Abstract
The crosslink interaction and rheological properties of transglutaminase (TGas) on the soy protein isolate (SPI) and the effect on patty application were studied. The TGas concentration as 0, 1, 3, 5, or 10% without incubation, or incubation at 50 °C for 1 or 4 h were measured. The molecular size of the SPI tended to increase with TGas. However, there were no differences in the SDS-PAGE results between adding 5 or 10% TGas. The rheological properties of the SPI and TGas interaction showed shear-thinning behavior with n values less than 1. The increase of TGas concentration and incubation time increased the solid-like properties according to the storage modulus increased and tanδ decreased, especially for 5% TGas with 1 or 4 h incubation time. The plant-based patties composed of TGas at 0, 1, 3, or 5% and incubated at 25 °C/1 h, 25 °C/2 h, or 4 °C/24 h were investigated. The color changed with increasing TGas concentration and incubation time. The samples hardness tended to increase with higher TGas concentrations. Cohesiveness and springiness increased when incubated at 25 °C for 2 h, especially using 5% TGas. The rheological properties and the textural properties would be highly correlated with the concentration of TGas. The structural characteristics using confocal laser scanning microscopy showed the clustering of green colors representing protein components with 3 and 5% TGas addition that might have been due to the noticeable aggregation of the protein. Thus, the crosslinking between SPI and TGas affected rheological properties, texture and structure of the plant-based patties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global food crisis and food problems arising from climate change have increased every year. Alternative food choices to solve this problem include plant-based protein. The growth in demand for vegetarian and vegan food products has propelled the market size for vegan food to an estimated USD 19.7 billion in 2020 and it is expected to reach USD 36.3 billion by 2030 [1]. Soy protein, as a plant protein containing water-soluble and insoluble protein, has functional properties, including the gels produced having high water holding capacity, hydrophobic interaction, foamability, and surface elastic behavior [2]. Soy protein isolates have been used as ingredients for meat analogs, such as sausage, burger, or meat muscle, according to their functional ingredients and the production process [2]. Textured vegetable proteins (TVP) products have been transformed into a meat-like texture by using the extrusion technique, commonly utilizing soy protein to which provides chewiness and is fibrous and similar to real-meat texture [3]. However, in the production of sausage or burger, the protein needs to be mixed with other ingredients, including an emulsion phase, in which the protein isolate or modified starch is coalesced into patties.
Transglutaminase (TGas) is an enzyme widely used for crosslinking protein through an acyl-transfer reaction, leading to building an ε-(γ-glutamyl) lysine isopeptide bond [4]. The interaction of TGas might affect the rheological properties and physical properties in a product application through the cross-linking causing protein modification due to the interaction between an amino group on the protein bound lysine residues and a carboxyamide group on the protein-bound glutamine residues [5]. In applications, many products have used TGas to improve the properties (due to their crosslinking network inside the TGas matrix) of protein substrates such as egg proteins, fish proteins, pea proteins, and soy proteins [4, 6, 7]. TGas has been used in many food industries such as meat, cheese, yogurt, or bread [8, 9]. TGas could improve the gel network structure of myofibrillar protein by increasing the water holding capacity of the gel [10]. Wang et al. [11] reported the oat noodles contained proteins and TGas could decrease cooking loss and increase elasticity. TGase could also restructure meat by binding together fragments or small pieces of fish or meat through the interaction of caseinate and TGase to become a dense system and binding as a fish fillet or large beefsteak [7]. Moreover, the interaction mechanism of TGas would depend on ingredients in a system such as the interaction between TGas and Alginate would improve the quality of soy-based meat analogs by using in the optimal levels of these two binding agents [6]. At present, TGas is widely used in the meat industry to restructure meat with strong cohesion due to it having an inexpensive source and TGas also has been recognized as safe (GRAS) [9]. However, the interaction between TGas and protein isolate would not have been clear in some characteristics, especially the changing condition affected rheological properties and lack of condition knowledge for application during food processing. Thus, the objective of this study was to investigate the effect of the TGas concentration and incubation time on the cross-linking and rheological properties of soy protein isolate to understand the interaction changing during various conditions, and the changing behavior of TGas concentration and incubation time on the physical properties in a plant-based patty application.
Materials and methods
Materials
Soy protein isolate (SPI; protein 90.3%, moisture 6.0%, ash 4.8%) was purchased from Krungthepchemi (F014SP; Krungthepchemi; Thailand). Texture vegetable protein (TVP) was extruded from the SPI adjusted to 25% (wet basis) using a twin-screw extruder (Chareon Tut Co. Ltd.; Thailand) with 450 rpm screw speed, 50 rpm feeding paddle, highest extruder barrel temperature at 140 °C, and a short (4 mm diameter) expansion die. Transglutaminase (TGas; transglutaminase- ACTIVA® TG-B; Ajinomoto Ltd.; Japan) was used as a crosslink enzyme.
Sample preparation for investigated crosslink properties
Each sample was prepared using SPI 5% (w/w) in distilled water. Then, TGas at concentrations of 0, 1, 3, 5, or 10% of SPI was added. The solutions were stirred for 10 min and then incubated at 50 °C for 1 h or 4 h with an incubation shaker at 120 rpm. After the end of the incubation time, the TGas was inactivated at 90 °C for 30 min in a water bath, after which the solutions were dried to a powder using freeze-drying (Lyovapor L-200, Buchi). The sample powders were kept in aluminum foil at −18 °C before analysis [12].
Studying protein patterns of samples using Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Proteins were extracted and separated from samples using Tris–HCl buffer with a concentration of 50 mM at pH 8.8 at a sample-to-buffer ratio of 1:50 (by volume) and mixed thoroughly before centrifugation for 30 s at 14,000 × g at 4 °C. Then, 50 μl of protein extract was mixed with 50 μl of 2 × Laemmli sample buffer without the 2-mercaptoethanol for samples without cross-linking agents. The samples with cross-linking agents were mixed with the extract with 50 μl of 2 × Laemmli sample buffer containing 5% (v/v) of 2-mercaptoethanol. The samples were heated at 100 °C for 5 min. Protein standards (Mark 12TM; Invitrogen; Thermo Fisher Scientific; USA) and the samples were loaded onto a 12% SDS-PAGE gel (with a protein sample load of 20 mg per well); then, the gel was run at 200 V for 40 min. After completing the electrophoresis, the SDS-PAGE gel was washed three times with distilled water and the gel was stained using SimplyBlue™ SafeStain (Invitrogen), with images of the SDS-PAGE gel taken using a gel scanner [13].
Rheological properties
Analysis of the rheological properties was performed using a rheometer (MCR 302; Anton Paar; Austria) in two modes (steady shear test and oscillatory frequency sweep), using a parallel-plate measuring system having a centerline diameter of 50 mm and a gap size set at 1 mm [14].
Steady shear test: The steady shear test measured the viscosity of the sample over a range of shear rates from 0.01 to 100 s−1. The data obtained were fitted to the Herschel-Bulkley model, as described by Eq. (1), to determine the values of the flow behavior index (n) and the consistency index (k) of the sample.
where: τ is the shear stress (Pa), τ0 is the yield stress (Pa), γ is the shear rate (s−1), n is the flow behavior index, and k is the consistency index (Pa·sn).
Oscillatory strain sweep: The experiment was performed at a constant frequency of 0.1 Hz with a strain sweep of 0.01–10% at 25 °C. The storage or elastic modulus (G′, Pa) and loss or viscous modulus (G′′, Pa) were monitored. The limit of the linear visco-elastic region (LVR) was determined to carry out the frequency sweep test.
Oscillatory frequency sweep: The oscillatory frequency sweep was conducted by varying the frequency from 0.1 to 20 Hz with strain of 0.1% at a controlled temperature of 25 °C. Storage modulus (G′), loss modulus (G′′), and loss tangent (tanδ = G′′/G′) values were recorded. The frequency-dependent G′ data were fitted to the power-law gel model as described by Eq. (2), which were modified from method of [15] to determine the values of S′ and n′ for the sample:
where: ω is the frequency (Hz), n' is the exponent used for modelling G', and S' is the gel strength (Pa·sn).
Plant-based meat patty preparation
The TVP was soaked with water for around 30 min and then the water was drained from the TVP. A TVP sample (60 g) from the extrudate product was mixed with TGas at concentrations 0, 1, 3, or 5%, and 40 g of an emulsion blended paste (modified starch, coconut oil, canola oil, yeast extract, and beetroot powder) to form ground meat patties. Then, 60 g of ground meat patties were placed into a cylindrical mold (size 16 X 67 mm). For widely used process applications, this research tries experimenting both at room temperature (25 °C) and refrigerator temperature (4 °C). The patties were incubated at 25 °C for 1 h, 25 °C for 2 h, or 4 °C for 24 h before being kept at − 20 °C until cooked. The frozen patties were thawed at 4 °C for 24 h and then were cooked to an internal temperature of 70 °C. The patties were allowed to cool to room temperature for 10 min before analysis.
Color
The color of the plant-based patty samples was measured using a colorimeter (CR-400/CR-410; Minolta; Japan), with the CIE or L*a*b* system. The L* axis represents lightness, with values ranging from 0 to 100, where 0 represents black and 100 represents white, the a* axis describes color from green (−a*) to red (+ a*), and the b* axis describes color from blue (−b*) to yellow (+ b*).
Cooking yield
The cooking loss of the plant-based patties was investigated during cooking. Then, the samples were heated at 120 °C for 3 min on each side until the center of the samples was 70 °C. After that, the samples were cooled at room temperature for 10 min until they had reached room temperature. Next, the samples were weighed before and after cooking [16]. The cooking yield of samples was calculated from Eq. (3):
Texture properties
Texture profile analysis of the plant-based patties was performed using a material tester (TA plus; Lloyds; England). A 45 mm cylinder probe was used to determine 70% compression at a rate of 1 mm/s. Samples were evaluated for hardness, cohesiveness, and springiness. Eight texture analyses were performed on each sample [17].
Structural characteristics using confocal laser scanning microscopy
The structural characteristics of the plant-based patties samples treated with TGas were studied using a confocal laser scanning microscope (CLSM; Zeiss LSM 510 META; Breda, the Netherlands). Samples of plant-based patties were analyzed that had been marinated with TGas at 0, 1, 3, and 5% concentrations at a temperature of 25 °C for 2 h. The samples were stained with fluorescein isothiocyanate isomer at a concentration of 0.001% w/v (green for proteins staining), rhodamine-B-isothiocyanate at a concentration of 0.001% w/v (red for lipids staining), and Nile Blue A at a concentration of 0.001% w/v (blue for carbohydrates staining). The stained plant-based patties were tested at 488 nm (Ar) and 633 nm (He), with the results being recorded in the ranges 400–565 nm and 600–700 nm, respectively (modified from [18]).
Statistical analysis
All the samples were analyzed statistically using the SPSS version 22 software (IBM SPSS Statistics; Somers; NY; USA). Different means were investigated using ANOVA and Duncan’s multiple range tests at a level of significance of 0.05. Correlation of variables was determined using Pearson Bivariate Correlations at the 0.01 and 0.05 significance levels.
Results and discussion
Studying protein patterns of samples using SDS-PAGE
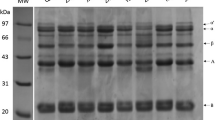
The protein patterns of SPI that had been fermented with TGas at different concentrations (0, 1, 3, 5, or 10%) for 1 or 4 h are shown in Fig. 1. In the absence of cross-linking agents, the protein profile consisted of proteins with approximate sizes of 17, 35, and 75 kDa. This result aligned with the report of Wagner et al. (1996) that the major protein components of soybeans were 11S (glycinin) and 7S (β-conglycinin), with 11S comprising proteins of approximately 20 and 40 kDa [19].
Protein patterns of SPI having undergone incubation with different TGas different enzyme ratios of 0% (Lanes 1 and 2), 1% (Lanes 3 and 4), 3% (Lanes 5 and 6), 5% (Lanes 7 and 8), and 10% (Lanes 9 and 10) for 1 h (Lanes 1, 3, 5, 7, 9) or 4 h (Lanes 2, 4, 6, 8) and marker (M lanes). A: displays SPI pattern with addition of cross-linking agents, and B: displays SPI pattern without addition of cross-linking agents
The molecular size of the SPI tended to increase when incubated with TGas, resulting in the protein sizes exceeding 250 kDa. This result was in agreement with Truong et al. [20], who found that the intensive-stained banding for the cross-linking (large) whey protein polymers formed when the TGas reaction time exceeded 1 h, with larger protein polymers forming as the incubation time increased. TGas enzymes stimulated the acyl transfer reactions between the carboxyl groups of the glutamine amino acids and the amino groups of the lysine amino acids in the proteins as a result of the cross-link formation [7]. This result led to the aggregation of proteins into larger structures. From the results, the TGas concentration and incubation time influenced the cross-linking of protein, with the higher enzyme concentrations leading to better cross-linking, while the longer incubation times also positively impacted the formation of cross-links. The result was similar to Al-Asmar et al. [4] who reported a chickpea protein was a more effective substrate with increased TGas concentration, due to the increase in higher molecular mass polymers. However, the results from SDS-PAGE did not differ between 5 and 10% TGas addition when interacting with SPI. Thus, only the concentrations of 0, 1, 3, and 5% of TGas were studied in the next part of the experiment.
Rheological properties
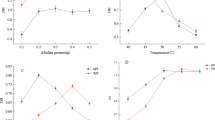
The SPI treated with TGas at concentrations of 0, 1, 3, or 5% were studied for their rheological properties. From Fig. 2a., the apparent viscosity of all samples decreased as the shear rate increased, indicating shear-thinning behavior, except for the untreated SPI sample (0% TGas). This result suggested that the cross-linked protein network was disrupted and that the macromolecules (protein molecules) were elongated and aligned in a high shear field, resulting in the reduction in the viscosity because of the reduced volume fraction [21]. Figure 2b. shows the flow curve (shear stress against shear rate) of TGas-treated SPI samples well fitted to the Herschel-Burkley model (Eq. 1) with regression coefficients (R2) of more than 0.98 (Table 1), except for the sample at a TGas concentration of 5% and an incubation time of 4 h with lowest R2 of 0.83. The shear-thinning behavior was exhibited by the flow behavior index (n) values for all samples that were less than 1, as shown in Table 1. A lower n value indicates that flow behavior has greater shear thinning characteristics. However, the untreated SPI sample (0% TGas) exhibited the highest n value, which was close to 1, indicating behavior closer to Newtonian flow. In contrast, the SPI samples that had undergone enzyme treatment had n values less than 1, and these n values tended to decrease as enzyme concentration and incubation time increased. This may have been due to the increase in the enzyme concentration and incubation time promoted the formation of disulfide bonds of intra- and inter-chain cross-linking soybean protein molecules, and increased protein molecular size, confirmed by SDS-PAGE analysis (Fig. 1.), resulting in the formation of a dense or strong protein gel network structure. This led to an increase in apparent viscosity, consistency index, yield stress, and shear-thinning behavior (Table 1). These results concurred with Wu et al. [10], who reported that TGas enhanced a tight protein (sodium caseinate, soy protein isolate, and egg white protein) gel network formation to improve the gel strength, while TGas also induced an increase in the viscosity and gelation of whey protein [20].
The strain of 0.1% was in the linear viscoelastic region (LVR) for all samples (Fig. 3a.) and this strain value (0.1%) was chosen for oscillation frequency test. Characteristics of a protein gel network are related to rheological properties—the value of G' (storage modulus) and tan δ (loss tangent)—as shown in Fig. 3b. and c.). G' indicates gel rigidity or gel strength. G' increased as the TGas concentration and incubation time increased (Fig. 3b). In particular, an increase in incubation time clearly enhanced the G' value. The increase in G' with increasing TGas enzyme concentration and incubation time indicated an increase in the solid-like properties of the samples, which might have been caused by the enhanced entanglement and interconnection of protein molecules in addition to the enhanced junction zones of the structural protein network. The value for G'0.1 Hz of the sample with a TGas concentration of 5% and an incubation time of 4 h was the highest of all the samples, indicating the stronger gel matrix formation (Fig. 3b.). However, the G'0.1 Hz value of the sample with a TGas concentration of 1% and incubation times of 1 and 4 h was not significantly different from the un-treated sample (0% TGas). This may have been because the TGas concentration at 1% was too low and insufficient to induce the proteins to interact and become entangled to form a gel network.
a Strain sweep diagram at a constant frequency of 0.1 Hz; b and c: Storage modulus (G') and loss tangent (tanδ) at a frequency of 0.1 Hz with strain of 0.1%, respectively; and d: Frequency-dependent rheological parameters (G', G'', and tanδ) of TGas-treated SPI with varied enzyme concentrations and incubation times. *Significantly different with same TGas concentration at 95% confidence level. Different capital letters (A, B) and lowercase letters (a, b, c) with same incubation time of 1 h or 4 h, respectively
In Fig. 3c, tanδ decreased as the TGas concentration and the incubation time increased. A decrease in tanδ was indicative of the viscoelastic structure of the protein, which became more solid-like in its behavior. In particular, using TGas concentrations of 3% and 5% and an incubation time of 4 h, resulted in tanδ values that were close to 0.1. A tan δ value less than 0.1 indicates a semi-solid gel [22]. These results suggested that the protein network structure would become more rigid and solid-like and exhibit less liquid-like behavior as the enzyme concentration and incubation time increased, which was also reflected in the increase in G' (Fig. 3b.). In addition, the un-treated sample (0% TGas) was a flowable, low viscous paste because there was no TGas to catalyze the protein cross-linked interaction and protein network formation, resulting in its tanδ value being the highest (~ 0.7), as shown in Fig. 3c. The tanδ value was higher than 0.1, indicating a less solid-like behavior [14]. However, the tanδ value (~ 0.7) of the sample treated with TGas at a low concentration (1%) and for a short incubation time (1 h) was quite close to that of un-treated sample (0% TGas), indicating that the low TGas concentration and short incubation time were not sufficient to enhance the protein molecular interaction.
The data presented in Fig. 3d. shows that the G', G′′, and tanδ values of all samples were dependent on frequency, since the relaxation processes occurred even within short time scales [23] and the sample structure was deformed during increased in frequency [22]. However, the G' values of the un-treated sample (0% TGas) and the samples treated with TGas for 1 h (1% and 3%) and 4 h (1%) were clearly dependent on the frequency (1–50 Hz), with a high value of n’ in the range 1.6375–3.8066 (as shown in Table 2) because these samples were flowable and had a less semi-solid structure (low G', Fig. 3b.), which had less protein molecule interconnection, and junction zones may be easily disrupted even with a low frequency or shear rate application [14]. On the other hand, the samples treated with TGas for 1 h (5%) and 4 h (3% and 5%) showed smaller changes in G', G”, and tanδ (as shown in Fig. 3d). Their n' values were lower, ranging from 0.4022 to 0.6970 (as shown in Table 2). This suggested that the gel network structure of the protein became more rigid as the TGas concentration was higher than 3%, resulting in fewer changes in the internal structure of the protein gel as the frequency increased. This result was in agreement with Udomrati et al. [14], who found that the less semi-solid system was more dependent on frequency than were the high semi-solid samples. The S' value related to the strength of the protein gel, with the S' value increasing as the TGas concentrations and incubation times increased (Table 2). According to the Pearson Bivariate Correlation between TGas incubation (concentration and incubation time) and rheological properties of SPI gel in the present study was shown in Table 3. The concentration of TGas for using in SPI gel system affected viscosity, flow behavior index, and especially n’. Moreover, the S’ highly correlated with other properties (viscosity, flow behavior, and consistency index). Thus, the differences in the G', n', and S' values between the samples subjected to different TGas concentrations and incubation times indicated that the internal structure of the protein gel would play a crucial role in the rheological properties of the samples.
Based on the rheological determination, it was concluded that the SPI protein molecules could be cross-linked to form the strongest protein gel network at a TGas concentration of 5% with an incubation time of 4 h. The rheological data were used to evaluate the appropriate conditions (TGas concentration and incubation time).
Application in plant-based meat patties – Color
The TGas was used to treat plant-based meat patties using different concentrations (0, 1, 3, or 5% addition) and different incubation conditions (not incubated, 25 °C/1 h, 25 °C/2 h, or 4 °C/24 h). The cooked plant-based meat patties were analyzed for color, cooking yield, and texture properties.
The plant-based meat patties samples not treated with the TGas were incubated at 25 °C for 2 h or at 4 °C for 24 h which produced a significant difference in lightness values compared to samples that were not incubated. Furthermore, the incubated sample at 25 °C for 2 h showed the effect of reducing lightness values with increasing the TGas concentration (Fig. 4.). This result was consistent with the findings of Forghani et al. [24], who found that using microbial transglutaminase (MTGas) at concentrations of 0.5% and 0.75% resulted in a reduction in the lightness of plant-based burgers compared to burgers without MTGas, due to the MTGas playing a role in preventing protein denaturation during cooking, since such denaturation led to a brownish color.
Color of plant-based meat patties after cooking. *Different capital letters (A, B) indicate significant differences in incubation conditions with same TGas concentration at p ≤ 0.05. Different lowercase letters (a,b,c) indicate significant differences in TGas concentration with same incubation conditions at p ≤ 0.05
When TGas was added to the plant-based patties at higher concentrations, there was a significant decreasing trend in the a* values for all incubation conditions. In particular, the sample with 3% TGas and incubated at 25 °C for 2 h had a significant decrease in the a* value compared to the other incubation conditions. Incubation at 25 °C for 1 h produced changes in the b* value. The TGas-added samples could reduce the yellow color compared to samples without TGas. However, MTGas addition could not significantly influence the L* and a* color parameters of beef gels, and the color measuring of raw meat would not be possible to predict the impact of MTGas after cooking [25]. These differences in color values could be attributed to variations in the formulation or composition, such as protein content, fat content, moisture content, or other ingredients, as well as to the cooking process of the plant-based meat product [24].
Application in plant-based meat patties – Cooking yield
Cooking yield is used a parameter to investigate the quality of the product after cooking, where cooking yield is the weight remaining in the product, while cooking loss is the weight loss of the product. Cooking loss and cooking yield represent shrinkage in the product during cooking, which is an important factor and an indicator for evaluating the juiciness and yield of the final product [12, 26]. The cooking yield results of plant-based patties with the addition of TGas enzymes and with incubation under different conditions were shown in Table 4. Increasing the concentration of TGas enzymes resulted in an increase in the cooking yield for all incubated conditions. With each TGas concentration, incubation at 25 °C for 2 h tended to result in the highest cooking yield for the plant-based patties samples.
The increasing trend in cooking yield was similar to the results in the research by Pietrasik [25], who reported that the addition of 0.5% MTGas in beef gel improved water-holding properties, the meat became smoother, and water release decreased compared to the samples that did not have MTGas added. In addition, Forghani et al. [24] reported that burgers made from vegetables had reduced shrinkage when 0.5% or 0.75% MTGas had been added. However, Lantto et al. [27] found the cooking loss increased with an increase in the concentration of TGas enzymes added to chicken nuggets. The addition of TGas might lead to increased protein–protein interactions, resulting in decreased protein-water interactions due to enzyme-induced cross-linking [6]. The effects of TGas on binding properties might depend on the concentration and type of TGas used [25].
Application in plant-based meat patties – Textural properties
The effects of the TGas concentration and incubation conditions on the textural characteristics of plant-based meat patties were shown in Table 5. Normally, the strength of the structure of the plant-based meat patties under compression was indicated by their hardness [28]. The hardness of the plant-based meat patties tended to increase with a higher TGas concentration, particularly when the plant-based meat patties had been incubated at 25 °C for 1 or 2 h. Incubation at 25 °C for 1 h resulted in an increase in hardness with TGas addition, especially when using 5% TGas addition. Hence, the observed increase in hardness after the first hour of incubation might be attributed to the optimum time for TGas activity. This result was consistent with a report by Setiadi et al.[29] that the addition of a 2% transglutaminase enzyme concentration to textured soy protein increased the hardness of the sample.
For the adhesiveness, the significant decreasing showed with the using of 1% TGas addition at 25 °C for 1 h. There was a significant difference in cohesiveness with 3% TGas addition when incubated at 25 °C for 2 h. The change in hardness and cohesiveness might be due to concentrations of TGas, which were similar to Setiadi et al. [29] who reported that using 2% TGas could increase hardness but decrease the cohesiveness of texturized soy protein. The trend of gumminess and springiness increased with increasing of TGas concentration in the plant-based meat patties, especially with 5% TGas addition. The increasing effect of the added TGas was similar to the results of Pietrasik [25], who reported that gel samples containing transglutaminase had increased hardness, cohesiveness, and springiness than samples without transglutaminase, with varying the amount of transglutaminase affecting the textural characteristics of cooked meat emulsions. The increased springiness, gumminess, and chewiness of TGas added plant-based meat compared to the control might be due to the inducing of new cross-linking in the soy protein structure from denatured protein to be attributed to textural modification and improvement of the cohesiveness of system [6, 30]. However, the using of TGas would depend on a matrix in the system such as the addition of MTGase might not significantly change the texture properties of Atlantic salmon meatballs [31]. The change of texture profile analysis parameter of the patty could be enhanced crosslink structure by enzyme [26]
According to the Pearson Bivariate Correlation between TGas incubation (concentration and incubation condition) and texture properties of the patty in the present study was shown in Table 6. The concentration of TGas for use in patty highly affected gumminess springiness, and chewiness. The incubation condition was also correlated with hardness and adhesiveness. These correlation results of TGas concentration were similar to both the SPI gel system and patty in which the TGas concentration showed a higher effect on the properties of the system than the incubation condition (Table 3 and Table 6).
Application in plant-based meat patties – structural characteristic
The structural characteristics were investigated using CLSM of the plant-based meat patties incubated at 25 °C for 2 h with TGas enzyme added at concentrations of 0, 1, 3, or 5% (Fig. 5.). The red color represented the lipid components, the green color represented the protein components, and the blue color represented the carbohydrate components.
The color distribution appeared more scattered in the plant-based meat patties without TGas addition, with a more pronounced clustering of components when TGas was added, especially at concentrations of 3 and 5%. In these cases, there was noticeable aggregation of biomacromolecules in the plant-based meat patties and the green color (representing protein components) became more distinct when the level of TGas increased. Additionally, the blue color in all experimental samples increased with the addition of TGas, which might have been due to the composition of the modified starch (used as a binder) and the commercial TGas enzyme (containing maltodextrin) in the system. The structure of the plant-based meat patties was similar to the network pattern of plant-based protein in plant-based hamburger and plant-based chicken meat products, as the distribution of fat appeared to interweave with the components of the samples [18]. Fat or binders would be obtained as a protein–fat interphase in recombinant plant-based meat alternatives with good texture, juiciness, and chewiness for selecting optimized structural quality [28]. The results in (Fig. 5) could be observed that the protein components (represented by the green color) remained integrated as a cohesive structure with the other parts of the sample. The structure of the 5% TGas concentration added to plant-based meat patties might have been influenced by the high hardness, springiness, and cooking yield for the same incubation conditions. The addition of transglutaminase to plant protein would form a covalent crosslink between primary amines (lysine and glutamine) resulting in the protein network structure accumulating a high moisture content in the plant-based product, with a positive effect on water binding and shrinkage of the product, which could contribute to the texture of soy protein after fat and moisture loss [24].
Conclusion
The TGas concentration and incubation time influenced the cross-linking of protein, with higher enzyme concentrations leading to better cross-linking, while longer incubation times also positively impacted the formation of cross-links. However, the result from SDS-PAGE were the same for 5% and 10% TGas. Thus, later experimentation would focus on only on concentrations of 0, 1, 3, or 5% of TGas. The interaction of SPI solution and TGas led to an increase in apparent viscosity and in the shear-thinning behavior which enhanced solid-like properties (increased G' and decreased tanδ) due to the increased enzyme concentration and longer incubation time producing a more rigid protein gel structure due to the protein molecules becoming cross-linked to form a viscoelastic protein gel network. The characteristics of the plant-based meat patties samples could be altered by different added TGas concentrations and incubation conditions. The highest cooking yield for plant-based patties resulted from incubation at 25 °C for 2 h. The hardness of the plant-based meat patties tended to increase with a higher TGas concentration, especially following incubation at 25 °C for 1 or 2 h. Cohesiveness and springiness increased with incubation at 25 °C for 2 h. Thus, adding 5% TGas with incubation at 25 °C for 2 h might be the best conditions for plant-based meat patties in this experiment. From rheological properties and texture properties, the concentration of TGas was an important factor affecting the characteristics of the system, as shown by clearly correlated results. The structural characteristics of the plant-based meat patties observed using CLSM indicated there was aggregation of the protein components with increased levels of TGas enzyme. The results from this research should help to elucidate the interaction of SPI and TGas enzyme incubation and their effects on the characteristic structure and texture in such practical food-based applications especially, using 5% TGas with incubation at 25 °C for 2 h was preferred to develop hardness cohesiveness and springiness. However, the limitations of this research would be held on the interaction of TGas. The other compositions of the matrix would be important for interaction in the system and affect changing shelf life.
Data availability
Not applicable.
References
M. Benković, A. Jurinjak Tušek, T. Sokač Cvetnić, T. Jurina, D. Valinger, J. Gajdoš Kljusurić, Gels 9, 921 (2023)
K. Kyriakopoulou, J.K. Keppler, A.J. van der Goot, Foods 10, 600 (2021)
S. Featherstone, (2015) In Complete Course Cann. Relat. Process. Fourteenth Ed., edited by S. Featherstone Woodhead Publishing, Oxford, pp. 147–211
A. Al-Asmar, C. Giosafatto, L. Panzella, L. Mariniello, Coatings 9, 331 (2019)
P.D. Ribotta, C.M. Rosell, Starch - Stärke 62, 373 (2010)
E.-J. Lee, G.-P. Hong, Food Sci. Biotechnol. 29, 777 (2020)
K. Yokoyama, N. Nio, Y. Kikuchi, Appl. Microbiol. Biotechnol. 64, 447 (2004)
K. Vasić, Ž Knez, M. Leitgeb, Int. J. Mol. Sci. 24, 12402 (2023)
M. Kieliszek, A. Misiewicz, Folia Microbiol. (Praha) 59, 241 (2014)
M. Wu, Q. Yin, J. Bian, Y. Xu, C. Gu, J. Jiao, J. Yang, Y. Zhang, Gels 9, 910 (2023)
F. Wang, W. Huang, Y. Kim, R. Liu, M. Tilley, J. Cereal Sci. 54, 53 (2011)
Y.-N. Zhang, X.-H. Zhao, Int. J. Food Prop. 16, 1257 (2013)
M. Hayakari, Y. Kondo, H. Izumi, Anal. Biochem. 84, 361 (1978)
S. Udomrati, P. Tungtrakul, N. Lowithun, and D. Thirathumthavorn, Sci. Eng. Health Stud. 22030007 (2022).
P. Kumar, C. Kaur, S.G. Rudra, B. Arora, Int. J. Gastron. Food Sci. 31, 100638 (2023)
M. Serdaroğlu, B. Nacak, M. Karabıyıkoğlu, G. Keser, Korean J. Food Sci. Anim. Resour. 36, 744 (2016)
T.W. Crowe, L.A. Johnson, T. Wang, J. Am. Oil Chem. Soc. 78, 775 (2001)
L. Godschalk-Broers, G. Sala, E. Scholten, Foods 11, 2227 (2022)
V. Biscola, A.R. de Olmos, Y. Choiset, H. Rabesona, M.S. Garro, F. Mozzi, J. Chobert, M. Drouet, T. Haertlé, B.D.G.M. Franco, Microbes 8, 635 (2017)
V.-D. Truong, D.A. Clare, G.L. Catignani, H.E. Swaisgood, J. Agric. Food Chem. 52, 1170 (2004)
Q. Lin, Z. Liu, H. Xiao, L. Li, F. Yu, W. Tian, in Comput. Comput. Technol. Agric. III. ed. by D. Li, C. Zhao (Springer, Berlin, Heidelberg, 2010), pp.407–419
J. Ahmed, Rheological properties of foods, In mathematical modeling of food processing (Farid, M.M., ed.) (New York, UK: CRC Press, n.d.).
J. A. Lopes da Silva and M. Anandha Rao, Rheological Behavior of Food Gel Systems, In Rheology of fluid and semisolid foods (Anandha Rao, M., ed) (Maryland, USA: Aspen Publishers, Inc., n.d.)
Z. Forghani, M.H. Eskandari, M. Aminlari, S.S. Shekarforoush, J. Food Sci. Technol. 54, 2203 (2017)
Z. Pietrasik, Meat Sci. 63, 317 (2003)
K. Sakai, Y. Sato, M. Okada, S. Yamaguchi, Sci. Rep. 11, 16631 (2021)
R. Lantto, E. Puolanne, K. Katina, M. Niemistö, J. Buchert, K. Autio, Eur. Food Res. Technol. 225, 75 (2007)
D. Zhao, L. Huang, H. Li, Y. Ren, J. Cao, T. Zhang, X. Liu, Foods 11, 2202 (2022)
S. Setiadi, W. Sah, and N. Alisha, E3S Web Conf. 67, 03043 (2018).
H. Kim, M.-Y. Lee, J. Lee, Y.-J. Jo, M.-J. Choi, Foods 11, 3337 (2022)
C. O. Altan, D. Kocatepe, M. S. İPar, B. Çorapçi, B. Köstekli̇, and H. Turan, J. Agric. Fac. Gaziosmanpasa Univ. 40, 41 (2023).
Acknowledgements
This work was supported by Thailand Institute of Scientific and Technological Research [Grant numbers: 6718102051].
Author information
Authors and Affiliations
Contributions
Sunsanee Udomrati: Rheological properties analysis and writing. Thidarat Pantoa: Crosslink analysis. Waraporn Sorndech: Review and editing. Thongkorn Ploypetchara: Conceptualization, preparation and application in patty analysis, writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Udomrati, S., Pantoa, T., Sorndech, W. et al. Investigation of transglutaminase incubated condition on crosslink and rheological properties of soy protein isolate, and their effects in plant-based patty application. Food Measure (2024). https://doi.org/10.1007/s11694-024-02846-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11694-024-02846-7