Abstract
Background
Aiming to clarify the mechanism of weight loss after the restrictive bariatric procedure of sleeve gastrectomy (LSG), the volumes and pressures of the stomach, of the removed part, and of the remaining sleeve were measured in 20 morbidly obese patients.
Methods
The technique used consisted of occlusion of the pylorus with a laparoscopic clamp and of the gastroesophageal junction with a special orogastric tube connected to a manometer. Instillation of methylene-blue-colored saline via the tube was continued until the intraluminal pressure increased sharply, or the inflated stomach reached 2,000 cc. After recording of measurements, LSG was performed.
Results
Mean volume of the entire stomach was 1,553 cc (600–2,000 cc) and that of the sleeve 129 cc (90–220 cc), i.e., 10% (4–17%) and that of the removed stomach was 795 cc (400–1,500 cc). The mean basal intragastric pressure of the whole stomach after insufflations of the abdominal cavity with CO2 to 15 mmHg was 19 mmHg (11–26 mmHg); after occlusion and filling with saline it was 34 mmHg (21–45 mmHg). In the sleeved stomach, mean basal pressure was similar 18 mmHg (6–28 mmHg); when filled with saline, pressure rose to 43 mmHg (32–58 mmHg). The removed stomach had a mean pressure of 26 mmHg (12–47 mmHg). There were no postoperative complications and no mortality.
Conclusions
The notably higher pressure in the sleeve, reflecting its markedly lesser distensibility compared to that of the whole stomach and of the removed fundus, indicates that this may be an important element in the mechanism of weight loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleeve gastrectomy (SG), a new procedure for weight loss, was initially devised to constitute the first stage of bariatric surgery for the superobese or high-risk patient. The intention was to achieve a significant weight loss that would reduce the perioperative risks prior to the performance of a more extensive mixed restrictive and malabsorptive operation [1, 2]. Recently, some surgeons have begun to perform LSG as a sole procedure not only for superobese patients but also for those with a BMI of less than 50 [3, 4]. The bariatric concept for this operation is to minimize the capacity of the stomach by resection. Yet to be determined, however, are the intraluminal pressure and volume of the remaining stomach. The report of Han et al. [5] is the first to mention sleeve volume, found by them to be 50–60 cc; however, they measured the volume by instilling saline through a gastric tube without controlling the drainage or the pressure inside the gastric lumen. In their more recent report, Weiner et al. [6] noted that the measurement of sleeve volume by the application of fluids is technically difficult, and they therefore calculated the sleeve volume by measurement of the total volume of the stomach and the volume of the removed stomach.
The mechanism responsible for the early satiety following sleeve gastrectomy is still unclear. A number of factors may be involved: alterations in hormonal levels, impaired gastric motility of the remaining stomach, or elevated pressure within the sleeve.
The present study was therefore undertaken to investigate the pressure and the volume of the sleeve following laparoscopic sleeve gastrectomy in relationship to the entire stomach and the removed fundus.
Materials and Methods
Participants
Enrolled in this prospective noncontrolled study initiated in October 2007 were 20 consecutive morbidly obese patients scheduled to undergo LSG, all of whom had given their informed consent. Their demographic data including BMI and comorbidities are presented in Table 1.
All of the patients met the standard criteria for bariatric surgery, having a BMI of 35–40 kg/m2 and obesity-related comorbidities or a BMI of >40 kg/m2 irrespective of the presence or absence of comorbid conditions. Excluded were individuals having previously undergone bariatric surgery, as well as those with chronic liver disease, chronic renal disease, or heart failure. The preoperative work-up included blood tests, chest radiography, electrocardiogram, abdominal ultrasound and endocrinological, breath test for detection of Helicobacter pylori, nutritional, and psychological/psychiatric evaluation.
Operative Procedure and Measurements of Volume and Pressure
Under general anesthesia, all the patients were placed in the lithotomic position. A gastric calibration tube (Ethicon Endo-Surgery, Cincinnati, OH, USA) with a 10-cc balloon for measuring the basal pressure of the empty stomach was inserted. Pneumoperitoneum was induced with CO2 using a Veres needle and maintained at a pressure of 15 mmHg. Five trocars were inserted into the peritoneal cavity [4].
Measuring of volume and pressure was performed in three stages: (1) in the entire occluded stomach, (2) in the removed gastric fundus, and (3) in the remaining “sleeve” after LSG.
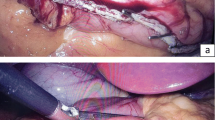
The pylorus was occluded with an atraumatic laparoscopic clamp. An orogastric tube with an inflatable 10-cc balloon was used to occlude the GE junction and also for instillation of methylene-blue-colored saline. The volume of the calibrating tube (20 cc) was added to the total volume measured. The tube was connected to a manometry device. The entire stomach was filled with the saline and the volume and pressure were recorded sequentially. The infusion of saline into the stomach was continued until the intraluminal pressure increased sharply, or the inflated stomach reached 2,000 cc and blocked the operating field.
After completion of the measurements, the Harmonic Scalpel™ (Ethicon Endo-Surgery) was used to open the gastrocolic ligament adjacent to the stomach, starting 6 cm from the pylorus. The greater curvature of the stomach was freed up to the cardio-esophageal junction. A 50-Fr orogastric tube was then inserted by the anesthesiologist into the stomach and directed towards the pylorus. The gastrectomy was performed using a laparoscopic linear stapler–cutter device (Endocatter, Endopath, Ethicon Endo-Surgery) with one 4.1 mm green load firings for the antrum, followed by five to seven sequential 3.5 mm blue loads for the remaining gastric corpus and fundus. The staple line was reinforced with an absorbable running suture. The resected part of the stomach was removed from the peritoneal cavity and the wounds closed.
The volume and pressure of the remaining stomach (the sleeve) were determined by the same method as that used for the whole stomach, and a drain was placed in the left subdiaphragmatic space. The volume and pressure of the resected part of the stomach (after it was removed) were determined using the same method. The resected specimen was sent for pathological evaluation.
In the first six patients, the measurements of the volume of the stomach, the sleeve and the remnant were recorded only at the beginning and at the end of the infusion of the saline, whereas in the next 14 patients pressure and volume determinations were made at several points along the inflation maneuver for greater precision.
On the second postoperative day, radiological examination was carried out by swallowing water-soluble contrast medium. This was done to check for any postoperative leakage from the suture line and also to measure the volume of the gastric sleeve and compare it to the volume measurement obtained during operation. After the initial three patients had undergone radiological examination, it was judged to be an unreliable test for volume determination of the sleeve in view of the technical difficulties encountered, and its inaccuracy resulting from the immediate flow into the duodenum.
Statistical Analysis
The study protocol was approved by the local Ethical Committee. Results are expressed as mean ± standard deviation. A P value <0.05 considered to be significant. Correlations between variables were determined by Spearman’s correlation coefficient (Table 2).
Results
The volume and pressure assessment of the 20 patients enrolled in the study is presented in Figs. 1, 2 and 3. These figures demonstrate the positive correlation found between these variables, which reflect the distensibility, as also appeared in Table 2.
Due to the technical difficulties encountered in securing complete occlusion of the gastro-esophageal junction and the pylorus, three of the 20 patients had to be withdrawn from analysis in the initial stage because of leakage. Figure 1 demonstrates the relationship between the pressure and the volume in the entire stomach in the 17 patients, where any additional volume resulted in a relatively insignificant increase in pressure (R = 0.46, P < 0.1). The mean end volume was 1,553 ± 472 cc, and the mean pressure at that point was 34 ± 6 mmHg.
As shown in Fig. 2, the changes in pressure and volume of the removed portion of the stomach were similar to those in the intact stomach. Here too a small addition in volume resulted in only a small insignificant increase in pressure (R = 0.48, P < 0.1): the mean end volume was 795 ± 251 cc, and the mean pressure at that point was 26 ± 12 mmHg. Infusion of colored saline into the removed stomach was continued until the appearance of leakage through the staple line. This figure shows the data of 15 of the 17 patients enrolled in the study (in two cases, it proved technically impossible to inflate the removed stomach in order to evaluate the volume and the pressure, due to disruption of the suture line).
Figure 3 demonstrates the behavior of the sleeve. In contrast to the intact stomach or resected portion, in the sleeve, a small addition in volume resulted in a much earlier significant elevation of the pressure (R = 0.73, P < 0.005). The mean end volume was 129 ± 35 cc, and the mean pressure at that point was 43 ± 7 mmHg. This figure shows the data of 14 of the 17 patients enrolled in the study (three patients were excluded from the study due to leakage of fluids through the duodenum and the esophagus).
Table 3 presents the average value of the volume and the pressure in the entire stomach, removed part and the sleeve. One can see that in addition to the high pressure, the volume of the sleeve (129 ± 35 cc) is somewhat less than 10% of the mean volume of the entire stomach (1553 ± 472 cc).
The histology of the resected stomach was normal in ten patients. Chronic gastritis was present in nine patients, and in one 31-year-old patient, the histologic finding was surprisingly MALT lymphoma, found to be cured by the operation. Helicobacter pylori was found incidentally in four patients and treated before surgery. There were no postoperative complications such as hemorrhage from the staple line or leakage, no stenosis, and no mortality.
Discussion
The primary motor function of the stomach is to receive, store and prepare food for digestion. This task is made possible by the accommodation reflex, which through active relaxation of the gastric fundus allows for a volume increase without a rise in intragastric pressure (IP) and thus enables the stomach to accommodate large volumes during food intake [7–9]. The stomach consists of the following functional parts: two valves (lower esophageal sphincter and pylorus), antrum (pumping mechanism) and the corpus or fundus (reservoir). The fundus is the most easily expanded part of the reservoir with only two thin layers of muscle [6]. Our measurements of the volume and pressure of the whole stomach, of the remaining sleeve, and of the resected portion, clearly demonstrated that the part removed is indeed the most expansible part of the stomach. The distensibility of the total stomach and of the excised fundus was found to be tenfold higher than that of the sleeve (Table 2). The volume of the sleeve is less than 10% of the volume of the total stomach. These findings constitute the first reported evidence that it is the distensible region of the stomach that is removed in this operation.
It is more than likely that the high intraluminal pressure resulting from the relatively small volume and markedly lesser distensibility of the sleeve is responsible for the early satiety following LSG. Indeed, our clinical experience would appear to support this assumption: many of our patients have reported that following the operation they experienced a feeling of fullness even after ingestion of a very small amount of food. Thus, it would appear that the size of the gastric sleeve is an important factor in achieving long-term success with this operation. Prevention of segmental dilatation may also contribute to its long-term success. It would therefore appear to be of importance to make the sleeve as narrow as possible, ensuring the complete removal of the fundus, which has the greatest potential for later dilatation.
It is of interest that, in contrast to previous reports [6], the data of our patients clearly show that the total gastric volume cannot be calculated by adding that of the sleeve to that of the removed fundus. Since the gastric distensibility differs in each part of the stomach, the volume of the sleeve added to that of the removed part is not the total gastric volume when measured under the same pressure. In this respect, the estimates of sleeve volume reported by Han et al. [5] and obtained by the infusion of saline via a nasogastric tube, without either clamping the pylorus or obstruction of the gastro-esophageal junction and without controlling the pressure, are likely to be inaccurate. On the other hand, Braghetto et al. [10] measured the volume of the sleeve and of the resected stomach by instillation of saline with methylene blue through a nasogastric tube. Similar to our technique, they transiently blocked the flow into the duodenum by occlusion of the pylorus with an atraumatic laparoscopic clamp, but neither occluded the GE junction to prevent flow into the esophagus nor controlled the infusion by pressure.
The present study reports for the first time a reproducible and a reliable technique that enables us to measure precisely the volume and the pressure of the different segments of the stomach. Important features of this technique are complete occlusion of the pylorus and the gastro-esophageal junction as well as infusion with colored saline to facilitate diagnosis of a leakage. Moreover, the pressure measurement apparatus is connected to the infusion set for controlling the volume determination. In studies involving organs of varying distensibility, the measurement of the volume must be measured in relation to or controlled by pressure.
The findings in this study strongly suggest that the mechanism of weight loss following the sleeve gastrectomy procedure is due mainly to a restricted calorie intake, which results from the combination of the small capacity, low distensibility of the sleeve and the resultant immediate high intraluminal pressure. There are, however, other mechanisms that must be taken into consideration, such as hormonal changes. In recent years there have been a few reports, based on small series, which have shown that after LSG there is a decrease in levels of ghrelin, an appetite-stimulating hormone produced in the gastric fundus [11, 12]. Furthermore, in their report of a prospective double-blind study comparing the Roux-en-Y gastric bypass (LRYGBP) and LSG, Karamanakos et al. [13] suggest that LSG is superior to LRYGBP not only because of volume restriction but also due to the markedly increased fasting and postprandial levels of PYY, a hormone inducing a feeling of satiety, as well as to the significantly decreased fasting and postprandial ghrelin levels.
Our study has provided important basic information but there remain several aspects of the early satiety achieved by LSG that remain to be clarified. It is possible that this satiety is due not only to the high intraluminal pressure but conceivably could also express an effect on gastric motility as well as on levels of gastric hormones. A more comprehensive assessment would require data on postoperative motility and hormone levels. Further work in this field is needed to achieve a better understanding of this novel bariatric procedure.
References
Mognol P, Chosidow C, Marmuse JP. Laparoscopic sleeve gastrectomy as an initial bariatric operation for high-risk patients: initial results in 10 patients. Obes Surg. 2005;15:1030–3.
Cottam D, Qureshi FG, Mattar G, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859–63.
Roa PA, Kaidar-Person O, Pinto D, et al. Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes Surg. 2006;16:1323–6.
Givon-Madhala O, Spector R, Wasserberg N, et al. Technical aspects of laparoscopic sleeve gastrectomy in 25 morbidly obese patients. Obes Surg. 2007;17:722–7.
Han SM, Kim WW, Oh JH. Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients. Obes Surg. 2005;15:1469–75.
Weiner RA, Weiner S, Pomhoff I, et al. Laparoscopic sleeve gastrectomy—influence of sleeve size and resected gastric volume. Obes Surg. 2007;17:1297–305.
Mayer EA. The physiology of gastric storage and emptying. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 3rd ed. New York, USA: Raven Press; 1994. p. 929–76.
Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504:479–88.
Piessevaux H, Coulie B, Caenepeel P, et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–52.
Braghetto I, Korn O, Valladares H, et al. Laparoscopic sleeve gastrectomy: surgical technique, indications and clinical results. Obes Surg. 2007;17:1442–50.
Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–9.
Kotidis EV, Koliakos G, Baltzopoulos VG, et al. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—a prospective study. Obes Surg. 2006;16:1425–32.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg. 2008;247:401–7.
Acknowledgment
The authors thank Nahum Beglaibter M.D for fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yehoshua, R.T., Eidelman, L.A., Stein, M. et al. Laparoscopic Sleeve Gastrectomy—Volume and Pressure Assessment. OBES SURG 18, 1083–1088 (2008). https://doi.org/10.1007/s11695-008-9576-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9576-x