Abstract
Background
Laparoscopic sleeve gastrectomy (SG) is a popular bariatric procedure for treating morbid obesity. This study aimed to investigate the relation between SG and lipid metabolism in an obese diabetic rat model.
Methods
Forty-five male Zucker diabetic fatty (ZDF) rats were divided into three groups: sham-operated (SO) control, gastric banding (GB), and SG. Six weeks after surgery, metabolic parameters, including plasma adiponectin level, small bowel transit, mRNA expression of peroxisome proliferator-activated receptor (PPAR)-α and PPARγ in the liver, skeletal muscle and white adipose tissue, and that of adiponectin in white adipose tissue, and triglyceride (TG) contents in the liver and skeletal muscle were measured.
Results
Metabolic parameters in the GB and SG groups were significantly improved compared with those in the SO group. However, plasma total cholesterol (TC) and free fatty acid (FFA) concentrations were significantly lower while the plasma adiponectin level was significantly higher in the SG group than in the GB and SO groups. In addition, small bowel transit time was significantly shorter in the SG group than in the other two groups. Furthermore, in the SG group, mRNA expression of PPARα in the liver and skeletal muscle and that of adiponection and PPARγ in white adipose tissue were significantly higher, while TG in the liver and skeletal muscle were significantly lower, compared with those in the other two groups.
Conclusions
These results suggest that SG improves lipid metabolism compared with GB, although there were no significant differences in the effect on weight loss between the two procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has been suggested to be the greatest threat to human health by World Health Organization [1]. It induces various lifestyle-related diseases, such as type II diabetes mellitus (T2DM), hyperlipidemia, hypertension, and fatty liver disease, and is causing a major health burden in terms of morbidity and mortality [2]. Treatment for morbid obesity includes diet, behavioral modifications, and drug therapy; however, their effectiveness on the weight loss remains limited [3]. Bariatric surgery is accepted as the most effective treatment for morbid obesity [4]. It includes restriction and malabsorption and is currently performed primarily as a laparoscopic procedure [5]. Laparoscopic sleeve gastrectomy (SG) is rapidly expanding worldwide as a restrictive bariatric procedure that is effective for the treatment of morbid obesity [6–8]. The procedure causes rapid and extensive weight loss by decreasing the gastric volume and ghrelin levels, shortening the gastrointestinal transit time, and improving glucose metabolism [8]. Recently, we reported a high gastric emptying rate and improved glucose metabolism following SG in an obese diabetic rat model [9]. However, the effect of SG on lipid metabolism remained unclear.
Peroxisome proliferator-activated receptor (PPAR), cloned from cDNA of mouse liver in 1990, participates lipid metabolism [10] and comprises of three subtypes: α, δ, and γ [11]. PPARα is mainly expressed in liver, kidney, and muscle; it decreases lipid accumulation and has an anti-inflammatory effect [12]. PPARγ is specifically expressed in adipose tissue and is associated with adipocyte differentiation [13]. However, it is unclear how bariatric surgery influences the tissue expression of PPARs. Therefore, the aim of the present study was to evaluate the relation between SG and lipid metabolism, including gastrointestinal food transit and tissue expression of PPARs, in a Zucker diabetic fatty (ZDF) rat model and compare these results with the results of two control models, a gastric banding (GB) model, which is namely a simple restriction model, and a sham-operated (SO) model.
Materials and Methods
Animals
Forty-five male ZDF rats were obtained from Charles River Japan, Inc. (Saga, Japan) and housed in individual cages with ad libitum access to standard rat chow (CE-2, Clea Japan, Tokyo, Japan) and tap water (24 ± 2 °C), at 50 ± 10 % humidity, and a 12-h-light cycle (7:00 am–7:00 pm). Fourteen days before surgery, the rats were acclimated to the local facilities. This study was approved by the Animal Committee of Oita University (Oita, Japan) and conformed to the Guidelines for Animal Experimentation of Oita University.
Surgical Procedure
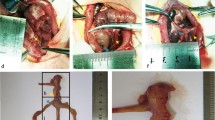
The rats were divided into three groups (n = 15/group): an SO control group, a GB group, and an SG group. The rats were fasted for 24 h before surgery, and the surgery was performed under anesthesia (4 % sevoflurane; Maruishi Pharmaceutical Co., Osaka, Japan). The methods for performing GB have been described previously [14, 15]. Rat stomach consists of forestomach (an upper part, squamous epithelium) and glandular stomach (a lower part, glandular epithelium; Fig. 1a). A 5-mm incision was made on the midline between the upper and lower portions of the stomach and the incision lines were closed using polydioxanone sutures (5-0 PDS®; Ethicon, Tokyo, Japan; Fig. 1b). Next, a gastric band made of nylon (Insulok; Hellermann, Tyton, Tokyo, Japan) was tied around the suture line. To avoid band slippage, the incised stomach was fixed above the nylon band with 5-0 PDS® (Fig. 1c). SG was performed as described previously [9, 16]. Briefly, the greater curvature from the antrum to the fundus across the forestomach and glandular stomach was incised, and approximately 90 % of the forestomach and 70 % of the glandular stomach were removed (Fig. 1d). The incision line in the stomach was then closed using 5-0 PDS® in three layers to create the gastric sleeve (Fig. 1e). The SO control rats were underwent laparotomy, and their stomachs were elevated and returned to the abdominal cavity. Body weights and food intake were measured (Animal Scale; Clare, Tokyo, Japan) weekly, in all the groups (at 10:00 am).
Surgical procedures of gastric banding and sleeve gastrectomy Scheme of a rat stomach (a). Gastric banding: b A 5-mm-length incision was made in the stomach at the borderline between the upper and lower parts (arrow), and sutured. c The nylon gastric band was tied around the lower part of stomach below the gastroesophageal junction (arrow). Sleeve gastrectomy: d The greater curvature from the antrum to the fundus across the forestomach and glandular stomach was incised (dot line), and e approximately 90 % of the forestomach and 70 % of the glandular stomach was removed
Biochemical Tests
Blood samples were collected 6 weeks after surgery. Blood glucose data were evaluated using a commercial test kit (Accu-Chek; Sanko Junyaku Co. Ltd., Tokyo, Japan). Total cholesterol (TC), triglyceride (TG), and free fatty acid (FFA) levels were estimated using an H7180 automatic biochemical analyzer (Hitachi, Tokyo, Japan). Enzyme-linked immunosorbent assay (ELISA) kits were used to evaluate plasma insulin levels (rat insulin ELISA kit; Shibayagi, Gunma, Japan), high molecular weight (HMW) adiponectin (mouse/rat high molecular weight adiponectin ELISA kit; Shibayagi), glucagon-like peptide-1 (GLP-1; YK160 GLP-1 EIA; Yanaihara Institute Inc., Shizuoka, Japan), and glucose-dependent insulinotropic polypeptide (GIP; YK251 Rat GIP (Active) ELISA kit; Yanaihara Institute Inc.). To evaluate GLP-1 and GIP, total blood must be treated with dipeptidyl dipeptidase (DDP)-IV inhibitor at the moment of extraction. DDP-IV is a peptide in the blood that causes the degradation of GLP-1. To evaluate insulin resistance, the homeostasis model assessment ratio (HOMA-R) was calculated by the formula: HOMA-R = fasting glucose (mmol/l) × fasting insulin (μU/ml)/22.5 [17].
Small Bowel Transit
Small bowel transit was measured as described previously [18]. The rats were given 5 % indigo carmine (10 ml/kg) by oral gavage. Thirty minutes later, they were sacrificed and laparotomy was performed to remove the small intestines. The distance from the pyloric ring to the blue stained intestine was measured.
Oral Fat Loading Test
An oral fat loading test was performed as described previously [19]. In brief, the rats were orally administered 7 ml/kg of 20 % intralipid fluid solution (Fresenius Kabi Japan, Tokyo, Japan). Blood samples were collected hourly for 5 h by catheterization of the internal jugular vein following administration of the intralipid fluid solution. The TG level was measured using an H7180 automatic biochemical analyzer (Hitachi).
Quantitative Real-Time PCR for mRNA Quantification of PPARα, PPARγ, Adiponectin, and Uncoupling Protein 1
Total RNA isolation was performed as described previously [20]. Quantitative real-time PCR was performed as described previously [20] with a Light Cycler system (Roche Diagnostics, Lewes, East Sussex, UK). The sequences of the used primers are listed in Table 1. Data were analyzed using the LightCycler analysis software (Roche), and a standard curve correlating cycle number with the amount of formed products was plotted for each sequence of interest. mRNA expression of PPARα, PPARγ, adiponectin, and uncoupling protein 1 (UCP-1) was then normalized to that of rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western Blotting
Western blotting was performed as described previously [20]. Rabbit anti-PPARα, anti-PPARγ, anti-adiponectin, anti-UCP1, and β-actin polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted 1:10,000 in TBS/Tw, were used the primary antibodies and incubated for 60 min at 37 °C. The membranes were placed in TBS/Tw and then incubated with rabbit antibody (Santa Cruz) for 30 min at 37 °C. Antibody-labeled proteins were detected with an Image Quant LAS 4000 mini imaging system (GE Healthcare Japan, Tokyo, Japan). β-actin was used to standardize the protein on the blots in each sample. Densitometry was performed using Image J software (Wayne Rasband, National Institute of Mental Health, Bethesda, Maryland, USA), and each datum was expressed as a ratio to β-actin.
Tissue TG Contents
Tissue TGs were measured as described previously [21]. In brief, skeletal muscle and liver samples (200 mg, each) were homogenized using a tissue homogenizer and then centrifuged at 10,000×g for 10 min at 4 °C. The TG contents of the samples were then determined using a commercial kit (Triglyceride E-test kit; Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Tissue Histological Analysis
Liver and skeletal muscle samples were frozen at −80 °C and stained with Oil red O to reveal intracellular lipids. Thus, a pathologist, who was blinded to other details, evaluated all histological sections at ×400.
Statistical Analysis
All data are expressed as means ± standard deviation. All data were evaluated using one-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons. A P value <0.05 was considered to be statistically significant. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) II software (SPSS, Inc, Chicago, IL, USA).
Results
Changes in Body Weight and Food Intake
The body weights 5 and 6 weeks after surgery in the GB and SG groups were significantly lesser than those in the SO group; however, there was no significant difference in weight between the GB and SG groups (Fig. 2a). Weekly food intake in the GB and SG groups was significantly decreased compared with that in the SO group between weeks 2 and 6 after surgery (Fig. 2b). However, there was no significant difference in weekly food intake between the GB and SG groups.
Changes in body weight and food intake. a Changes in body weight after surgery in the sham-operated (SO), gastric banding (GB), and sleeve gastrectomy (SG) groups. Each group consisted of ten rats. *P < 0.01 versus the SO group. b Weekly food intake after surgery. SO sham operated, GB gastric banding, SG sleeve gastrectomy. Each group consisted of ten rats. *P < 0.01 versus the SO group
Changes in Metabolic Parameters and Hormones
Mean plasma levels of glucose, TC, TG, FFA, insulin, HMW adiponectin, GLP-1, GIP, and HOMA-R 6 weeks after surgery are shown in Table 2. The levels of glucose, TC, TG, FFA, insulin, GIP, and HOMA-R in the GB and SG groups were significantly lower than those in the SO group. Compared with the GB group, the SG group showed significantly lower levels of TC and FFA. The HMW adiponectin levels in the GB and SG groups were significantly higher than the level in the SO group, and the level in the SG group was significantly higher than that in the GB group. The GLP-1 level in the SG group was also significantly higher than those of the GB and SO groups. HOMA-R data in the GB and SG groups were significantly lower compared with SO group. The HOMA-R in the SG group tended to be lower than that in the GB group, although there were no significant differences between the two groups.
Small Bowel Transit and Oral Fat Loading Test
The distance from the pyloric ring to the blue stained intestine in the SG group was significantly greater than that in the SO and GB groups (Fig. 3a). The plasma TG levels after oral gavage of intralipid solution in the SG group were significantly lower than those in the SO group at 1–5 h (Fig. 3b). Compared with the GB group, the SG group showed a significantly lower TG level at 2–4 h. Compared with that in the SO group, the TG level in the GB group was low at hours 1, 4, and 5.
Small bowel transit and oral fat loading test. a Small bowel transit 6 weeks after surgery. SO sham operated, GB gastric banding, SG sleeve gastrectomy. Each group consisted of ten rats. *P < 0.01. b Oral fat loading test 6 weeks after surgery. The TG levels were measured 1–5 h after oral gavage of intralipid solution (10 mg/kg). SO sham operated, GB gastric banding, SG sleeve gastrectomy. Each group consisted of five rats.*P < 0.01 versus the SO group; #P < 0.01 and ##P < 0.05 versus the GB group
Expression of PPARα, PPARγ, Adiponectin, and UCP-1
PPARα mRNA expression in the liver and skeletal muscle in the SG group was significantly higher than that in the SO and GB groups (Fig. 4a, c). PPARγ and adiponectin mRNA expression of retroperitoneal fat in the SG group was significantly higher than that in the other two groups (Fig. 4f, g). UCP-1 mRNA expression of brown fat tissue from interscapular region in the SG group was significantly higher than that in the other two groups (Fig. 4h).
mRNA expression of PPARα, PPARγ, adiponectin, and UCP1 mRNA expression of PPARα and PPARγ in the liver (a, b), PPARα and PPARγ in the skeletal muscle (c, d), PPARα and PPARγ in the retroperitoneal fat (e, f), adiponectin in the retroperitoneal fat (g), and UCP1 in the brown fat tissue (h) was quantified by real-time polymerase chain reaction (PCR) and expressed as a ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). SO sham operated, GB gastric banding, SG sleeve gastrectomy. Each group consisted of ten rats. *P < 0.01, **P < 0.05
Protein expression of PPARα, PPARγ, adiponectin, and UCP-1
Western blot analyses were performed to confirm protein expression of PPARα (expressed as a 55-kDa-band), PPARγ (expressed as a 55-kDa-band), adiponectin (expressed as a 92-kDa-band), and UCP1 (expressed as a 32-kDa-band; Fig. 5). PPARα protein expression in the liver (Fig. 5a, b) and skeletal muscle (Fig. 5c, d), PPARγ (Fig. 5e, f) and adiponectin (Fig. 5g, h) protein expression in retroperitoneal fat, and UCP1 protein expression in brown fat tissue (Fig. 5i, j) in the SG group were significantly higher than that in the SO and GB groups.
Protein expression of PPARα, PPARγ, adiponectin, and UCP-1 Western blot analyses were performed to confirm protein expression of PPARα in the liver (a, b) and skeletal muscle (c, d), PPARγ (e, f) and adiponectin (g, h) in the retroperitoneal fat, and UCP-1 in the brown fat tissue (i, j). Data were expressed as a ratio to β-actin. SO sham operated, GB gastric banding, SG sleeve gastrectomy. Each group consisted of 6 rats. *P < 0.01, **P < 0.05
Tissue TG Contents and Histological Changes of Liver and Skeletal Muscle
TG contents in the liver and skeletal muscle in the SG group were significantly lower than those in the SO and GB groups (Fig. 6a, b). Intracellular lipids in the liver samples were recognized in the SO and GB groups but not in the SG group under the light microscope (×400; Fig. 6c). In the skeletal muscle, intracellular lipids were recognized in the SO group but not in the GB and SG groups under the light microscope (×200; Fig. 6d).
Tissue triglyceride contents and histrological changes of liver and skeletal muscle Tissue triglyceride contents were measured in the a liver and b skeletal muscle. The Oil red O staining showed intracellular lipids in the c liver (×400) and d skeletal muscle (×200) under the microscope. SO sham operated, GB gastric banding, SG sleeve gastrectomy. Each group consisted of ten rats. *P < 0.01, **P < 0.05
Discussion
Recently, Schauer et al. [22] compared bariatric surgery with intensive medical therapy in obese patients and reported that the glycated hemoglobin levels, use of glucose-lowering drugs, and the index for homeostasis model assessment of insulin resistance in the laparoscopic gastric bypass and SG groups were significantly improved compared with those in the medical-therapy group. Sirbu et al. reported that TC, TG, and low density lipoprotein cholesterol (LDL-C) levels after laparoscopic SG were significantly decreased compared with the preoperative levels [23]. The use of laparoscopic SG has rapidly increased worldwide as a bariatric procedure, and several studies have reported its long-term effects. Himpens et al. [6] reported that the percent excess weight loss (%EWL) 6 years after laparoscopic SG was 57.3 %, and Bohdjalian et al. [24] reported that %EWL 5 years after laparoscopic SG was 55.0 %. The morbidity of laparoscopic SG was reportedly 3.2–7.3 %, and the mortality of laparoscopic SG was 0–0.7 % [25–27]. Chambers et al. [28] compared SG with gastric bypass and reported that SG had equal effects of weight loss and secretion of GLP-1 and insulin. Hady et al. showed that blood levels of glucose, insulin, TC, TG, and LDL-C after laparoscopic SG was significantly decreased compared with the preoperative levels, in the early postoperative period (3 months) when the sufficient weight loss was not gained [29]. SG could improve glucose and lipid metabolism in the early postoperative period. Recently, we compared SG with GB using a rat model and found that SG significantly improved insulin resistance and glucose metabolism [9]. Therefore, SG may influence glucose and lipid metabolism regardless of weight change.
There have been several reports that evaluated food transit following bariatric surgery [8, 30–34]. Following SG, small bowel transit time and gastric emptying half-time were shortened, and as a result, T2DM may be improved [8]. We also demonstrated higher gastric emptying and greater small bowel transit in the SG group compared with the GB and SO groups in our previous and present studies [9]. These effects can activate GLP-1 and insulin secretin. In addition, the processes of gastric emptying and bowel transit may influence lipid metabolism, as measured by the oral fat loading test for estimating lipid absorption in vivo. Yamada et al. [19] indeed demonstrated how the pancreatic lipase inhibitor, cetilistat, absorbed oral fat solution using this method. This study suggested that food transit time was significantly shortened after SG and lipid absorption was significantly decreased.
PPARα regulates target genes of fatty acid oxidation and lipid metabolism. Laeter et al. [35] reported that a PPARα agonist, Wy 14 643, significantly improved metabolic parameters and steatosis and ballooning of the liver in a diabetic mouse with non-alcoholic steatohepatosis. Furthermore, Abdelmegged et al. [36] reported that mice with protected expression of PPARα who had fed a high-fat diet showed greater levels of lobular inflammation, higher NAFLD scores, and increased levels of malondialdehyde and tumor necrosis factor α in the liver. However, there are few reports demonstrating the relation between bariatric surgery and PPAR expression [37–39]. PPARγ mRNA expression in the tissues of the small bowel, stomach, and visceral adipocytes are inversely associated with body mass index (BMI) [39]. PPARγ gene expression is increased in the rat model after excision of adipose tissue, compared with the controls [37]. However, Costa et al. [38] reported that open Roux-en Y gastric bypass decreases PPARγ1–3 mRNA expression in human visceral adipocytes. Therefore, the influence of bariatric surgery on PPAR expression remains uncertain.
Adiponectin, which is secreted by adipocytes, plays an important regulatory role in glucose and lipid metabolism [40, 41]. PPARs increase adiponectin concentrations through adiponectin mRNA induction in adipose tissue, which increases adiponectin-induced PPARα upregulation in liver and skeletal muscle, by increasing the activity of endogenous ligands [40, 42–44]. Furthermore, upreglated PPARα decreased TG contents in the liver and skeletal muscle [45, 46]. Administration of thiazolidinediones (TZDs), which are synthetic PPARγ ligands, significantly increased the plasma adiponectin concentrations in humans and adiponectin mRNA expression in the adipose tissues of obese mice [42]. Inversely, elevated circulating adiponectin increased PPARγ expression in the adipose tissue and improved insulin sensitivity in a transgenic mouse model [47]. Therefore, PPARγ activation may be associated with various processes including weight loss and adiponectin activation.
Although interaction between PPARs and incretins has been still unclear, there are a few reports about the relation. Metformin, which is used for the treatment of type 2 diabetes, directly increased GLP-1 receptor expression in INS-1 beta cells via PPARα dependent mechanism [48]. In high fat diet-fed GIP receptor-deficient mouse, adiponectin mRNA expression in white adipose tissue and PPARα mRNA expression in muscle are significantly increased compared with the high fat diet-fed wild type mice [49].
In the present study, the SG group increased mRNA and protein expression of PPARα and PPARγ compared with the SO and GB groups. In addition, SG increased plasma adiponectin levels and mRNA expression of adiponectin in the visceral fat. Reduction in food transit time may lead to decrease fat absorption, which introduces downsize of adipocytes, decrease of FFA concentration, and adiponectin activation in the visceral fat tissue. Furthermore, activated adiponectin can activate PPARα in the liver and skeletal muscle, and combined with lipid malabsorption, may reduce TG content in the liver and skeletal muscle. In addition, activated adiponectin together with weight loss may activate PPARγ in adipose tissue. Besides, activated adiponectin can activate UCP-1 expression in the brown fat tissue [50], and activated UCP-1 may increase energy expenditure level.
The present study used to two control models, GB and SO. The GB model was used as a simple restriction model. In this study, SG improves lipid metabolism compared with GB in an obese diabetic rat model, although there was no difference in the weight loss between the two procedures. The improvement of lipid metabolism after SG may be induced by lipid marabsorption and activation of adiponectin and PPARs. Therefore, SG appeared to have additional effects on lipid metabolism compared with GB. However, this study used the rodent model, which is different from human. Therefore, our results have limitations to apply human lipid metabolism, and further clinical studies are necessary to investigate the relation between SG and lipid metabolism. In conclusion, these results suggest that SG improves lipid metabolism compared with GB, although there were no significant differences in the effect on weight loss between the two procedures.
References
Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43.
Strum R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–6.
Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93.
Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–9.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19:1605–11.
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–24.
Gluck B, Mobitz B, Jansma S, et al. Laparoscopic sleeve gastrectomy is a safe and effective bariatric procedure for lower BMI (35.0-43 kg/m2) population. Obes Surg. 2011;21:1168–71.
Shah S, Shah P, Todkar J, et al. Prospective control study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010;6:152–7.
Masuda T, Ohta M, Hirashita T, et al. A comparative study of gastric banding and sleeve gastrectomy in an obese rat model. Obes Surg. 2011;21:1774–80.
Issemann I, Green S. Activation of a number of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;18:645–50.
Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996;12:335–63.
Toyoda T, Kamei Y, Kato H, et al. Effect of peroxisome proliferator-activated receptor-α ligands in the interaction between adipocytes and macrophages in obese adipose tissue. Obesity. 2008;16:1199–207.
Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–56.
Endo Y, Ohta M, Kai S, et al. An obese rat model of bariatric surgery with gastric banding. Obes Surg. 2007;17:815–9.
Endo Y, Ohta M, Hirashita T, et al. Additional effect of visceral fat resection in an obese rat model of gastric banding. Obes Surg. 2009;157:243–50.
Lopez PP, Nicholson SE, Burkhardt GE, et al. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. J Surg Res. 2009;157:243–50.
Mattews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Suzuki S, Ramos EJB, Goncalves CG, et al. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–90.
Yamada Y, Kato T, Ogino H, et al. Cetilistat (ATL-962), a novel pancreatic lipase inhibitor, ameliorates body weight gain and improves lipid profiles in rats. Horm Metab Res. 2008;40:539–43.
Tominaga M, Ohta M, Kai S, et al. Increased heat shock protein 90 expression contributes to impaired adaptive cytoprotection in the gastric mucosa of portal hypertensive rats. J Gastroenterol Hepatol. 2009;24:1136–41.
Arakawa M, Masaki T, Nishimura J, et al. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocrinol J. 2011;58:161–70.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76.
Sirbu A, Copăescu C, Martin S, et al. Six months results of laparoscopic sleeve gastrectomy in treatment of obesity and its metabolic complications. Chir (Bucur). 2012;107:469–75.
Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535–40.
Deitel M, Gagner M, Erickson AL, et al. Third International Summit : current status of sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:749–59.
Bellanger DE, Greenway FL. Laparoscopic sleeve gastrectomy, 529 cases without a leak: short-term results and technical considerations. Obes Surg. 2011;21:146–50.
Manenacos E, Stamou KM, Albanopoulos K, et al. Laparoscopic sleeve gastrectomy performed with intent to treat morbid obesity: a prospective single-center study of 261 patients with a median follow-up 1 year. Obes Surg. 2010;20:276–82.
Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–8.
Hady HR, Dadan J, Gołaszewski P, et al. Impact of laparoscopic sleeve gastrectomy on body mass index, ghrelin, insulin and lipid levels in 100 obese patients. Wideochir Inne Tech Malo Inwazyjne. 2012;7:251–9.
Melissas J, Koukouraki S, Askoxylakis J, et al. Sleeve gastrectomy: a restrictive procedure ? Obes Surg. 2007;17:57–62.
Bended N, Livshitz G, Mindlin L. Gastric emptying half-time following silastic ring vertical gastroplasty: a scintigraphic study (preliminary results). Obes Surg. 1996;6:459–62.
Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy–volume and pressure assessment. Obes Surg. 2008;18:1083–8.
Behrns KE, Soper NJ, Sarr MG, et al. Anatomic, motor, and clinical assessment of vertical banded gastroplasty. Gastroenterology. 1989;97:91–7.
Melissas J, Daskalakis M, Koukouraki S, et al. Sleeve gastrectomy-a “food limiting” operation. Obes Surg. 2008;18:1251–6.
Laeter CZ, Yeh MM, Van Rooyen DM, et al. Peroxisomeproliferator-activated receptor-α agonist, Wy 14 643, improves metabolic indices, steatosis and ballooning in diabetic mice with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2012;27:341–50.
Abdelmegeed MA, Yoo SH, Henderson LE, et al. PPARα expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutl. 2011;141:603–10.
Coelho DF, Gualano B, Artioli GG, et al. Exercise training attenuates lipectomy-induced impaired glucose tolerance in rats. Endocr Regul. 2009;43:107–16.
Costa SD, Rohden F, Hammes TO, et al. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARγ1-3 mRNA expression in human visceral adipocytes. Obes Surg. 2011;21:356–61.
Brody F, Hill S, Celenski S, et al. Expression of ectonucleotide pyrophosphate phosphodiesterase and peroxisome proliferator activated receptor gamma in morbidly obese patients. Surg Endosc. 2007;21:941–4.
Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6.
Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolodinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–262.
Maeda N, Takahashi M, Funahashi T, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9.
Combs TP, Wagner JA, Berger J, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007.
Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7.
Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8.
Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95.
Combs TP, Pajvani UB, Berg AH, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–83.
Maida A, Lamont BJ, Cao X, et al. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia. 2011;54:339–49.
Naitoh R, Miyawaki K, Harada N, et al. Inhibition of GIP signaling modulates adiponectin levels under high-fat diet in mice. Biochem Biophys Res Commun. 2008;376:21–5.
Masaki T, Chiba S, Yasuda T, et al. Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice. Diabetes. 2003;52:2266–73.
Conflicts of Interest
The authors declare they have no conflicts of interests, and no sources of funding or material support were provided for the performance of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawano, Y., Ohta, M., Hirashita, T. et al. Effects of Sleeve Gastrectomy on Lipid Metabolism in an Obese Diabetic Rat Model. OBES SURG 23, 1947–1956 (2013). https://doi.org/10.1007/s11695-013-1035-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-1035-7