Abstract
Background
In South Korea, the number of severely obese patients has increased. An economic study comparing bariatric surgery with nonsurgical interventions has not been published for Asia.
Objectives
This study was conducted to evaluate the cost effectiveness of bariatric surgery as compared to nonsurgical interventions for severe obese Korean people.
Methods
We used the Markov model to compare the lifetime expected costs and quality-adjusted life years (QALYs) between bariatric surgery and nonsurgical interventions from Korean Healthcare system perspectives. Our target cohort consisted of severe obese people defined as having a body mass index of 30–<40 kg/m2 in South Korea. The starting age of the cohort was 30 years old, and the cycle length was 1 year. Nonsurgical interventions included a physician visit, exercise, diet, and pharmacotherapy. A discount of 5 % was applied in cost and QALY. The incremental cost-effectiveness ratio (ICER) of bariatric surgery compared to nonsurgery interventions was calculated.
Results
The cost-utility analysis study indicated that bariatric surgery had US$1,522 incremental costs and 0.86 incremental QALYs as compared to nonsurgical interventions. Through the base case analysis, ICER was US$1,771/QALY. The sensitivity analyses were performed using a variety of assumptions, and the robustness of the study results was also demonstrated.
Conclusion
The study indicated that bariatric surgery was a cost-effective alternative to nonsurgical interventions over a lifetime, providing substantial lifetime benefits for severely obese Korean people.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In South Korea, the number of adult patients with severe obesity (body mass index ≥30 kg/m2) increased about 1.5 times from 2.4 % in 1998 to 3.9 % in 2007–2009 according to the Korean National Health and Nutrition Examination Survey IV (KNHANES IV) data, a national representative survey [1]. Severe obesity is defined as a body mass index (BMI) of ≥35 kg/m2 in Western countries, but according to Asian guidelines, the severely obese have a BMI of ≥30 kg/m2 [2, 3]. A different guideline by ethnicity is a cause for more vulnerability for obesity related disease in Asians as compared to Western persons. Generally, Asians have a higher prevalence for diabetes, hypertension, and dyslipidemia at a lower BMI in comparison to Western people [4].
In addition, the societal costs of obesity are substantial in South Korea. It estimated at about 1.8 billion, which is 3.7 % of health care costs and 0.22 % of the gross domestic product (GDP) [5], even though the prevalence is lower, with severe obesity (4 %) as compared with Western results (30 %) [1, 6].
Bariatric surgery was a very popular option for Western obese subjects as noted in the results of the National Institute of Health [7], which resulted in 220,000 subjects who underwent bariatric surgery in the USA in 2008 [8]. Asians including South Koreans have a very short history with bariatric surgery, and relatively few subjects have undergone bariatric surgery. In South Korea, the number of subjects who underwent bariatric surgery increased by 622 % in 7 years (from 125 in 2003 to 778 in 2009) [9]. Thus far, there has been relatively little data for the efficacy and safety of this surgery for Asians [10, 11]. Furthermore, there are numerous economic evaluations about bariatric surgery for procedures performed in Western countries, but no study exists in Asia, in particular Korea.
Thus, the present study aimed to evaluate the cost effectiveness of bariatric surgery, which is comprised of laparoscopic adjustable gastric banding (LAGB), laparoscopic Roux-en-Y gastric bypass (LRYGB), and laparoscopic sleeve gastrectomy (LSG), to nonsurgical interventions in severe obese Korean people.
Methods
Framework of Economic Model
We conducted a cost-utility analysis using lifetime expected costs and quality-adjusted life years (QALYs) that compared bariatric surgery with nonsurgical interventions. The Korean Healthcare system perspectives, which consider only medical expenditure, were applied for this study. The surgery group included adults who underwent bariatric surgery (LAGB, LRYGB, and LSG); these three types of surgery were almost 90 % of all bariatric surgeries in South Korea [9]. The nonsurgical intervention group included patients who were treated by conventional therapy such as pharmacotherapy and lifestyle modification therapy (exercise, diet, and etc.) at medical centers. Our target cohort consisted of severe obese people defined as having a BMI of 30–<40 kg/m2 with the starting age of 30 years in the model.
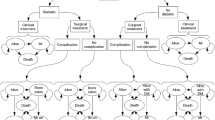
The model was a combined decision tree and Markov process model (Fig. 1). In the decision tree model, severe obese patients were treated with bariatric surgery or a conventional intervention, and then followed for 1 year. In the surgery arm, patients underwent one of three types of obesity surgery, and they could either die due to surgery complication or survive. Some patients among the surviving patients may have required corrective surgery within a year of the initial surgery. For the second year, all patients moved into the Markov transition model with a lifetime horizon.
The health status was comprised of five states such as no comorbidity, mild/moderate comorbidity (diabetes and/or hypertension and/or, dyslipidemia), severe comorbidity (myocardial infarction and/or ischemic heart disease, and/or stroke), death due to coronary vascular disease (CVD), and death due to other cause. In this study, the comorbidities related to obesity included diabetes, hypertension, dyslipidemia, myocardial infarction (MI), ischemic heart disease (IHD), and stroke according to the reference of Korean Obesity Treatment Guideline 2010 (level I, grade A) and previous studies [3, 12–16]. We assumed that a patient could simultaneously experience a maximum of three kinds of obesity-related comorbidities according to the severity of the comorbidity. Throughout the period, patients could die of an age-specific death or obesity comorbidity-related death during their lifetime. The cycle length was 1 year. The incremental cost per the additional QALY gained of bariatric surgery compared to nonsurgery interventions was calculated.
The study was approved by the Investigation Review Board of each hospitals and National Evidence-based Healthcare Agency (NECA).
Weight Change of Obese People
The weight change over time after surgery or a nonsurgical intervention was obtained from medical chart review from eight medical centers [19]. Between 9 and 15 months after the intervention, the mean BMI of the surgery group and nonsurgery group was 26.39 kg/m2 (i.e., state of obesity according to Korean guideline) and 31.74 kg/m2 (i.e., state of severe obesity), respectively. In the Swedish Obese Subject (SOS) study, a mean weight change percentage from baseline was observed for over 20 years [15]. The SOS study showed that the change in weight was the biggest at 1 year, but it increased slightly and was maintained until 20 years after first year. Thus, the present study assumed that the surgery group remained obese, while the nonsurgery group and the band removal after surgery remained morbidly obese. Revision surgeries such as sleeve gastrectomy or gastric bypass after band removal were not considered in our model. In addition, it was supposed that the people who got the reoperation due to the complication after bariatric surgery or sleeve gastrectomy remained obese. We assumed that the probability of having obesity-related comorbidities is different whether patients were obese or severely obese.
Health-Related Quality of Life and Clinical Data
Model inputs including clinical data, transition probability of comorbidities, and health-related quality of life are presented in Table 1.
In the first year, the distribution of surgery type and the reoperation rate were taken from a representative population survey [9]. The probabilities of death during the operation, band removal, and reoperation were obtained from a medical chart review of eight tertiary hospitals [17]. The transition probability of comorbidities and the utility weight for each obese status was obtained gain using KNHANES IV (2007–2009) data [1]. This survey included information on BMI, comorbidities, and utility weights of EuroQol-5 dimension (EQ-5D) in the personal level. Using these data, the transition probabilities of comorbidities for the severe obese status (i.e., BMI of 30–<40 kg/m2) were calculated using a logistic regression. Severe obese people had a 2.467 and 1.429 odds ratio for a mild/moderate comorbidity (MC) and severe comorbidity (SC) when compared with obese people (BMI of 25–<30 kg/m2) after an adjustment for age. The transition probabilities of MC or SC in severe obese people were calculated by multiplying the probabilities of MC or SC in obese people in a given age by odds ratio. For example, the probability of MC in obese people aged 30–39 years old was 0.058. The probability of MC in severe obese people aged 30–39 years old was calculated by multiplying 0.058 by 1.429.
We calculated the utility weight for an obese and severely obese state using tobit regression, which is generally used for the analysis of utility weight [18, 19]. Obese patients had utility weights of 0.901, and the utility weights for severe obese patients were calculated by subtracting 0.029 from the utility weights of obese patients in KNHANES IV data [1]. Likewise, utility weights for MC and SC were obtained by subtracting 0.012 and 0.123 from those of noncomorbid patients.
EQ-5D was developed by EuroQol Group for estimating the health states as general instrument [20]. The five dimensions consist of mobility, self-care, usual activity, pain/comfort, and anxiety/depression. Each dimension was answered with three levels of response, and total scores had range from 0 (death) to 100 (perfect health) using the tariff of Lee et al. [21, 22].
The rate of death due to CVD and other causes were calculated in the Complete Life Table in 2010 published by the Korean statistical office [23]. Death due to CVD included death due to CHD or stroke.
Costs
The cost data were collected from the survey of bariatric surgeons and family medicine doctors of nine medical centers and the Korean National Health Insurance Statistics (Table 2) [17, 24]. The estimates for the first year time horizon were based on health resource use and cost data from a survey of physicians from nine medical centers. The direct medical costs of the surgery group were associated with the initial operation of three types of surgery, band removal related operation, reoperation, and a regular checkup for 1 year. Nonsurgical interventions, costs for physician visits, counseling for exercise and diet, and pharmacotherapy were considered for 1 year. After 1 year, the medical costs related with weight control were not applied.
The costs of comorbidities were considered when they moved to the Markov model for the second year. The costs of comorbidities were obtained from the Korean National Health Insurance Statistics [24]. The cost of MC and SC comorbidities were considered to be the number of concomitant diseases based on KNHANES IV data [1]. For example, severe obese people had more of a possibility of having diabetes, hypertension, and hyperlipidemia as compared to obese people. In other words, the cost for MC of severe obese people is higher than MC of obese people. The costs estimates are based on 2011 and adjusted for inflation using the Health Care Component of the Consumer Price Index for Korea, when necessary [25].
Cost-Effectiveness Analysis
The main result of the present study was an incremental cost-effectiveness ratio (ICER) of bariatric surgery compared with conventional therapy. The ICER was calculated by dividing the incremental costs (i.e., difference in costs between two treatments) by incremental QALY. In other words, an ICER can be explained as the additional costs to be paid in achieving the additional effectiveness of target treatment with well-established previous treatment. In the base case, analysis was determined to represent our best scenario from literature and discussions with clinical and methodological experts. A discount of 5 % was applied in cost and QALY according to Korean pharmacoeconomic guideline [26].
Sensitivity Analysis
We conducted one-way and probabilistic sensitivity analyses to examine the impact of a variation in parameters on the resulting ICER. Through one-way sensitivity analysis, the impact of 1-year costs, utility weight, transition probabilities, discount rate, and time horizon to see the weight control effects were investigated. To quantify the comprehensive effect of uncertainty, a probabilistic sensitivity analysis was tested using a Monte Carlo simulation. The simulation was recalculated 10,000 times, where each of the parameter estimates was sampled from its distribution for each model recalculation. Triangular distributions were used for utility weights and transition probabilities. Normal distributions were used for cost data because the present study estimated the costs from the survey. The parameter values and their ranges used in the sensitivity analysis are shown in Table 1. The model was programmed in TreeAge Pro 2009 (TreeAge Software Inc., Williamstown, MA, USA).
Results
Base-Case Analysis
The cost-utility study indicated that bariatric surgery was more effective and more costly compared with non-surgery treatment for severe obesity in South Korea. Bariatric surgery had incremental costs of US$1,522 and Incremental QALYs of 0.86 when compared with non-surgical interventions over lifetime horizon (Table 3). Through the base case analysis, ICER was US$ 1,771/QALY.
Sensitivity Analysis
The sensitivity analyses were performed using a variety of, and robustness of the study results were also demonstrated (Table 3). In the aspects of cost, two one-way sensitivity analyses were conducted. First, continuous treatments when patients remained in a severely obese status in the nonsurgical intervention group make surgery dominant option. Second, when costs of MC or SC in obese (i.e., surgery group) and severe obese (i.e., nonsurgery intervention group) status were applied in equal amounts, not considering the difference of the number of comorbidities between obese and severe obese status, ICER was about US$5,000/QALY.
Regarding utility weight, when we applied larger difference between obese and severe obese status than base-case (0.085 vs. 0.029) based on utility value by BMI level in the literature [27], ICER increased by US$832/QALY. When the transition probability of MC and SC in obese and severe obese people was changed into a lower and upper level, ICER was slightly decreased or increased (US$1,526/QALY or US$2,707/QALY).
Bariatric surgery was dominance when discount rates of 0 and 3 % were applied. However, surgery had reduced incremental QALY and increased ICER (US$17.639/QALY) when the time horizon reduced by 15 years. ICER was smaller as the time horizon expands. While ICER was US$3,639/QALY when the starting age of cohort was 20 years old, surgery became the dominant alternative when the starting age was 40 years old.
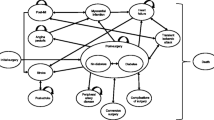
The tornado diagram that presents a one-way sensitivity analysis results indicated that time horizon and discount rate were important parameters influencing ICER value (Fig. 2). The probabilistic sensitivity analysis results showed that surgery was a cost-effective alternative as compared to non-surgery treatment if a willingness to pay was more than about US$200 (Fig. 3).
Discussion
Through the cost-utility analysis, ICER of bariatric surgery compared with nonsurgery treatment was US$1,771/QALY, suggesting that bariatric surgery was cost-effective option when considered the threshold of ICER in South Korea. The threshold of ICER in South Korea was not officially determined, but a guideline of cost effectiveness by WHO that recommended the one to three times the GDP per capital [28]. Therefore, it was US$20,000 conservatively because the GDP per capital was US$21,539 in South Korea in 2011, and the recent research also found approximately US$19,000 as the threshold of ICER in a South Korean representative population [29].
Our study results were likely to be consistent with previous studies which demonstrated cost effectiveness in Western countries. In USA, Crag and colleagues performed a cost-effectiveness analysis of gastric bypass in the treatment of morbid obese patients (BMI, 40–45 kg/m2) from payer perspective compared with no treatment [13]. As a result, gastric bypass was not cost-saving but a cost-effective alternative over a lifetime. The ICERs were respectively US$5,000–16,000/QALY and US$10,000–35,600/QALY for woman and men, depending on age and initial BMI. According to the type of surgery, the ICERs of LRYGB and LAGB were respectively US$14,680–18,543/QALY and US$8,878–11,604/QALY in the treatment of morbid obese patients (BMI, 40 kg/m2) compared with nonsurgical intervention [14]. Studies performed in Europe present that bariatric surgery is more preferable in terms of cost effectiveness from a payer perspective. The LRYGB and LAGB were a dominant alternative in Germany and France and cost-effective alternatives (₤1,517/QALY and ₤1,929/QALY in LRYGB and LAGB) in UK compared with conventional treatment in severe obese patients (BMI, ≥35 kg/m2) with type II diabetes over the 5 years [30]. In Finland, bariatric surgery was strong dominant compared with ordinary treatment from a healthcare provider’s perspective with a time horizon of 10 years [31].
Even though bariatric surgery is a cost-effective option for severely obese patients, the result of cost effectiveness is slightly different among countries. It is already known that a cost-effectiveness analysis is affected by disease epidemiology, availability of healthcare resources, variation in clinical practice, and relative price or costs, which is challengeable to generalize study results across countries [32].
Nonetheless, there are some explanations for the different value for ICERs for further understanding. A US study had a higher ICER than other studies (i.e., EU studies or our study) [30]. This may be explained by the following reasons. Subjects in the USA were severely obese without chronic diseases, and the benefits of weight loss in the USA seems to be smaller than our and EU studies. In addition, the cost for bariatric surgery was higher in the USA. Generally, the USA had much higher medical costs [33]. The difference of cost between bariatric surgery and nonsurgical treatment was much greater in the USA (i.e., about US$15,000–30,000 in USA vs. US$1,500 in Korea).
Although France, Germany, and UK applied the same QALY in the study of Ackroyd et al. [30], the different cost system among countries resulted in the various results of ICER. Whereas bariatric surgery in France and Germany is cost saving with a higher QALY than conventional therapy, the UK had about ₤1,517/QALY. The difference of costs among the three countries was caused by the different payment systems. While France and Germany used a diagnosis-related group (DRG) to calculate bariatric surgery costs, the UK used micro-costing method.
In Finland, bariatric surgery was a dominant option because the cost of nonsurgery treatment was considered over 10 years [31]. The difference in the result was caused due to the cost of nonsurgical treatment was conservatively considered for only first year in our model. When patients with severe obesity in nonsurgical intervention applied continuous treatment in a sensitivity analysis, surgery was dominant.
In the present study, the combined model of the decision tree and Markov model has the strength to be able to reflect at once the effects due to various comorbidities and treatment pathway in surgery or conventional therapy option. Generally, a previous model considered to limit single comorbidity in one state [12, 34, 35]. For example, comorbidities were distinguished as two states such as moderate and severe state, but they did not consider a mixture of comorbidities (i.e., hypertension, diabetes, and dyslipidemia). In our study, the percentage of hypertension, diabetes, and dyslipidemia were different by the state of obesity using the national representative data [1]. While patients with severe obesity (BMI>30 kg/m2) had 88.3 % hypertension, 32.4 % diabetes, and 22.3 % dyslipidemia, patients with obesity (25 kg/m2≤BMI<30 kg/m2) had 82.3 % hypertension, 29.7 % diabetes, and 20.8 % dyslipidemia. Thus, the present model applied the mixture of diabetes and/or hypertension and/or dyslipidemia in MC, MI, and/or IHD and/or stroke in SC. Thus,this model could estimate reliable results reflecting the best clinical reality.
In addition, it is the key achievement of this study estimating the cost effectiveness of bariatric surgery in the Asian population. The cost effectiveness of bariatric surgery in patients with severe obesity (BMI, ≥35 kg/m2) is known in USA or Europe, and not well known in Asia (BMI≥30 kg/m2). Until now obesity, was considered a disease of the West, but recently, its occurrence is an increasing issue for obese patients in Asia. Thus, it is necessary to identify cost effectiveness of bariatric surgery of obese people in Asia. In that regard, the present study is able to make evidence in treatment of severe obese patients in Asia.
This study has some limitations. First, we used a cross-sectional national representative data (i.e., KNHANES) to calculate the utility weight and prevalence of concomitant disease based on a BMI level. However, subjects with a BMI of 30–<40 kg/m2 were relatively small (about 600 subjects, 3.5 %) in this database. Those values for utility weight and the prevalence of concomitant disease of severe obese people may not be representative for subjects who have a BMI of 30–<40 kg/m2 due to small sample size. Furthermore, we could not consider the impact of high BMI during lifetime because we used cross sectional data. Younger people had a higher BMI than the elderly due to birth cohort effects. Younger birth cohorts (i.e., people who were born late) had a higher BMI than older birth cohorts (i.e., people who were born early) [36]. Elderly people may have more concomitant disease even though they have lower BMI level, and we used multivariable regression to see the association between obesity and comorbidity after an adjustment for confounding factors such as age. Multivariable logistic regression was used to calculate the prevalence of concomitant disease, and multivariable tobit model was used to calculate utility weight for the status of obesity and severe obesity. Second, we considered only limited concomitant diseases related with obesity. Obesity may be related with not only those diseases but also several other diseases such as asthma, several cancers, musculoskeletal diseases, and mental diseases [2, 3]. Furthermore, we did not consider nonmedical costs such as decreased productivity. Thus, these results may underestimate the cost of the economic benefit of bariatric surgery. Lastly, the base analysis performed during lifetime, under the benefit of weight reduction lasted lifetime in bariatric surgery in the present study. However, the weight reduction of surgery continues over 20 years in previous study [15]. Therefore, a sensitivity analysis conducted with a 20-year time horizon, additionally for 15, 30, and 40-year time horizon. When the time horizon is longer, bariatric surgery is a more cost-effectiveness alternative. It accounted for greater initial costs of bariatric surgery and can impact comorbidities over the lifetime. The improved comorbidities by surgery may reduce the lifetime medical costs to treat the comorbidities. However, if time horizon was applied for short-term period such as 5 or 10 years, the reduced costs due to the improved comorbidity may not be substantial.
Conclusions
The present study showed that bariatric surgery is an estimated cost-effectiveness alternative over a lifetime as compared to conventional therapy from a healthcare system perspective. Surgery was more costly than nonsurgery, but it reduced the morbidity and mortality associated with obesity and improved QALY. In conclusion, bariatric surgery of severe obese patients (BMI, ≥30 kg/m2) was cost effective in South Korea.
References
Korean National Health and Nutritional Examination Survey IV 2007–2009. Korean Ministry of Health and Welfare Affairs. 2010.
Obesity guidance in the prevention, identification, assessment and management of overweight and obesity in adults and children. National Institute for Health and Clinical Excellence. 2006.
Management of obesity, 2010 Recommendation. Endocrinol Metab. 2010;25:301–304.
Ramachandran A, Snehalatha C, Viswanathan V, et al. Risk of noninsulin dependent diabetes mellitus conferred by obesity and central adiposity in different ethnic groups: a comparative analysis between Asian Indians, Mexican Americans and Whites. Diabetes Res Clin Prac. 1997;36:121–5.
Kang JH, Jeong BG, Cho YG, et al. Socioeconomic costs of overweight and obesity in Korean Adults. J Korean Med Sci. 2011;26(12):1533–40.
Freedman DS. Obesity—United States, 1988–2008. MMWR Surveill Summ. 2011;60(01):73–7.
Gastrointestinal surgery for severe obesity Reference NIH conference. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–961.
Metabolic & bariatric surgery (fact sheet). American Society for Metabolic and Bariatric Surgery. 2011. http://www.asbs.org/Newsite07/media/asmbs_fs_surgery.pdf. Accessed 21 Nov 2012.
Lee SK. The current status of bariatric surgery in South Korea. Korean Society for the study of obesity 33th conference. Seoul, 2010.
Lomanto D, Lee WJ, Goel R, et al. Bariatric surgery in Asia in the last 5 years (2005–2009). Obes Surg. 2012;22(3):502–6.
Lee WJ, Wang W. Bariatric surgery: Asia-Pacific perspective. Obes Surg. 2005;15(6):751–7.
Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost—the clinical effectiveness and costeffectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technology Assessment 2009;13(41).
Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. Am J Med. 2002;113(6):491–8.
Salem L, Devlin A, Sullivan SD, et al. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis. 2008;4(1):26–32.
Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65.
Jeong BG, Moon OR, Kim NS, et al. Socioeconomic costs of obesity for Korean adults. Korean J Prev Med. 2002;35(1):1–12.
Heo YS, Kwon JW, Lee HJ, et al. The clinical effectiveness and economic analysis of bariatric surgery for severe obesity. National Evidence-based Collaborating Agency. 2011.
Petrie D, Doran C, Shakeshaft A, et al. The relationship between alcohol consumption and self-reported health status using the EQ5D: evidence from rural Australia. Soc Sci Med. 2008;67(11):1717–26.
Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20.
The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Hurst NP, Kind P, Ruta D, et al. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol. 1997;36(5):551–9.
Lee YK, Nam HS, Chuang LH, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value in Health. 2009;12(8):1187–93.
Complete Life Table 2010. Korean Statistical Office. 2010. Available at: http://kostat.go.kr Accessed 15 Sept 2012.
Korean national health insurance statistics. Health Insurance Review and Assessment. 2011.
Korean Statistical Information Service, Consumer Price Index in Healthcare. 2011. http://kosis.kr. Accessed 21 Nov 2012.
The guideline of Pharmacoeconomics in South Korea. Health Insurance Review and Assessment. 2011.
Hakim Z, Wolf A, Garrison LP. Estimating the effect of changes in body mass index on health state preferences. PharmacoEconomics. 2002;20(6):393–404.
Choosing interventions that are cost effective (WHO-CHOICE). World Health Organization. http://www.who.int/choice/costs/CER_thresholds/en/index.html. Accessed 21 Nov 2011
Ahn JH, Kim YH, Shin SJ, et al. Research on methodologies for evidence based healthcare decision making process in Korea. National Evidence-based Collaborating Agency. 2010.
Ackroyd R, Mouiel J, Chevallier JM, et al. Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes Surg. 2006;16(11):1488–503.
Mäklin S, Malmivaara A, Linna M, et al. Cost-utility of bariatric surgery for morbid obesity in Finland. Br J Surg. 2011;98(10):1422–9.
Drummond MF. Comparing cost-effectiveness across countries: the model of acid-related disease. PharmacoEconomics. 1994;5:60–7.
Mark W. Stanton MA. The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality Advancing Excellence in Health Care 2006;19.
Galani C, Al M, Schneider H, et al. Uncertainty in decision-making: value of additional information in the cost-effectiveness of lifestyle intervention in overweight and obese people. Value Health. 2008;11(3):424–34.
Trueman P, Haynes SM, Felicity LG, et al. Long-term cost-effectiveness of weight management in primary care. Int J Clin Pract. 2010;64(6):775–83.
Kwon JW, Song YM, Sung J, et al. Varying patterns of BMI increase in sex and birth cohorts of Korean adults. Obesity (Silver Spring). 2007;15(2):277–82.
Acknowledgments
This study was completed as part of the health technology assessment report (project no. NA2011-003) funded by the NECA in Korea. The results of this project underwent the appraisal process involving orthopedist, family medicine specialists, methodologists, and governmental officials. No other sources of funding were used to assist in the preparation of this article. Hyun Jin Song, Jin Won Kwon, Yong Jin Kim, Sung-Hee Oh, Yoonseok Heo, and Sang-Moon Han have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
H.J. Song and J.W. Kwon contributed equally to this work.
Rights and permissions
About this article
Cite this article
Song, H.J., Kwon, J.W., Kim, Y.J. et al. Bariatric Surgery for the Treatment of Severely Obese Patients in South Korea—Is it Cost Effective?. OBES SURG 23, 2058–2067 (2013). https://doi.org/10.1007/s11695-013-0971-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-0971-6