DM) is a progressive disease with a gradual increase in insulin resistance and beta cell failure which often requires intensification of medical therapy.
Pories et al., in his landmark article, highlighted that surgery can be a more effective treatment for T2DM than medical treatment alone [2]. Bariatric procedures with malabsorptive component lead to a remission rate of 70–80 % in obese patients with type 2 diabetes [3]. Laparoscopic sleeve gastrectomy (LSG), a less complex procedure, has also shown a beneficial effect with resolution rates of 50–80 % [4, 5]. These impressive results have led to consideration of LSG as a treatment option for T2DM in patients with a body mass index (BMI) <35 kg/m2. The few prospective studies assessing the response of LSG in patients with BMI <35 kg/m2 have shown remarkable improvement in diabetes without any major complication [6, 7].
Diabetic remission occurs even before significant weight loss, and it appears that there are other weight-independent mechanisms involved [8]. It is postulated that, through change in the gut anatomy, there is change in nutrient and neuronal and hormonal signalling which result in improved glucose homeostasis. Improvement in gut hormones like incretins (glucoregulatory hormones and mediators of the enteroinsular axis) is a key factor in diabetic improvement in patients undergoing Roux-en-Y gastric bypass (RYGB). Recent evidences suggest that LSG also leads to similar improvement in incretins [9, 10]. While in gastric bypass anatomic gut rearrangement leads to incretin response, a faster gastric emptying and an earlier presentation of food to gut are hypothesized as the reasons among patients undergoing LSG [11].
The objectives of this study were to assess the impact of LSG on patients with T2DM and with a BMI of 30–35.0 kg/m2 and to evaluate the possible mechanism for improvement of glycaemic state which included alterations in gastric emptying time and hormones such as glucagon-like peptide 1 (GLP-1), ghrelin and leptin.
Subjects and Methods
After approval of study protocol by our institutional ethics committee, 20 non-morbidly obese patients (BMI 30–35.0 kg/m2) with T2DM on medication were recruited. All patients provided written consent. After a detailed evaluation, they underwent LSG at our institution, a tertiary care academic centre with an established bariatric surgery program.
A questionnaire was used to obtain information about duration of diabetes and usage of oral hypoglycaemic agents and insulin requirements and medication usage for other co-morbidities including high blood pressure, dyslipidemia and hypothyroidism. Height and weight were measured with participants wearing light clothing and no shoes. BMI was calculated at the time of registration and 3, 6, 12 and 24 months after surgery.
All patients underwent assessment of diabetic status [fasting plasma glucose (FPG) and glycosylated haemoglobin (HbA1c)] and oral glucose tolerance test (OGTT) pre-operatively and 3, 6, 12 and 24 months after surgery. Following a 12-h overnight fasting, serum insulin and plasma glucose levels were obtained at 0, 30, 60, 90 and 120 min after ingesting 75 g of glucose. The area under the curve (AUC) for plasma glucose and plasma insulin was measured. Early insulin secretory response was calculated using the insulinogenic index [(30 min insulin − fasting plasma insulin (FPI)) / (30 min glucose − FPG)]. Insulin resistance was calculated by homeostatic model assessment of insulin resistance (HOMA-IR) (FPG × FPI / 22.5). Whole-body insulin sensitivity was evaluated using the Matsuda index [10,000 / (FPG × FPI × Gm × Im)0.5 where Gm and Im are mean post-meal values during the first 120 min of the meal tolerance test]. The disposition index was calculated as a product of insulinogenic index and Matsuda index.
Gastric emptying time for each patient was measured at baseline, 3–6 months, 12 months and 24 months after surgery. For computing the gastric emptying time, patients ingested a dumpling made of grounded rice labelled with 99mTc-sulfur colloid after an overnight fast. Immediately after ingestion of the meal, the subject laid supine on the table of the triple-head camera, and imaging was started. Time activity curves were generated using a gastric region of interest for each of the views.
Serum GLP-1, ghrelin and leptin were measured at 0, 30 and 120 min, respectively, after liquid oral meal with 270 kcal and 67.5 g of carbohydrates pre-operatively, 3 and 6 months after LSG. The plasma was separated after 20 min and stored at −40 °C until assay for ghrelin, leptin and GLP-1 was performed. GLP-1 levels were measured by commercially available kits which measures the non-radioactive quantification of total GLP-1. Ghrelin levels were measured using a commercially available human enzyme-linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals). The minimum detectable concentration of ghrelin by these kits were 0.09 ng/ml, while the intraassay and interassay variation was <10 and <15 %, respectively. Leptin concentrations were quantified using a commercially available ELISA kit (Diagnostics Biochem Canada Inc). The intraassay and interassay variation was 3.7–5.5 and 5.8–6.8 %, respectively.
Resolution of diabetes was defined as FPG <126 mg/dl and HbA1c levels <6.5 without any hypoglycaemic agent.
Technique of Lap Sleeve Gastrectomy
The standard technique for sleeve gastrectomy was used for all 20 patients. After retracting the liver with the Nathanson retractor, division of greater curvature vascular supply of the stomach was carried out using an energy device. The left crura were completely exposed, and the entire fundus was mobilized. A 36-French gastric calibration tube was inserted, and a gastric sleeve was created by sequential firings of linear stapler (Endo GIA, US Surgical, Norwalk, CT) starting at 4 cm from the pylorus and continued till the angle of His. The staple line was not reinforced. Intraoperative air insufflation test was done to check for a leak.
Data Analysis
All data was analysed using IBM SPSS Statistics 20. Continuous variables were described as means and standard deviations. Quantitative data were compared using Student’s t test for paired samples. Correlations were studied by Spearman’s correlation coefficient. A p value of less than 0.05 was taken as statistically significant.
Results
Baseline Characteristics
Of 20 patients, 11 were male and 9 were female. Age, BMI and duration of diabetes were 41.6 ± 9.5 years (21–60), 33.4 ± 1.2 kg/m2 (30.9–34.9) and 3.2 years (1–22), respectively. Fasting plasma glucose, HbA1c and fasting C-peptide level were 171.1 ± 56.8 mg/dl (80–272), 8.7 ± 1.6 % (6.4–11.7) and 3.78 + 1.04 ng/ml (1.9–5.05), respectively. Fourteen patients were on oral hypoglycaemic agents (OHAs) alone, while six patients were requiring both OHA and insulin. The average number of OHAs and insulin required were 1.8 ± 0.7 and 69.7 ± 17.5 units, respectively. All patients underwent successful laparoscopic operation with an average operating time of 116.25 ± 23.56 min. There were no major post-operative complications including leak, stricture or bleeding. The duration of follow-up ranged from 3 to 36 months with the average duration of follow-up being 17.6 months. Ten patients had completed 2 years of follow-up at the time of writing this paper.
Impact of LSG on Weight Loss
Percentage excess weight loss (EWL) at 3, 6, 12 and 24 months was 66.4 ± 14.7, 95.4 ± 18.8, 95.5 ± 17.1 and 80.9 ± 19.2 %, respectively. The average BMI decreased from 33.4 ± 1.3 to 27.9 ± 1.5, 25.4 ± 1.6, 25.5 ± 1.5 and 26.7 ± 1.8 kg/m2 during the same time periods.
Impact of Surgery on Type 2 Diabetes Mellitus
Surgery had a favourable outcome on diabetes as summarised in Table 1. All patients (100 %) showed improvement in diabetes after surgery. The resolution rate in our study was 60, 70.6, 50 and 50 % at 3, 6, 12 and 24 months, respectively. The mean FPG levels decreased from 171.1 ± 56.8 at baseline to 128 ± 39.7, 106.6 ± 22, 136.5 ± 45.2 and 122.5 ± 45.4 at 3, 6, 12 and 24 months, respectively. The mean HbA1c values of 8.7 ± 1.6 decreased to 6.5 ± 1.1, 6.0 ± 1, 6.7 ± 1.4 and 6.7 ± 1.5 at 3, 6, 12 and 24 months, respectively.
Table 2 shows the comparison of baseline clinical characteristics between those who had complete remission after surgery and those without complete remission. Patients with complete remission had a shorter duration of diabetes, were not on insulin and had better-controlled diabetic status as compared to those without remission.
Impact of LSG on Glucose Homeostasis
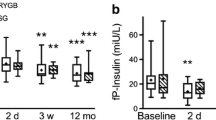
OGTT showed favourable changes in glucose homeostasis after surgery (Table 3). Insulin secretion curves showed a significant change in the pattern of insulin secretion after surgery (Fig. 1). Plasma insulin level 30 min after oral glucose showed a statistically significant increase after surgery. Preoperatively, serum insulin levels achieved a peak level at 120 min, while after surgery, maximum insulin levels were achieved at 30 min after glucose load. HOMA-IR, insulinogenic index, Matsuda index and disposition index showed similar change after surgery. All these changes were statistically significant except the disposition index at 6 months (Table 4).
Impact of LSG on Gastric Emptying Time
The time required for half of the solid meal to leave the stomach (T1/2) was significantly decreased following LSG, indicating that the stomach empties solid food rapidly into the duodenum. The mean T1/2 before surgery was 38.4 ± 13 min and was 20.3 ± 7.6, 20.7 ± 9.5 and 20.6 ± 4.4 min at 3–6, 12 and 24 months, respectively. All these changes were statistically significant (Fig. 2).
Impact of Surgery on Gut Hormones
GLP-1
Both fasting and meal-stimulated GLP-1 levels increased significantly after surgery. However, the most significant increase in GLP-1 level was seen at 30 min after the meal as seen in Fig. 3.
Leptin and Ghrelin
The AUC for ghrelin decreased significantly (73 % decline) from 16.8 at baseline to 4.5 at 6 months after surgery (p < 0.001). The AUC for leptin declined to 50 % after 6 months (baseline AUC for leptin 17.0 vs AUC for leptin 6 months after surgery 7.8, p value 0.007).
Correlation
Change in the gastric emptying time showed a positive correlation with change in 30-min serum insulin level (correlation coefficient 0.6). Change in 30-min GLP-1 level correlated positively with change in 30-min serum insulin level (correlation coefficient 0.67). However, these positive correlations did not reach a statistical significance due to the small sample size of the study.
Discussion
In our study, LSG resulted in improvement of T2DM in all the patients. A complete remission was achieved in 50 % of patients at the end of 12 and 24 months. In similar prospective studies, Lee et al. and Abbatini et al. reported resolution rates of 50 and 88.8 %, respectively, at 12 months after LSG [6, 7]. The variable remission rates seen in the literature are mainly due to the variation in the baseline diabetic parameters (duration of diabetes, baseline fasting C-peptide levels, glycaemic control and medication usage). Diabetes duration has been considered a very important determinant for achieving remission after LSG. As evident from our study, patients with shorter duration of disease, with better-controlled diabetic status and without insulin therapy are more likely to achieve remission after surgery. The overall rate of resolution is comparatively lower in our study, which can be explained by the heterogeneity of our study population. The subgroup of patients with insulin usage prior to surgery, poorly controlled diabetes and disease of prolonged duration failed to achieve complete remission. But, these patients had remarkable improvement after surgery. There was cessation of insulin therapy within the first month of surgery and significant reduction in FPG, HbA1c and amount of medication usage.
In T2DM, early-phase (glucose-induced) insulin secretion becomes defective at an initial stage [12]. This has significant impact on post-prandial glucose metabolism. In this study, there was marked improvement in the early phase of insulin release after surgery, as evident from a significant increase in 30-min serum insulin level and insulinogenic index. Even though the amount of total insulin secreted remained unchanged after surgery, the insulin secretion pattern changed dramatically. The early surge in serum insulin level effectively counteracted the post-prandial hyperglycaemia. After the surgery, there was a significant drop in fasting plasma insulin and HOMA-IR values, which suggests a reduction in insulin resistance. Moreover, there was a significant increase in Matsuda index indicating improvement in whole-body insulin sensitivity.
In addition to impact on resolution of diabetes, the possible mechanisms of resolution of diabetes were also evaluated. Meal-stimulated GLP-1 response which is deficient in diabetes [13] increased significantly after surgery with most dramatic improvement seen in 30-min post-meal GLP-1 level. GLP-1 is an incretin released by the intestine in response to food. Amongst its many effects including appetite suppression and increasing beta cell mass, it has glucoregulatory function and stimulates insulin secretion in a glucose-dependent fashion [14]. The 30-min post-meal GLP-1 level positively correlated with the 30-min serum insulin level, which suggests that GLP-1 is an important mediator of anti-diabetic impact of LSG. Nannipieri et al. also concluded that restored GLP-1 response was one of the chief determinants of diabetes remission after RYGB and LSG [15]. The probable reason for defective incretin response (and hence defective insulinaemic and glycaemic responses) in T2DM is delayed small intestine glucose delivery due to gastroparesis (a prevalent condition amongst diabetics) [16]. LSG resulted in rapid gastric emptying which coincided with increased GLP-1 and insulinomimetic effect. Direct evidence that increased GLP-1 is due to faster gastric emptying after LSG comes from a murine study [11].
Significant reduction in serum ghrelin and leptin was noted after LSG. Ghrelin is an orexigenic hormone, and a decrease in ghrelin after surgery leads to appetite suppression and resultant weight loss. Ghrelin may also influence the glucose homeostasis by inhibiting the insulin secretion. In a murine study, suppression of ghrelin reduced fasting glucose and fasting insulin level and improved glucose tolerance [17, 18]. An increased level of leptin is observed in obese individuals. Leptin and insulin have a bi-directional feedback, and a high level of leptin causes attenuation of inhibitory effects of leptin which leads to hyperinsulinaemia and diabetes. This hyperinsulinaemia leads to increased adipogenesis and leptin production. LSG tends to break this cycle and improves insulin sensitivity [19].
Conclusion
LSG had significant impact on T2DM without causing any major complication. It resulted in improvement of glycaemic status in 100 % of patients, and 50 % of patients achieved complete resolution. Patients with lesser duration of diabetes, with better baseline glycaemic control and not requiring insulin are most likely to achieve remission. The possible mechanism responsible for glycaemic control appears to be an immediate rise in post-prandial GLP-1 levels induced by accelerated gastric emptying, leading to an increase in early insulin secretion. Decreased ghrelin level after LSG may have a significant role in improving glycaemic status, and it needs further evaluation. The weight loss leading from surgery results in decreased levels of serum leptin, leading to decreased inflammatory markers and increased sensitivity of insulin.
The strengths of our study include the fact that it is a prospective study with primary end point being impact on T2DM. The study has evaluated the possible mechanisms responsible for this impact. Limitations of our study include small sample size, lack of control group and shorter duration of follow-up. However, encouraged by these promising results, we plan to continue further studies with longer duration of follow-up.
References
Shaw JE, Sicree RA, Zimmet PZ. Global estimates for the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50.
Kim S, Richards WO. Long-term follow-up of the metabolic profiles in obese patients with type 2 diabetes mellitus after Roux-en-Y gastric bypass. Ann Surg. 2010;251:1049–55.
Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9.
Peterli R, Wölnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–41.
Lee WJ, Ser KH, Chong K, et al. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147:664–9.
Abbatini F, Capoccia D, Casella G, et al. Type 2 diabetes in obese patients with body mass index of 30–35 kg/m2: sleeve gastrectomy versus medical treatment. Surg Obes Relat Dis. 2012;8:20–4.
Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–8.
Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301:R15–27.
Roslin MS, Dudiy Y, Weiskopf J, et al. Comparison between RYBG, DS, and VSG effect on glucose homeostasis. Obes Surg. 2012;22:1281–6.
Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):E424–32.
Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologica. 2001;44:929–45.
Vilsbøll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13.
Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–30.
Nannipieri M, Baldi S, Mari A, et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98:4391–9.
O’Donovan DG, Doran S, Feinle-Bisset C, et al. Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3431–5.
Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32.
Bose M, Olivan B, Teixeira J, et al. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg. 2009;19:217–29.
Woelnerhanssen B, Peterli R, Steinert RE, et al. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy—a prospective randomized trial. Surg Obes Relat Dis. 2011;7:561–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved and carried out in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Vigneshwaran, B., Wahal, A., Aggarwal, S. et al. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30–35.0 kg/m2: a Prospective Study. OBES SURG 26, 2817–2823 (2016). https://doi.org/10.1007/s11695-016-2226-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2226-9