Abstract
The main objective of this study was to investigate the effects of microbial-transglutaminase (MTGase 0–0.75%)/sodium-caseinate (SC 0–2%) as crosslinker agents on proximate analysis, binding properties (expressible moisture and shrinkage), texture analysis, electrophoretic patterns, instrumental color, and sensory properties of veggie burgers. Addition of SC and MTGase positively affected shrinkage and expressible moisture. It also increased hardness, springiness, chewiness, and cutting-force of burgers. Presence of SC had no effects on cohesiveness of burgers. Total protein and ash of samples were increased by treatment with SC. The lightness (L*) of samples was significantly decreased by 0.75% MTGase. No significant influence of SC on samples color parameters was observed. The results indicated that distinct protein bands were not formed on the SDS–PAGE of burger samples and resulted in a smearing pattern on the gel. When soy-protein was incubated with MTGase, a progressive decrease in the intensity of the bands corresponding to the subunits 7S and 11S globulins was observed concomitant with disappearance of A3 and B3 bands. Electrophoresis pattern of gluten was slightly changed after MTGase treatment. There were significant differences in color, taste, appearance, mouth feel, and overall acceptability between treated and control samples. Results suggest that production of veggie burgers using MTGase alone or in combination with SC brings about covalent cross-linking between homologous and heterologous proteins to form high-molecular weight polymers, thereby improving the mechanical properties of veggie burgers and profoundly increases the acceptability of the end product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Institute of Medicine suggests that human adults need a minimum of 0.8 g protein for every kilogram of body weight per day (Johnson 2013). Red meat is a major food source of protein, but many studies have shown that higher consumption of red meat is linked to increased risk of cancers, diabetes, and cardiovascular diseases. The use of healthier protein sources, especially plant sources in place of red meat, can lower the risk of these diseases. In addition, the rise in the number of vegetarians necessitates the increased use of plant sources of protein, such as grains, nuts, and seeds (Pan et al. 2012).
The change in eating pattern requires new products which are attractive to consumers but does not deprive them of the nutrients normally supplied by meat products (Wild et al. 2014). Soy protein and wheat gluten are the most common raw materials for meat-like products (Ahirwar et al. 2015). The nutritional benefits and functional properties of plant proteins make them good candidates for the manufacture of healthy fast foods. The development of these new products as a substitute for meat that are acceptable to the consumers is a challenge for food producers (Van Trijp and Van Kleef 2008). The textural characteristics of meat analogues, such as softness and pasty texture, incohesive and poor chewiness are critical limiting factors in consumer’s acceptance of these products (Baer and Dilger 2014).
Meat industry is efficiently using different texturizing agents such as alginate, casein, fibrinogen and thrombin, transglutaminase, etc. to improve textural attributes, water-holding capacity and appearance of restructured meat products (Hong and Xiong 2012). Microbial transglutaminase (MTG or MTGase) catalyzes covalent cross-link formation between primary amines (such as ε amino group of lysine) and the amide group of glutamine residues to form high molecular weight polymers, resulting in improvement of mechanical properties. MTGase is used as a texturizing agent in several meat products, such as sausages (Baer and Dilger 2014), burgers (Velioğlu et al. 2010), and restructured meats (Kilic 2003; Herranz et al. 2013) to enhance the network structure of the products. Nevertheless, few studies have dealt with the possible influence of the application of MTGase/caseinate as a crosslinking agent on the textural properties of veggie burgers. Previous studies have shown that casein is the best substrate for MTGase (Kilic 2003) and has good potential for improving texture of meat analogues, Therefore, the main objective of this work was to investigate the effects of MTGase/SC on the improvement of physicochemical, electrophoretic patterns and sensory properties of veggie burgers.
Materials and methods
Materials
Textured soy protein (TSP) (Sonic Co., India.), spices (Meat Creaks, Germany), carrageenan and xanthan (BLG, China) and sodium caseinate (Pegah Fars Dairy, Shiraz, Iran) were purchased. Gluten, hydrogenated soybean oil, sunflower oil, and other ingredients (onion, turmeric, and baking powder) were also purchased from the local market (Shiraz, Iran). MTGase was from Ajinomoto Co. (ACTIVA WM, Ajinomoto Europe Sales GmbH, Germany). The composition of commercial enzyme was 1% of MTGase and 99% maltodextrins (activity of ≈96 U/g).
Preparation of veggie burgers
Figure 1 shows the flow chart for preparation of veggie burgers. When all of the ingredients were weighted, an initial mixture containing water soaked TSP and hydrogenated vegetable oil were ground in a grinder with a 5 mm diameter mesh (Pars-Khazar Co., Model MT1200, Iran) and then were mixed in a mixer (Pars-Khazar Co. Model GMT-05 B2, Iran) for 2 min. Powdered ingredients were subsequently added to the mass and mixed for 10 min (Fig. 1). Then the mixture was divided into 12 portions for further implementing 12 types of treatment plan. MTGase solution was prepared as described in Ajinomoto Co. manual. MTGase (at four levels: 0, 0.25, 0.5, and 0.75%) and sodium caseinate (at three levels: 0, 1, and 2%) were added and mixed for 3 more minutes. One hundred gram portions of the pastes were machine-shaped into burgers. The burgers were stored at room temperature for 1 h, after which the samples were packed in PE/PA (Polyethylene/Polyamide) 75 micron plastic bags and stored at 4 °C for 24 h to complete the enzyme activity. All analyses were performed in three replications.
Proximate analysis
The content of protein (AOAC 981.10), moisture (AOAC 950.46), ash (AOAC 992.16), total fat (AOAC 991.36), and dietary fiber (AOAC 992.16) was determined using AOAC method (Horwitz and Horwitz 2000).
pH of raw veggie burgers was measured by making a 10% (w/v) suspension of each sample in distilled water. pH was determined with a portable pH meter (Model 501-05 Barcelona, Spain) in triplicate (Dzudie et al. 2002).
Evaluation of binding properties
Expressible moisture (EM)
EM was determined using a press technique as described by Wang and Zayas (1992) with slight modification. In this study digital image processing was used to determine the expressible moisture of burgers. A 0.3 g sample was placed between 2 filter papers (Whatman No. 2) and was pressed between 2 glass plates under 1 kg weight. After 20 min, the upper plate was removed and photos were taken from the filter papers (Wang and Zayas 1992). The area of the pressed batter and the total area of the moistened paper (cm2) from the digital pictures were measured using Digimizer, an image analysis software (Version 4.3.5, MedCalc Software, Belgium). EM was expressed as the percentage ratio of moisture released from the sample to the initial sample weight.
Shrinkage
Shrinkage was measured using an image processing method (Velioğlu et al. 2010). The image size was 1900 × 1900 pixels and the pictures included two parts, black (background) and white (veggie burger). Images were processed using Matlab R2015a (The MathWorks, Inc., Natick, MA) to provide the areas of burgers before and after cooking. Percentages of shrinkage were calculated using the following formula:
Texture analysis
The effect of adding MTGase and SC on the texture of veggie burgers was studied using a texture analyzer (Brookfield, CT3, UK). Burgers were equilibrated to room temperature for 1 h and cut into small cubes (1 × 1×1 cm). Samples were compressed to 70% of their original height in two cycles with 2 s intervals between cycles. Crosshead speed of 20 cm/min was performed by cylindrical aluminum probe (diameter: 10 cm). Hardness [peak force on first compression (g)], cohesiveness (ratio of the active work done under the second force–displacement curve to that done under the first compression curve), springiness [distance the sample recovered after the first compression (mm)], and chewiness [hardness × cohesiveness × springiness (g mm)] were determined from the resulting force/deformation curves (Canto et al. 2014). Nine samples per treatment were measured. The load cell capacity was set to 250 N.
Instrumental cutting force (g) determinations were made on a total of 9 veggie cooked burgers per replication. Burgers were fried for 7 min (3.5 min per side) using 9 g oil at medium intensity flame. After cooling to 23 °C each sample was cut into 6 × 2.5 cm sections. Cutting force of each piece of burger was measured using texture analyzer with Warner–Bratzler blade (Berry and Bigner 1996; Baer and Dilger 2014). Cut speed was 200 mm/min. The load cell capacity used was 1000 N.
Instrumental color evaluation
The cooked samples were equilibrated to laboratory temperature (23 ± 2 °C) and then L* (lightness), a* (redness “+” or greenness “−”), and b* (yellowness“+” or blueness “−”) of samples were measured by a computer vision system according to the method described by Girolami et al. (2013). A 7.1-megapixel digital camera (Canon, Ixus70, Japan) was used for photographing and the camera setting was adjusted to the followings: shutter speed 1/6 s, manual operation mode and flash off. Lighting was achieved with four fluorescent lamps (Philips Master Graphica TLD 965). The camera was located vertically at a distance of 30 cm from the sample. Lighting was achieved with four fluorescent lamps (Philips Master Graphica TLD 965). Adobe Photoshop CS3 software was employed for image analysis. The size of samples was 6 × 2.5 cm (Girolami et al. 2013).
Protein–protein interactions (electrophoresis analysis)
Slab sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was performed based on the discontinuous buffer system of Laemmli with some modifications as described by Aminlari and Majzoobi 2002. Frozen samples (burger batters, soy protein, and gluten) were allowed to thaw at room temperature and were subjected to SDS–PAGE (Aminlari and Majzoobi 2002). A 12% acrylamide separating gel (width × height × thickness = 14 mm × 14 mm × 1 mm), and a 4% acrylamide stacking gel was used. Treated protein samples (0.2% protein concentration) were mixed with an equal volume of SDS–PAGE sample buffer (4% SDS, 20% glycerol, 10% β-mercaptoethanol in 0.125 M Tris HCl, pH 6.8) and heated in boiling water for 5 min. Aliquots of 30 μg of protein per well were loaded onto the gel. The intensity of color for each band and their protein content were determined by ImageJ software (USA, version 1.46r).
Sensory evaluations
Sensory evaluations were carried out by 20 trained panelists from the staff of Setareh Yakhi Asia, a food processing and marketing company (Ice-Asian co-star, BA Food, Shiraz, Iran, site: http://www.bafoods.com/). Ten women and ten men, aged between 20 and 35 years old, tested the veggie burgers for appearance, aroma, texture, overall acceptability, and mouth feel. Panelists were trained according to the guidelines of American Meat Science Association. The degree of like or dislike for samples was evaluated by acceptance test. Samples were fried and served warm to the panelists. Panelists received the samples in a randomized order (based on random 3-digit numbers). Unsalted crackers and water were given between samples to clear the mouth of residual flavor (Meilgaard et al. 2006).
Statistical analysis
All data were statistically analyzed using the one-way analysis of variance (ANOVA) procedure of the SAS 9.2 system (SAS Institute Inc., Cary, NC). The analysis was carried out in three replications. Duncan’s multiple range tests were applied to determine which of the differences were significant (P < 0.05).
Results and discussion
Proximate composition
Proximate analysis of the veggie burgers after 24 h of refrigerated storage are presented in Table 1. Addition of SC and MTGase had no significant effects on moisture, fat content, and pH of burgers (P > 0.05). Total protein and ash of samples were significantly affected by the addition of SC (P < 0.05). Cofrades et al. (2011) evaluated the quality features of low-salt restructured poultry containing microbial transglutaminase and seaweed and found that the addition of 3% Sea-Spaghetti and MTGase/caseinate had no significant effects on moisture, protein, and fat contents of the products. Pietrasik et al. (2007) investigated the effect of addition of four non-muscle proteins (blood plasma, sodium caseinate, soy protein isolate, and gelatin) on pH values of the pork gels processed with microbial transglutaminase. They showed that the pork gel containing sodium caseinate had the highest pH values and MTGase addition had no effect on pH of the samples. Canto et al. (2014) also reported that MTGase had no effect on the pH of the products.
Binding properties
One of the important features of burgers and other meat products is the ability to hold water and other juices in the product before and after cooking (Yang et al. 2007). In the current investigation, the cooking loss and shrinkage were used to measure the amount of juices that were lost during cooking, and the expressible water was a measure of batter stability of burgers before cooking and during storage. Table 1 shows the impacts of SC and MTGase on the water and fat binding properties of veggie burgers.
The percentage of expressible moisture is inversely related to the water-holding capacity (WHC) that means, the lower expressible moisture is usually associated with the higher WHC. The amount of expressible water ranged from 26.59 to 35.49%. The percent of expressible moisture of the veggie burgers was lower than meat based products, such as hamburger patties (Velioğlu et al. 2010) or crab meat (Martínez et al. 2014). Previous researches documented that the use of plant proteins plays a great role to enhance water retention capacity and yield of meat products (Pietrasik et al. 2007; Dzudie et al. 2002) and probably for this reason WHC of the burgers was higher than meat-based products. The addition of MTGase and SC separately and in combination, significantly decreased the expressible moisture of burgers. This parameter decreased with increasing the levels of SC and MTGase (P ≤ 0.05). Therefore the lowest expressible moisture was observed in samples containing 2% SC and 0.75% MTG and the highest EM was for the control sample. MTGase contributes to the formation of covalent crosslinks between primary amines, such as lysine and glutamine residues in SC and soy proteins, thereby enhancing the protein network structure and expressible moisture of the various protein-based products (Dzudie et al. 2002; Canto et al. 2014). However Pietrasik et al. (2007) showed that the effect of MTGase on water retention was dependent on the type of protein. Researchers reported that several proteins such as, soy protein isolate or blood plasma in combination with MTGase did not have any significant effect on water retention ability of pork gel, but sodium caseinate and gelatin were good substrates for MTGase and improved water retention of the gels. Pietrasik (2003) reported that the addition of MTGase had no significant effect on water holding of pork gel, although MTGase promoted the hardness of gels at higher levels of sodium caseinate. These contradictory results clearly demonstrate that the positive effect of MTGase on water binding properties of protein based products were dependent on the type of protein substrate and the selected preparatory steps (Krintiras et al. 2015).
Shrinkage of cooked burgers is of particular importance, as it not only destroys the figure of a product, but can also impact the economic aspect of meat and analogues meat products (Li and Wick 2001). The percentage of shrinkage varied between 8 and 14% (based on diameter). Overall, the percentage of shrinkage in veggie burgers was smaller than that reported by others in the meat-based products (Ahhmed et al. 2009a, b; Velioğlu et al. 2010). Velioglu et al. (2010) studied the effects of fat (15–30%), water (10–20%) and textured soy protein (3–9%) content on the shrinkage of hamburger patties after cooking. They reported that both fat and water content had significant effect on shrinkage and presence of textured soy protein in formulation is the main factor for minimizing fat and moisture loss. The results showed the application of increased SC levels (from 0 to 2%) had no significant effect on percentage of shrinkage of burgers (P > 0.05) but 0.5 and 0.75% MTGase reduced the percentage of shrinkage of veggie burgers (P < 0.05). No information has been found on the role of MTGase and SC addition on parameters of processed veggie burgers. The results of the studies on the effect of MTGase and SC in restructured meat products are contradictory. Su et al. (2000) reported that physical entrapment of fat globules within the hydrated soy protein or SC (2%) in the meat batters stabilized the meat emulsions during cooking and decreased shrinkage and cooking loss. Pietrasik (2003) found that the presence of increasing levels of MTGase (0–0.6%) decreased the cooking loss in cooked pork gels. However, Herranz et al. (2013) reported that the binding properties were strongly influenced by sodium content in processed muscle foods. Higher ionic strength enhances electrostatic repulsions and causes a loosening of the myofibrillar structure. Cofrades et al. (2011) also reported that when MTGase/caseinate was used in formulations with the low salt content products it did not affect percentage of shrinkage.
Color measurement
Table 2 shows that the samples did not differ with respect to redness (a*) and yellowness (b*) (P > 0.05). However, addition of 0.5 and 0.75% of MTGase reduced the lightness (L*) of samples significantly. Addition of SC had no significant effect on L* parameters of burgers but there was a synergistic effect on decrease in lightness of burgers when the enzyme and SC were applied at the same time (P < 0.05). The brown color of cooked burgers is attributed to the heat-induced denaturation of proteins. MTGase contribute to promoted denaturation of protein molecules (Canto et al. 2014). Probably for this reason, for the same frying time, veggie burgers which were treated with MTGase (0.5 and 0.75%) were darker (lower L* value) and appeared more cooked than those made without MTGase (control sample). No reports were found regarding the effect of MTGase and SC addition on veggie burgers color and the results of other studies on various meat products were remarkably contradictory. Several researches documented that MTGase levels had no effect on the color of various cooked meat products (Canto et al. 2014; Cofrades et al. 2011). However, Min et al. (2008) observed that b* values increased with the addition of MTGase in catfish patties. The differences between various reports could be attributed to the differences in the formulations (level of protein, fat, the addition of water, and other ingredients) and process of cooking of the various analogues meat products.
Texture analysis
Raw veggie burgers
The changes in texture features of raw burgers as a result of SC and MTGase addition are presented in Table 2. The results of texture profile analysis (TPA) indicated that the incorporation of SC and MTGase had significant effects on hardness, cohesiveness, springiness, and chewiness of the samples (P < 0.05). Presence of SC (from 0 to 2%) did not have any significant effect on cohesiveness of burgers. The effect of MTGase addition on measured textural properties of raw burgers was more than those produced without MTGase (P < 0.05). The addition of MTGase to formulations enhanced the cohesiveness of batters and improved their hardness, springiness, and chewiness. SC and MTGase addition showed synergistic interaction effects on TPA of samples, such that the veggie burgers made with MTGase plus SC had higher hardness, chewiness, fracturability, and cohesiveness values than other samples (P < 0.05). The highest textural properties were obtained in the presence of both SC-2% and MTG-0.75% treatment. The textural characteristics and WHC of protein based products can be improved by incorporation of sodium caseinate and microbial transglutaminase (Cofrades et al. 2011). In fact, formation of large polymeric protein aggregates due to cross-linking of adjacent protein molecules could be catalyzed by MTGase, thereby improving functional and textural characteristics, specially cohesiveness of the food products (Martínez et al. 2014). The texture analysis of noodles [plant protein based product, prepared by wheat flour, soy protein isolate (SPI), and microbial transglutaminase] showed that, MTGase incorporation has a positive effect on hardness and chewiness of the noodles. The increases in hardness, springiness, and chewiness of batters upon MTGase treatment were related to the formation of strong and tight protein network that could trap the starch granules of the wheat flour, limiting extra swelling of starch granules and inhibiting the softening of the texture (Aalami and Leelavathi 2008). Moreover, the gel forming ability of soy protein is intensified by MTGase and consequently is improved the network density of the soy protein based product (Gan et al. 2009).
Cooked veggie burgers
As Table 2 indicates, cutting force (hardness) of cooked burgers was lower than those of raw samples. The decreases in textural parameters and cutting force can be related to denaturation of proteins during cooking process which led to a decrease in water holding capacity, an increase in cooking loss and shrinkage of the protein network (Velioğlu et al. 2010). Changes in concentration of SC (from 0 to 2%) significantly increased hardness and force for fracture of batter values (from 659 to 747.24 g).
MTGase was more significantly effective in improving textural properties than SC and this effect was dependent on the enzyme concentration. Combination of SC and enzyme provided synergistic effect on hardness and fracture values of samples. The observed increase in textural features is attributed to a much stronger protein network formed with the addition of MTGase (Canto et al. 2014). Also, the heat processing of samples allows protein unfolding and exposure of reactive groups ultimately improves tenderness of the product (Canto et al. 2014). Our results support the findings of several previous investigations which indicated that the addition of MTGase alone or in combination with SC could enhance textural properties of various protein based products (Gan et al. 2009; Pietrasik 2003). However, Cofrades et al. (2011) reported that the addition of MTGase/caseinate did not have any significant effect on improving texture of low-salt restructured poultry. This could be due to the fact that the effect of MTGase on protein network is species specific (Ahhmed et al. 2009a, b). The poor texture and light color of conventional veggie burgers (control sample) were critical factors, which negatively affect consumer acceptance. Production of veggie burgers by using MTGase/caseinate proteins improves the texture and color properties of burgers which had profound influence on the acceptance of the end product.
Electrophoretic analysis
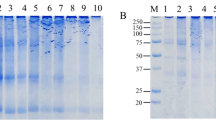
Electrophoresis experiments provide a denatured (SDS) and reduced (DTT) conditions to break down non-covalent and disulfide bonds. Therefore, this analysis reveals covalent bonds between proteins. The Electrophoresis profiles of soy burger batters treated with 0.0–0.75% MTGase and 0.0–2% SC are shown in Fig. 2a. The results indicated that distinct protein bands were not formed on the SDS–PAGE and formed smearing (poorly resolved protein) on the gel. Smearing is likely due to the presence of protein–carbohydrate derivatives, which will have a wide range of molecular weights and consequently produce a smear of bands (Davis et al. 2007) However, three zones of different molecular weights can be distinguished in the electrophoresis profile of MTGase treated samples; (1) high molecular weight zone (H zone: MW > 200 kDa), (2) a smear of middle molecular weight protein (M zone) and (3) low molecular weight zone (L zone: MW < 15 kDa). As seen in Fig. 2a, after enzymatic treatment, changes in the lanes were evident. All concentrations of MTGase treated samples showed H zone on the top of the gel, which was formed by polymerization of protein components in batter induced by MTGase, while H zone was not found in the control samples. In the lanes corresponding to the samples treated with MTGase, the polymer fraction (H zone) increased, while the smear (M zone) and monomer fractions (L zone) were less dense. Protein migration in electrophoresis experiment can mainly be supported by both hydrodynamic size and net charge. Since the SDS gives a negative charge to protein, the higher electrophoretic mobility mainly relies on the hydrodynamic size of protein molecule, which is changed by intramolecular cross-linking that catalyzed by MTGase. In other words, large molecular components formed by MTGase with or without sodium-caseinate could not migrate well on gel and provided an extra band on the top of the gel (Djoullah et al. 2016; Kilic 2003). When SC was added to the burger formulation, H zone was also not found (lanes 2 and 3). Addition of SC brings about non-covalent interaction between SC and other proteins of the burgers, thereby enhancing the mechanical properties of burgers, a process which is probably disrupted by DSD. Similar result is reported by Hu et al. 2015 on the effect of curdlan on SDS–PAGE pattern and the texture properties of hairtail (Hu et al. 2015). These authors suggested that hydrogen bonds between protein molecules were intensified with the increased level of curdling in hairtail gel and modified the gel texture.
a Electrophoretic patterns of soy burger batters incubated with MTGase and SC. MW molecular weight standards (in kDa), Control soy burger not-treated with MTGase, SC sodium caseinate, E microbial transglutaminase enzyme. b Line chart representing comparative effects of MTGase on different substrates
Also Fig. 2a shows that polymer content increased as a function of the MTGase level. The greatest polymerization was appeared in the lanes corresponding to 2% SC and 0.75% MTGase. This result was shown to be concomitant with improved physical properties of the burgers (Table 2). The increase of SC level in combination with MTGase resulted in increasing polymer content, suggesting that the SC is a good substrate for MTGase. Results of Fig. 2b supported this hypothesis that MTG reacts with soy, gluten, and SC. From this figure it can be concluded that MTGase had the largest effect on SC 2%.
Electrophoresis patterns of soy protein
The effect of different MTGase concentration on SDS–PAGE patterns of soy protein was also investigated (Fig. 3a, b). Soy proteins mainly consist of two important fractions; (1) High molecular weight fraction (glycinin), and (2) low molecular weight fraction (β-conglycinin). The glycinins (11S) are hexamer, each composed of two trimers of three monomers subunits. Each monomer in turn is made of an acidic and a basic polypeptide chains linked by disulfide bonds. Beta-conglycinins (7S) are trimers forrmed by various combinations of three polypeptide subunits: the α’, α, and β subunits. When soy protein was incubated with MTGase following electrophoretic changes were detectable:
Electrophoretic patterns of soy and gluten proteins incubated with MTGase and SC. a Electrophoretic patterns of soy proteins incubated with different concentrations of transglutaminase. b Soy protein bands at different concentrations of MTGase. B1–B8 protein bands numbered from bottom to top of the gel, respectively, B9 protein polymers formed by MTGase. c Electrophoretic patterns of gluten incubated with transglutaminase; d gluten bands at different concentrations of MTGase. B1–B6 protein bands numbered from bottom to top of the gel, respectively. B7 protein polymers formed by MTGase. E microbial transglutaminase enzyme, S soy protein, G gluten
-
1.
A progressive decrease of the subunits of 7 s globulin and finally the disappearance of ά subunit band at 0.75% enzyme. The attenuation of these bands might be due to the formation of intramolecular covalent bonds in the polypeptide chains and production of a more compact protein structure (Djoullah et al. 2016).
-
2.
The attenuation of acidic and basic subunits (A and B, respectively) of glycinin fraction in the enzyme treated sample and the elimination of A3 and B3 bands at 0.75% MTGase level. Other studies have shown that at the ionic strengths of 0.03–0.5, the acidic subunits were laid outside the glycinin complex and were more significantly affected by the enzyme, but the basic subunits were present in the interior of the glycinin molecule and remained resistant to the MTGase (Wu et al. 2016). However, in the current research, elimination of B3 band was observed, probably as a result of a longer incubation time (1 h at the room temperature followed by 24 h at 4 °C) which made B3 subunits more accessible to enzyme. Tang et al. 2006 reported that after 240 min treatment of soy protein with MTGase the density of basic subunits significantly decreased but this decline did not lead to removal of these bands. These authors considered that the SDS–PAGE patterns of the protein were relying on the incubation time (Tang et al. 2006).
-
3.
The formation of the large polymers (at the top of the gel; MW > 200 kDa) in the samples treated with MTGase. When SPI was incubated with MTGase, the monomer fraction was decreased while the polymer fraction was increased (Djoullah et al. 2016).
This shows that SPI is a substrate for transglutaminase and can participate in the polymerization (Fig. 2b).
Electrophoresis pattern of gluten
Gluten separated into three fractions on the SDS–PAGE based on their MW. These are the high-molecular weight subunits of glutenin (HMW-GS), a medium-molecular-weight group (omega-gliadins) and a low-molecular-weight group including α and γ-gliadins (LMW-GS) (Aminlari and Majzoobi 2002). As seen in Fig. 3c, d electrophoretic pattern was slightly changed after MTGase treatment. The medium-molecular-weight group (omega-gliadins) and LMW, showed density attenuation in samples containing MTGase as a function of enzyme level. The results of SDS–PAGE analysis in the present study are in agreement with the findings of several studies (Ahirwar et al. 2015). However, the production of high-MW bands in MTGase-treated samples was notably non-extensive. Three explanations for this phenomenon are suggested; (1) some peptides (especially < 14 kDa) in gluten remained in a compact structure, and a part of glutamine residue was not accessible for MTGase (2) Wheat protein is glutamine-rich and lysine-poor consequently it was resistant to intramolcular cross-linking induced by MTGase (Chin et al. 2009); (3) gluten contained cysteines which contributes to the formation of disulfide bonds (Aminlari and Majzoobi 2002).
In conclusion, SDS–PAGE patterns show that microbial transglutaminase catalyzed intra- and intermolecular cross-linking reaction of proteins from one source or two sources used in this research.
Sensory properties
The sensory characteristics (color, taste, appearance, mouth feel, and overall acceptability) are presented in Table 3. The sensory analysis was performed based on 5-point hedonic scale and scores higher than ‘3’ are considered acceptable. The sensory scores of every parameter for treated samples were higher than 3, as compared to the control. There was a significant difference (P < 0.05) in color, taste, appearance, mouth feel, and overall acceptability between treated and control samples. These results are in agreement with other reports, implying the addition of MTGase and SC enhances acceptance of various meat analogues samples. It seems that MTGase, through formation of crosslinks between protein molecules results in improvement of texture and quality attributes of veggie burgers, such as WHC, expressible moisture, cooking loss and shrinkage, thereby enhancing the sensory properties of samples and improving overall acceptability of veggie burgers.
Conclusion
High consumption of red meat and its products poses health problems. Substitution of red meat with plant proteins might be beneficial to the consumer’s health. The purpose of this research was to produce veggie burgers based on soy protein and to study the effects of microbial transglutaminase/caseinate (MTGase/SC) on physicochemical and sensory properties of veggie burger. The results indicated that the addition of MTGase alone or in combination with SC catalyzes the cross-linking reaction between primary amines and forms high-molecular weight polymers and subsequently, improves textural parameters and color of veggie burger and enhances the acceptance of the product.
References
Aalami M, Leelavathi K (2008) Effect of microbial transglutaminase on spaghetti quality. J Food Sci 73(5):C306–C312
Ahhmed A, Kawahara S, Ohta K, Nakade K, Soeda T, Muguruma M (2009a) Differentiation in improvements of gel strength in chicken and beef sausages induced by transglutaminase. Meat Sci 76(3):455–462
Ahhmed AM, Kuroda R, Kawahara S, Ohta K, Nakade K, Aoki T, Muguruma M (2009b) Dependence of microbial transglutaminase on meat type in myofibrillar proteins cross-linking. Food Chem 112(2):354–361
Ahirwar R, Jayathilakan K, Reddy KJ, Pandey M, Batra H (2015) Development of mushroom and wheat gluten based meat analogue by using response surface methodology. IJAR 3(1):923–930
Aminlari M, Majzoobi M (2002) Effect of chemical modification, pH change, and freezing on the rheological, solubility, and electrophoretic pattern of wheat flour proteins. J Food Sci 67(7):2502–2506
Baer AA, Dilger AC (2014) Effect of fat quality on sausage processing, texture, and sensory characteristics. Meat Sci 96(3):1242–1249
Berry B, Bigner M (1996) Use of carrageenan and konjac flour gel in low-fat restructured pork nuggets. Food Res Int 29(3):355–362
Canto AC, Lima BRC, Suman SP, Lazaro CA, Monteiro MLG, Conte-Junior CA, Freitas MQ, Cruz AG, Santos EB, Silva TJ (2014) Physico-chemical and sensory attributes of low-sodium restructured caiman steaks containing microbial transglutaminase and salt replacers. Meat Sci 96(1):623–632
Chin KB, Go MY, Xiong YL (2009) Konjac flour improved textural and water retention properties of transglutaminase-mediated, heat-induced porcine myofibrillar protein gel: effect of salt level and transglutaminase incubation. Meat Sci 81(3):565–572
Cofrades S, López-López I, Ruiz-Capillas C, Triki M, Jiménez-Colmenero F (2011) Quality characteristics of low-salt restructured poultry with microbial transglutaminase and seaweed. Meat Sci 87(4):373–380
Davis J, Gharst G, Sanders T (2007) Some rheological properties of aqueous peanut flour dispersions*. J Texture Stud 38(2):253–272
Djoullah A, Krechiche G, Husson F, Saurel R (2016) Size measuring techniques as tool to monitor pea proteins intramolecular crosslinking by transglutaminase treatment. Food Chem 190:197–200
Dzudie T, Scher J, Hardy J (2002) Common bean flour as an extender in beef sausages. J Food Eng 52(2):143–147
Gan C-Y, Ong W-H, Wong L-M, Easa AM (2009) Effects of ribose, microbial transglutaminase, and soy protein isolate on physical properties and in vitro starch digestibility of yellow noodles. LWT-Food Sci Technol 42(1):174–179
Girolami A, Napolitano F, Faraone D, Braghieri A (2013) Measurement of meat color using a computer vision system. Meat Sci 93(1):111–118
Herranz B, Tovar CA, Borderias AJ, Moreno HM (2013) Effect of high-pressure and/or microbial transglutaminase on physicochemical, rheological, and microstructural properties of flying fish surimi. IFSET 20:24–33
Hong G-P, Xiong YL (2012) Microbial transglutaminase-induced structural and rheological changes of cationic and anionic myofibrillar proteins. Meat Sci 91(1):36–42
Horwitz W, Horwitz W (2000) Official methods of analysis of AOAC international. AOAC international, Rockville, Maryland, USA (No. C/630.240 O3/2000)
Hu Y, Liu W, Yuan C, Morioka K, Chen S, Liu D, Ye X (2015) Enhancement of the gelation properties of hairtail (Trichiurus haumela) muscle protein with curdlan and transglutaminase. Food Chem 176:115–122
Johnson G (2013) Eat smart-metric edition. NoPaperPress LLC, Eugene, p 52
Kilic B (2003) Effect of microbial transglutaminase and sodium caseinate on quality of chicken döner kebab. Meat Sci 63(3):417–421
Krintiras GA, Göbel J, Van der Goot AJ, Stefanidis GD (2015) Production of structured soy-based meat analogues using simple shear and heat in a Couette cell. J Food Eng 160:34–41
Li C-T, Wick M (2001) Improvement of the physicochemical properties of pale soft and exudative (PSE) pork meat products with an extract from mechanically deboned turkey meat (MDTM). Meat Sci 58(2):189–195
Martínez MA, Robledo V, Velazquez G, Ramírez JA, Vázquez M, Uresti RM (2014) Effect of precooking temperature and microbial transglutaminase on the gelling properties of blue crab (Callinectes sapidus) proteins. Food Hydrocoll 35:264–269
Meilgaard MC, Carr BT, Civille GV (2006) Sensory evaluation techniques. CRC Press, Boca Raton
Min B, Green BW (2008) Use of Microbial transglutaminase and nonmeat proteins to improve functional properties of low NaCl, phosphate-free patties made from channel catfish (Ictalurus punctatus) belly flap meat. J Food Sci 73(5):E218–E226
Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB (2012) Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 172(7):555–563
Pietrasik Z (2003) Binding and textural properties of beef gels processed with κ-carrageenan, egg albumin, and microbial transglutaminase. Meat Sci 63(3):317–324
Pietrasik Z, Jarmoluk A, Shand P (2007) Effect of non-meat proteins on hydration and textural properties of pork meat gels enhanced with microbial transglutaminase. LWT-Food Sci Technol 40(5):915–920
Su YK, Bowers JA, Zayas JF (2000) Physical characteristics and microstructure of reduced-fat frankfurters as affected by salt and emulsified fats stabilized with nonmeat proteins. J Food Sci 65(1):123–128
Tang CH, Wu H, Yu HP, Li L, Chen Z, Yang XQ (2006) Coagulation and gelation of soy protein isolates induced by microbial transglutaminase. J Food Biochem 30(1):35–55
Van Trijp HC, Van Kleef E (2008) Newness, value and new product performance. Trends Food Sci Technol 19(11):562–573
Velioğlu HM, Velioğlu SD, Boyacı İH, Yılmaz İ, Kurultay Ş (2010) Investigating the effects of ingredient levels on physical quality properties of cooked hamburger patties using response surface methodology and image processing technology. Meat Sci 84(3):477–483
Wang C, Zayas J (1992) Comparative study of corn germ and soy proteins utilization in comminuted meat products. J Food Qual 15(2):153–167
Wild F, Czerny M, Janssen AM, Kole AP, Zunabovic M, Domig KJ (2014) The evolution of a plant-based alternative to meat. Agro Food Ind Hi Tech 25:1
Wu M, He Q, Hong Y, Wang S (2016) Preheating of kidney bean proteins enhances cross-linking and functional properties with chicken myofibrillar proteins induced by transglutaminase. LWT-Food Sci Technol 65:816–822
Yang H-S, Choi S-G, Jeon J-T, Park G-B, Joo S-T (2007) Textural and sensory properties of low fat pork sausages with added hydrated oatmeal and tofu as texture-modifying agents. Meat Sci 75(2):283–289
Acknowledgements
Funding was provided by Shiraz University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Forghani, Z., Eskandari, M.H., Aminlari, M. et al. Effects of microbial transglutaminase on physicochemical properties, electrophoretic patterns and sensory attributes of veggie burger. J Food Sci Technol 54, 2203–2213 (2017). https://doi.org/10.1007/s13197-017-2614-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2614-8