Abstract
This aim of this study was aimed to evaluate the impact of HHP (high hydrostatic pressure) pre-treatment on the drying behavior of cashew slices, water adsorption isotherms, on extraction kinetic of total phenolic compounds (TPC) and antioxidant activity (AA). The drying kinetics were performed for cashew slices without pre-treatment (control) and pre-treated with 200 MPa (HHP1), 350 MPa (HHP2) and 500 MPa (HHP3) at a temperature of 70 °C in an electric oven (1200 W). Drying kinetics experimental data were fitted using empirical and diffusive models (third type boundary condition). The kinetics of ultrasound-assisted (40 kHz and 132 W) extraction of total phenolic compounds (TPCs) were realized and was determined AA (ABTS• + , DPPH• and FRAP) and water adsorption isotherms. The application of pressure 500 MPa (HHP3) provided an increase in the moisture transport process, a higher drying rate and shorter process time (40%). The effective diffusivity ranged from 1.2546 × 10–8 m2 min−1 (control) to 3.2045 × 10–8 m2 min−1 (HHP3). The extraction of TPC he was higher in the time of 180 min, emphasis for HHP3 who presented 154.48 mg GAE 100 g−1. Higher retention percentages AA by the three methods were observed for the slices pre-treated (HHP3) and the adsorption isotherms which presented characteristic of the type II curves. Therefore, the results of this study provide information for the potential application of HHP as a drying pre- treatment.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Practical application
High hydrostatic pressure (HHP) can improve the performance of the drying process, minimizing thermal damage to the final products and promoting the improvement of their overall quality. This study was based on pretreatment with HHP, for which we investigated changes in drying parameters, extraction of phenolic compounds (TPC), antioxidant activity (AA) and water adsorption isotherms of cashew. It was observed that HHP and ultrasound-assisted extraction had a positive effect to increase TPC extraction from food materials. Therefore, HHP as a pretreatment for convective drying of cashew is a promising technology.
Introduction
Anacardium occidentale L. is native to Brazil and widespread to other tropical countries. It has relevant socioeconomic importance for the North and Northeast states due to the production of nuts which is highly appreciated all over the world. The peduncle, also called cashew, represents more than 90% of the total edible parts of the fruit, but it is considered an industrial residue because most of its production deteriorates in the soil after removing the nut. Although the peduncle (cashew) can also be consumed as juice, ice cream and other foods, only 10% of its production is used by the industry [1,2,3]. According to Souza et al. [4], cashew is a pseudo fruit with a short shelf life and very perishable having approximately 80% water in its composition.
Some studies with the application of conservation methods for cashew are available in the literature, such as: freeze-drying [5], convective drying [6], osmotic dehydration [7], spouted bed [8] and spray dryer [9]. Drying based on the use of hot air is the most widely used method to preserve fruits with high perishability and, consequently, obtain dry products [10]. However the drying process, it has some disadvantages, such as: high energy consumption which is usually provided by the use of fossil fuels or electricity which is a real problem for the process, long process time and decreased product quality (Gościnna et al., 2020). Considering the growing demand for cost reductions and minimal environmental impacts, different strategies are being studied to improve food drying, including the application of pre-treatments [11,12,13].
Emerging technologies or combined methods have been reported as pre-treatments to optimize the drying process [14]. These methods offer many benefits for food pre-treatment such as improving energy consumption and efficiency, product quality, and reduction in processing time [15]. High hydrostatic pressure (HHP) pre-treatment is a non-thermal method, which has gained commercial attention in the last decade, and is used to preserve nutritional and organoleptic characteristics of foods [16]. According to Hulle & Rao [17] HHP can be applied to improve water mass transfer during the drying process.
HHP treatment is governed by the Le Chatelier’s principle, which implies that reactions or phase transitions associated with a decrease of volume are favoured, while reactions accompanied with a volume in-crease are inhibited. HHP units consist of a horizontal HP vessel and an external pressure generating device. The pressure is transmitted uni-formly and instantaneously throughout the food system independent of its geometry and size. In general, small molecules like vitamins, minerals and aroma compounds are slightly affected under HHP process because no covalent bonds are split [18]. Furthermore, HHP has been used to extract bioactive compounds in some food matrices, being even denominated as “high hydrostatic pressure extraction” [19].
This pre-treatment can be used at different pressures ranging from 100 to 1000 MPa, with a range of applications comprising the pharmaceutical, metallurgical and food industries [20]. However, there are no studies reporting drying using high hydrostatic pressure (HHP) pre-treatment of cashew, this demonstrates the need for drying studies with innovative pre-treatment techniques. Therefore, this work aimed to evaluate the impact of high hydrostatic pressure pre-treatment (200–500 MPa) on the drying behavior of cashew slices at 70 ºC, on kinetic of ultrasound-assisted extraction of total phenolic compounds (TPC), antioxidant activity (AA) and water adsorption isotherms.
Materials and methods
Ripe cashew (Anacardium occidentale L.) was supplied by the Central Supply and Agricultural Services (CEASA). Cleaning and sanitizing were performed using an aqueous solution of sodium hypochlorite with a concentration of 200 mg L−1 of free chlorine for 15 min and then rinsed with running water. A stainless-steel knife was used to cut the cashew slices, and a digital caliper was used to guarantee that the cashew slices had a thickness of 5.0 mm (considering the geometry of a slab). The cashew slices presented an initial moisture content of 93.34% wet basis.
Pre-treatment
Cashew slices (5 mm thickness) were subjected to high hydrostatic pressure (HHP) pre-treatment following the procedure of Yucel et al. [21], Zhang et al. [22] and Zhang et al. [23]. Briefly, 300 g of cashew slices were packed in low density polyethylene bags before being placed in the 1 L chamber of a high hydrostatic pressure equipment (Hiperbaric SA, Burgos). The slices were subjected to high hydrostatic pressure (HHP) pre-treatment at a temperature of 25 °C, at pressures of 200 MPa (HHP1), 350 MPa (HHP2) and 500 MPa (HHP3), for 5 min (conditions defined by [20]. The slices without the HHP pre-treatment were taken as the control sample.
Drying kinetics
In triplicate, drying kinetics was performed for cashew slices (5 mm thickness) without pre-treatment (Control) and for slices submitted to pre-treatments of high hydrostatic pressure (HHP1, HHP2 and HHP3). The circular cashew slices (300 g) were uniformly distributed on metallic trays (dimensions of 15 × 30 cm) and drying was performed using a 1200 W electric oven (Semp Easy, model FO3015PR2) with dimensions 25 × 41.5 × 32.2 cm. The drying temperature used was 70 °C, and the temperature was controlled using a thermostat (conditions defines in preliminary experiments, data not shown). The moisture ratio of the drying process was calculated by Method No. 1934, [24]. The moisture loss was recorded by using a digital balance of 0.001 g accuracy (Bel, model M214AIH, São Paulo), drying process was continued until the constant reading of mass (equilibrium) was recorded for all cases.
Empirical models
The empirical functions of Lewis [25], Page [26], Henderson and Pabis [26], and Silva et alii [27] were adjusted to the experimental data sets by non-linear regression using LAB Fit software [28] which made it possible to determine the drying curves as a function of time. The results from the empirical models were evaluated and compared using the chi-squared (\(\chi^{2}\)) and the coefficient of determination (R2).
Diffusion model
The drying process of cashew slices under different conditions of study was described by a diffusion model, adjusted to the experimental data, considering the cashew slices as having the geometry of an infinite slab. According Santos et al. [29], when considering that the cashew slices are homogeneous and isotropic and that the convection mass transfer coefficient and the effective diffusion are constant throughout the drying process, the boundary condition of the third type can be used to solve the diffusion equation. For the third type boundary conditions, the analytical solution of the diffusion equation is given by the series shown in Eq. 1 [30] in which only the first sixteens terms of the infinite series were used:
where, L is the thickness of the infinite slab, Def is the effective mass diffusivity, and t is the drying time. The \(B_{n}\) coefficient is determined using Eq. 2.
where, Bi is the mass transfer Biot number given by Eq. 3:
The parameter h is the convective mass transfer coefficient. On the following Eqs. 1, 2, and 3. The \(\mu_{n}\) are the roots of the transcendental Eq. 4 which is the characteristic equation of the infinite slab.
The Eq. 1 was fitted to the experimental data using the optimization protocol proposed by Silva et al. [31]. Once the process parameters for the diffusive model were determined, the moisture distribution at a given moment can be determined according to Eq. 5 [13].
where, An is given by Eq. 6:
Extraction kinetics of total phenolic compounds
The kinetics of extraction of total phenolic compounds (TPC) were performed for fresh cashew slices, control and for those submitted to pre-treatments (HHP1, HHP2 and HHP3). The extraction procedure was assisted by ultrasound with indirect contact at a frequency of 40 kHz and power of 132 W (Unique, USC-2850A, Brazil), aqueous extracts were prepared in a 1:10 ratio (cashew:water), and 1 mL aliquots were withdrawals every 20 min for 180 min.
The TPC in the extracts was quantified by the spectrophotometric method with Folin-Ciocalteu by following the methodology of Osae et al. [15]. The absorbance was measured at 760 nm in a SP-2000 UV spectrophotometer (Spectrum, Shanghai, China), and the results expressed as mg of gallic acid equivalents (GAE) 100 g−1. Standard gallic acid solutions (6.25–100 mg mL−1 of gallic acid) were used to develop the standard curve (R2 = 0.984). The results obtained for extracting TPC from cashew slices under different conditions were fitted using an empirical function f (t, a, b) with two adjustment parameters, choosing the empirical equation obtained by the Peleg model (Eq. 7) [29, 32].
where: f is the content of total phenolic compounds (TPC); “a” and “b” are model parameters and t is the extraction time.
The fitting of the empirical equation to the experimental data was performed using the computer program LAB Fit software [28], in addition, the coefficient of determination (R2) and the chi-square function (\(\chi^{2}\)).
Antioxidant activity (AA)
The antioxidant determination was performed by the methods: ABTS• + (2,2’ – AZINO-BIS (3-ethylbenzo-thiazoline-6-sulfonic acid)), DPPH• (1,1‐diphenyl‐2‐picrylhydrazyl) e FRAP (Ferric reducing antioxidant power capacity). The antioxidant activity (ABTS• +) was determined using the method proposed by Re et al. [33] with modifications made by Rufino et al. [34]. Briefly, 30 µL aliquot of the extract was mixed with 3.0 mL of the ABTSº + radical. Absorbance was determined at 734 nm after 6 min of mixing. The result was expressed in µM trolox/g. The antioxidant activity of DPPH• was performed according to the methodology described by Maria do Socorro et al. [35] with adaptations. Briefly, 0.1 mL aliquots of the extract were mixed with 3.9 mL of DPPH (0.06 mmol/L). The absorbances of the samples were read at 515 nm every minute until stabilization. The result was expressed as percentage inhibition of DPPH (Inhibition (%)). The FRAP assay was performed according to method of Benzie and Strain [36]. Briefly, 90 mL aliquots of the extract were mixed with 270 mL of deionized water and 2.7 mL of FRAP reagent. The solutions were homogenized and kept in a water bath at 37 °C for 30 min. Absorbance was determined at 595 nm. The result was expressed as µM Fe2+/g.
Water adsorption isotherms
The cashew slices dried water adsorption isotherms (equilibrium water content versus water activity) were determined at 25 °C. The equilibrium water content (Xeq), expressed in percentage (%), on a dry basis, and was determined by the ratio between the water mass and the dry mass of the samples. Five mathematical models Peleg [37], GAB [38], Oswin [39] and Lang Steinberg Smith [40] were adjusted to the experimental data. In literature, these models have been successfully applied to describe the water adsorption isotherms of different food products. The results of the adjusted models to the experimental data for the water adsorption isotherms were evaluated using the determination coefficient (R2) and the mean percentage deviations (P) [41].
Statistical analysis
It was performed using Assistant beta 7.7 software (available as freeware from: http://www.assistat.com). Experiments were carried out with three repetitions (n = 3). Statistical significance (p < 0.05) was established by One-way ANOVA with Tukey’s HSD post hoc test.
Results and discussion
Drying kinetics and empirical models
To evaluate the effect the different pressures applied, initially the moisture data obtained in the drying process were converted into moisture ratio and the empirical mathematical models listed were adjusted to the data obtained experimentally. Table 1 shows the values obtained for the parameters of the adjusted mathematical models. The mathematical models of Page and de Silva et alii showed coefficient of determination (R2) values greater than 0.99 for all tested conditions. However, only the Silva et alii model had values low of the chi-square function (\(\chi^{2}\)) in the order of 10–2 for all applied pressures which increases the confidence level of the adjustment. Based on these results, the model by Silva et alii was chosen as the best model to represent the drying of cashew slices under the different conditions studied. Thus, the moisture content at any time during the drying process can be reliably estimated using this model.
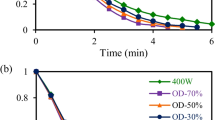
Figure 1 shows the drying kinetic curves for all conditions studied, adjusted to the model by Silva et alii. The total drying time of the control slices (without HHP) was about 5 h longer than the time of the slices pre-treated with HHP3 representing a reduction in processing time of 40%. However, when comparing the control slices with those pre-treated with HHP1 and HHP2, there was a reduction in process time of 16% and 32%, respectively. The significant effect pre-treatment with HHP on the drying curves is very clear where the drying time presented a decreasing behavior. Application of HHP causes permeabilization of the cell structure because of the cell disintegration, which leads to significant changes in the tissue structure resulting increased mass transfer rates and time drying reduced [42].
According to Almeida et al. (2021) a good approximation from empirical mathematical models is crucial to represent the drying rate curves using the derivative of these models. Therefore, the drying rates calculated by the derivative of the empirical model of Silva et alii are shown in Fig. 2. The results showed that the drying rate is proportional to water content since higher values were obtained in the initial moments of the process where the product had higher water content values. However, when the drying rate has zero value, water content decrease can be verified from start to finish of the process and an equilibrium condition is, in fact, reached. The results also showed that higher drying rates are linked to the slices pre-treated with HHP (HHP3 > HHP2 > HHP1 > Control). Same behavior was also observed in drying process of fruits as: green plums pre-treated with HHP (pressure ranging from 50 to 400 MPa) [42] and papaya [43], nectarine [44], guava [45] and strawberries [46], submitted different pre-treatments non-thermal.
Diffusion model
The effective diffusivity coefficient (Def), the convective mass transfer coefficient (h) and the Biot number (Bi) of the cashew slices pre-treated under different pressures (200-500 MPa) were determined by simulation (see Diffusion model section) and the results are shown in Table 2. The diffusion model and boundary condition of the third type are suitable for the drying kinetics of the cashew slices control and pre-treated with HHP. The chi-square function (\(\chi^{2}\)) showed low values, in the order of 10–2 and 10–3, and the coefficient of determination (R2) had values greater than 0.99 for every condition.
The cashew slices that were not pre-treated (control) had an effective diffusivity (Def) of 1.2546 × 10–8 m2 min−1. However, it was observed that the application of HHP3 provided an increase in diffusivity effective to 3.2045 × 10–8 m2 min−1, proving the drying time reduction in the electric oven. The increase in these values corresponds to the increase in the drying rate (Fig. 2B). According Verma et al. [47], HHP pre-treatment provides exposure of new surfaces further increasing mass transfer.
It can also be observed (Table 2) the convective mass transfer coefficient (h) had a behavior similar to the effective diffusivity, increasing its values from 2.3839 × 10–5 m min−1 (control) to 4.9340 × 10–5; 9.1330 × 10–5 and 11.0346 × 10–5 m min−1 for the slices pre-treated by HHP1, HHP2 and HHP3, respectively. In accordance to Ferreira et al. [48] the results obtained can serve as starting values for optimization processes regarding mass transport properties (Def e h) that involve a more realistic description of the drying process physics of cashew slices. It could be such as those using models rescue from numerical solutions of the diffusion equation that affect deformations by contraction and/or compositional variation (moisture content) on transport phenomena.
The values of the number of Biot (Bi) (4.75–16.00), obtained under different pre-treatment pressures, reveal a resistance to mass flow (water) on the surface of the samples. Therefore, they show that the solution of the diffusion equation can be adequate to describe the drying process. For that, it should be considered a boundary condition of the third type, even with limitations, and should not be considered the variation in the product dimensions and the non-linearities of the thermophysical properties [49].
It should also be inferred that the largest truncation error of the infinite series given by Eq. (1) occurs for t = 0 and this error varies according to the Biot number referred to drying [50]. To define the number of terms (nt) to be used in Eq. (1), the study by Silva et al. [50] was taken into account. These researchers observed that, for Bi = 0.001, only 1 term is needed to obtain X*(0) = 1.0 which is the expected value. On the other hand, when the number of Biot increases, the truncation error increases significantly. Thus, it is necessary to significantly increase the number of terms in the series to obtain negligible or at least acceptable truncation errors [48]. Once the process parameters for the diffusion model were set, the moisture distribution at a given time t was determined by solving the x-position dependent diffusion equation, with the origin established at the center of the cashew slice thickness dimensions.
In Fig. 3, it is possible to see the distribution of moisture content inside the cashew slices (control) and pre-treated with different pressures HHP. The one-dimensional domain (thickness) was divided into 100 parts, and the x coordinate of the center point of each part was replaced by Eq. (5) which allowed the moisture content determination of each of these 100 parts along the x direction (thickness). Over a period of 60 min, the dimensionless moisture content is quite variable for all conditions, being more pronounced in the pre-treatment with HHP3. It means that the effective diffusivity is greater in the drying of slices subjected to HHP3 as predicted by the model shown in Table 2, not promoting the formation of a high-water gradient in the sample. Consequently, it made for a shorter and more homogeneous drying process. Furthermore, it is also possible to observe that this model allows determining the internal stress of food materials [51].
Extraction kinetics of total phenolic compounds (TPC)
Phenolic compounds are abundant secondary metabolites in most fruits and vegetables and have in common an aromatic benzene ring with one or two hydroxyl group substitutions [52]. Table 3 shows the values obtained for the Peleg model adjusted to the experimental data of the kinetics of ultrasound assisted TPC extraction from fresh and dehydrated cashew slices under different conditions, in addition to the statistical parameters, coefficient of determination (R2) and chi-square function (\(\chi^{2}\)).
The coefficients of determination (R2) found were higher than (R2 > 0.97) and ranged from 0.977 to 0.991 and the values of the chi-square function (\(\chi^{2}\)) were in the order of 102 (1.852 × 102 ≤ \(\chi^{2}\) ≤ 5.401 × 102). This information indicated that the Peleg’s model presents a good fitting to the experimental data of the TPC extraction kinetics and proved to be adequate to describe the process. According by Santos et al. [29], Peleg's model can be interpreted as an equation that results from the second-order concentration rate law, making it possible to give a physical meaning to the parameters obtained by curve fitting. Peleg's model also proved to be suitable for extracting TPC from apple cubes [53], citrus industry residues [54] and cornelian cherry [55].
It can be seen in Fig. 4 that the highest concentrations of TPC were reached in the time of 180 min of extraction for all conditions studied, in which they presented values of 197.76 mg GAE 100 g−1 (Fresh), 105.13 mg GAE 100 g−1 (Control), 111.82 mg GAE 100 g−1 (HHP1), 119.76 mg GAE 100 g−1 (HHP2) and 154.48 mg GAE 100 g−1 (HHP3). According to Babotă et al. [56], this efficiency of longer extraction time comes from acoustic cavitation, which is the main process involved in ultrasound assisted extraction, inducing secondary changes in plant material through fragmentation, localized erosion, pore formation or shear forces. All these factors increase the breakdown of cell walls, increasing the contact of extractable compounds with the solvent and their partition in the extraction environments.
It can also be seen in Fig. 4 the effect of pre-treatment with HHP, in which the pre-treated samples showed higher TPC values compared to the control sample. Highlight for HHP 3, which showed lower TPC degradation. These results concluded that HHP efficiently improves TPC extraction from cashew slices. Similar effects were evidenced by Casazza et al. [57], Eroman Unni et al. [58] and Park et al. [59] in their studies, where they reported that the application of HHP also promoted higher levels of TPC. This can be explained by the fact that HHP contributes to the physical destruction of plant tissue and causes changes in cell wall permeability, promoting the extraction of intracellular compounds and increasing the bioavailability of phytonutrient yield [60], Park et al. al., [59]. According to Zhao et al. [61], HHP at pressures ranging from 200 to 600 MPa at moderate temperatures shows promise for preserving TPC and AA in fruits and vegetables.
According to the results of Table 3 and Fig. 4, it is evident that Peleg's model can adequately describe the experimental data and can be used to predict TPC levels throughout the extraction process.
Antioxidant activity (AA)
According Dede et al. [62], antioxidant activity is related to the antioxidant vitamins, carotenoids and polyphenol contents of fruits and vegetable products. Table 4 shows the values obtained for antioxidant activity by the ABTS• + , DPPH• and FRAP methods of fresh and dehydrated cashew slices. Drying at 70 ºC negatively affected the antioxidant activity of the dehydrated cashew slices, in which, reductions of 43%, 40% and 49% for the ABTS• + (9.695 µM trolox/g), DPPH• (16.364%) and FRAP (11.325 µM Fe2+/g) respectively, were achieved when comparing the control slices (without pre-treatment with HHP) with the fresh slices.
The application of different pressures of HHP (200–500 MPa) were effective in minimizing the reduction of the antioxidant activity of the dehydrated cashew slices. In this study, a positive correlation was found between pre-treatment and antioxidant activity, since samples HHP1, HHP2 and HHP3 showed higher values than the control treatment. However, there was no statistical difference (p > 0.05) between samples pre-treated with HHP1 and HHP2 which were only higher when compared to the control treatment. The pre-treatment HHP3 was the most efficient, reducing the AA by only 23%, 16% and 12% for the ABTS• + (13.016 µM trolox/g), DPPH• (23.059%) e FRAP (19. 456 µM Fe2+/g), respectively, when compared to fresh cashew slices.
This same behavior was also observed by Zhang et al. [63], in which the application of pressure 250 MPa increased the rate of free radical scavenging and by Torres-Ossandón et al. [52], where the retention of antioxidant activity significantly ncreased in the treatment with HHP above 300 MPa. Therefore, pre-treatment HHP3 had a significant positive effect in reducing the degradation of antioxidant properties of cashew slices. It is also believed that this behavior is directly related to that observed in the TPC (Fig. 4), as a result of the higher extraction achieved by the treatment HHP3.
Water adsorption isotherms
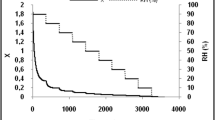
Table 5 presents the results obtained by fitting different mathematical models to the experimental data of water adsorption isotherms of cashew slices control and pre-treated with HHP at different pressures, as well as the coefficient of determination (R2) and the mean relative error (P%). The criteria for selecting the best model were the lowest P% and the highest R2. In this sense, the modeling results reported that the GAB model provided a satisfactory description of the water adsorption behavior with values of the coefficient of determination greater than 0.99 (R2 > 0.99) for all conditions studied and the lowest percentages of mean relative error, ranging from 0.936 to 1.746%. In the case of the other adjusted models [37], [39], [40], when jointly evaluating the statistical parameters (R2 and P%), it is observed that they did not reflect a close agreement between the experimental data and estimated adsorption and should not be chosen to represent the hygroscopic behavior of the material under study.
The parameter Xm of the GAB model presented values in the range of 7.801 to 25.011 when the pressure was increased from 200 to 500 MPa. According to Collazos-Escobar et al. [64], this parameter provides an estimate of the moisture content of the monolayer, which can be considered useful to guarantees stability during the storage fo material. Parameters C and K also showed a behavior defined according to the increase in pre-treatment pressure with HHP, presenting values in the range of 2.058 to 2.644 and 0.981 to 0.789, respectively. According to Lewicki [65] K values within the range of (0.24 ≤ K ≤ 1) also indicate a good fitting of the model to the experimental data. According to Zabalaga & Carballo [66], the results obtained in this study are important for predicting shelf life stability, which involves changes in humidity that may occur during storage and, therefore, is useful for the selection of packaging materials.
The goodness of fitting of the GAB model is illustrated in Fig. 5, in which the modeling and experimental results are plotted together. Higher levels of equilibrium water are observed with an increase in the pressure of the HHP, Al-Muhtaseb et al. [67] associates this fact with the low molecular weight food constituents (sugars) present in dehydrated cashews, which become more hygroscopic. According to the classification proposed by Brunauer et al. [68], the shape of the water adsorption isotherms of cahsew slices estimated using the GAB model (Fig. 5) is characteristic of type II curves (0 < K ≤ 1 and C ≥ 1) [69].
Conclusion
HHP pre-treatment evaluated in this research can be considered adequate for drying cashew slices. Slices dried at 70 ºC and pre-treated HHP3 showed greater reduction in total drying time (40%), compared to control and the empirical mathematical model by Silva et alii. was the best to represent drying. Furthermore, the pre-treatment HHP3 had the highest effective diffusivity (3.2045 × 10–8 m2 min−1) and highest mass transfer coefficient (11.0346 × 10–5 m min−1). The use of ultrasound was efficient in the TPC extraction process, however, maximum TPC values were quantified at 180 min, where highest retention of these compounds was observed for HHP3 who also presented higher values for AA for all tested methods (ABTS• + , DPPH• and FRAP). The adsorption isotherms were satisfactorily modeled by the GAB model, which presented characteristic of the type II curves. Therefore, pre-treatment with HHP can be considered as a potential alternative to thermal food processing.
Data availability
Research data are not shared.
References
T.L. Honorato, M.C. Rabelo, L.R.B. Gonçalves, G.A.S. Pinto, S. Rodrigues, Fermentation of cashew apple juice to produce high added value products. World. J. Microbiol. Biotechnol. 23(10), 1409–1415 (2007). https://doi.org/10.1007/s11274-007-9381-z
T. Prommajak, N. Leksawasdi, N. Rattanapanone, Biotechnological valorization of cashew apple: a review. Chiang Mai. Univ. J. Nat. Sci. 13(2), 159–182 (2014). https://doi.org/10.12982/CMUJNS.2014.0029
C.S. Tamiello-Rosa, T.M. Cantu-Jungles, M. Iacomini, L.M. Cordeiro, Pectins from cashew apple fruit (Anacardium occidentale): extraction and chemical characterization. Carbohyd. Res. 483(107752), 1–6 (2019). https://doi.org/10.1016/j.carres.2019.107752
E.F. Souza, M.R. Furtado, C.W. Carvalho, O. Freitas-Silva, L.M. Gottschalk, Production and characterization of Gluconacetobacter xylinus bacterial cellulose using cashew apple juice and soybean molasses. Int. J. Biol. Macromol. 146, 285–289 (2020). https://doi.org/10.1016/j.ijbiomac.2019.12.180
H. Rajkumar, N.D. Ganesan, Effects of freeze-drying process on the production of cashew apple powder: Determination of bioactive compounds and fruit powder properties. J. Food Process. Preserv. 45(6), e15466 (2021). https://doi.org/10.1111/jfpp.15466
J.P. Gouveia, R.S. de Moura, F.D.A. Almeida, A.M.D.V. Oliveira, M.M.D. Silva, Avaliação da cinética de secagem de caju mediante um planejamento experimental. Rev. Bras. de Eng. Agríc. e Ambient. 6, 471–474 (2002). https://doi.org/10.1590/S1415-43662002000300015
P.M. Azoubel, Â.A. El-Aouar, R.V. Tonon, L.E. Kurozawa, G.C. Antonio, F.E.X. Murr, K.J. Park, Effect of osmotic dehydration on the drying kinetics and quality of cashew apple. Int. J. Food Sci. Technol. 44(5), 980–986 (2009). https://doi.org/10.1111/j.1365-2621.2008.01783
D.C. Santos, E.N.A. de Oliveira, J.N. Martins, A.P.T. Rocha, Secagem da polpa de caju em secador de leito de jorro. Rev. Bras. de Tecnol. Agroind. (2015). https://doi.org/10.3895/rbta.v9n2.2028
D.S. Bastos, P.M. do Gonçalves, C.T. de Andrade, L.K.G. de Araújo, R.M.H.M. da Leão, Microencapsulation of cashew apple (Anacardium occidentale, L) juice using a new chitosan–commercial bovine whey protein isolate system in spray drying. Food Bioprod. Process. 90(4), 683–692 (2012). https://doi.org/10.1016/j.fbp.2012.04.005
G. Musielak, D. Mierzwa, J. Kroehnke, Food drying enhancement by ultrasound–A review. Trends. Food Sci. Technol. 56, 126–141 (2016). https://doi.org/10.1016/j.tifs.2016.08.003
J.A. Moses, T. Norton, K. Alagusundaram, B.K. Tiwari, Novel drying techniques for the food industry. Food. Eng. Rev. 6, 43–55 (2014). https://doi.org/10.1007/s12393-014-9078-7
M.L. Rojas, I. Silveira, P.E.D. Augusto, Ultrasound and ethanol pre-treatments to improve convective drying: drying, rehydration and carotenoid content of pumpkin. Food Bioprod. Process. 119, 20–30 (2020). https://doi.org/10.1016/j.fbp.2019.10.008
N.C. Santos, R.L.J. Almeida, M.D.F.D. de Medeiros, R.T. Hoskin, M.R. da Silva Pedrini, Foaming characteristics and impact of ethanol pretreatment in drying behavior and physical characteristics for avocado pulp powder obtained by foam mat drying. J. Food Sci. 87(3), 1–12 (2022). https://doi.org/10.1111/1750-3841.16123
D. Huang, K. Men, D. Li, T. Wen, Z. Gong, B. Sunden, Z. Wu, Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 63(5), 104950 (2020). https://doi.org/10.1016/j.ultsonch.2019.104950
R. Osae, C. Zhou, B. Xu, W. Tchabo, H.E. Tahir, A.T. Mustapha, H. Ma, Effects of ultrasound, osmotic dehydration, and osmosonication pretreatments on bioactive compounds, chemical characterization, enzyme inactivation, color, and antioxidant activity of dried ginger slices. J. Food Biochem. 43(5), e12832 (2019). https://doi.org/10.1111/jfbc.12832
P.A. Ramos-Parra, C. García-Salinas, C.E. Rodríguez-López, N. García, G. García-Rivas, C. Hernández-Brenes, R.I.D. de la Garza, High hydrostatic pressure treatments trigger de novo carotenoid biosynthesis in papaya fruit (Carica papaya cv. Maradol). Food Chem. 277, 362–372 (2019). https://doi.org/10.1016/j.foodchem.2018.10.102
N.R.S. Hulle, P.S. Rao, Effect of high-pressure pretreatments on structural and dehydration characteristics of aloe vera (Aloe barbadensis Miller) cubes. Drying. Technol. 34(1), 105–118 (2015). https://doi.org/10.1080/07373937.2015.1037887
N. Palláres, H. Berrada, J. Tolosa, E. Ferrer, Effect of high hydrostatic pressure (HHP) and pulsed electric field (PEF) technologies on reduction of aflatoxins in fruit juices. LWT-Food Sci Technol 142, 111000 (2021). https://doi.org/10.1016/j.lwt.2021.111000
J. Xi, S. Luo, The mechanism for enhancing extraction of ferulic acid from Radix Angelica sinensis by high hydrostatic pressure. Sep. Purif. Technol. 165, 208–213 (2016). https://doi.org/10.1016/j.seppur.2016.04.011
K.O.P. Inada, S. Nunes, J.A. Martinez-Blazquez, F.A. Tomás-Barberán, D. Perrone, M. Monteiro, Effect of high hydrostatic pressure and drying methods on phenolic compounds profile of jabuticaba (Myrciaria jaboticaba) peel and seed. Food Chem. 309, 125794 (2020). https://doi.org/10.1016/j.foodchem.2019.125794
U. Yucel, H. Alpas, A. Bayindirli, Evaluation of high pressure pretreatment for enhancing the drying rates of carrot, apple, and green bean. J. Food Eng. 98(2), 266–272 (2010). https://doi.org/10.1016/j.jfoodeng.2010.01.006
L. Zhang, L. Liao, Y. Qiao, C. Wang, D. Shi, K. An, J. Hu, Effects of ultrahigh pressure and ultrasound pretreatments on properties of strawberry chips prepared by vacuum-freeze drying. Food Chem. (2019). https://doi.org/10.1016/j.foodchem.2019.125386
L. Zhang, Y. Qiao, C. Wang, L. Liao, D. Shi, K. An, L. Shi, Influence of high hydrostatic pressure pretreatment on properties of vacuum-freeze dried strawberry slices. Food Chem. 331, 127203 (2020). https://doi.org/10.1016/j.foodchem.2020.127203
A.O.A.C., Official methods of analysis of AOAC International, 20th edn. (AOAC international, Rockville, 2016)
A. Kaleta, K. Górnicki, Evaluation of drying models of apple (var. McIntosh) dried in a convective dryer. Int. J. Food Sci. Technol. 45(5), 891–898 (2010). https://doi.org/10.1111/j.1365-2621.2010.02230.x
L.M. Diamante, R. Ihns, G.P. Savage, L. Vanhanen, A new mathematical model for thin layer drying of fruits. Int. J. Food Sci. Technol. 45(9), 1956–1962 (2010). https://doi.org/10.1111/j.1365-2621.2010.02345.x
W.P. Silva, C.M.D.P.S. Silva, J.A.R. Sousa, V.S.O. Farias, Empirical and diffusion models to describe water transport into chickpea (Cicer arietinum L.). Int. J. Food Sci. Technol. 48(2), 267–273 (2013). https://doi.org/10.1111/j.1365-2621.2012.03183.x
Silva, W.P., & Silva, C.M.D.P.S. (2008). LAB Fit Curve Fitting Software (Nonlinear Regression and Treatment of Data Program) V 7.2.50 (2008), online, available from world wide web: <www.labfit.net>, date of Accessed: 2020-April-10.
N.C. Santos, R.L.J. Almeida, G.M. da Silva, V.M.D.A. Silva, V.H.D.A. Ribeiro, A.C.D.O. Brito, L.M.S. Rodrigues, R.M.S. Santos, M.M.T. Saraiva, Impact of pre-treatments with ethanol and freezing on drying slice papaya: drying performance and kinetic of ultrasound-assisted extraction of phenolics compounds. J. Sci. Food Agric. 102(11), 1–10 (2022). https://doi.org/10.1002/jsfa.12119
A.V. Luikov, Analytical heat diffusion theory (Academic Press, Inc. Ltd, London, 1968)
W.P. Silva, J.W. Precker, C.M.D.P.S. Silva, J.P. Gomes, Determination of effective diffusivity and convective mass transfer coefficient for cylindrical solids via analytical solution and inverse method: application to the drying of rough rice. J. Food Eng. 98(3), 302–308 (2010). https://doi.org/10.1016/j.jfoodeng.2009.12.029
N. Milićević, P. Kojić, M. Sakač, A. Mišan, J. Kojić, C. Perussello, B. Tiwari, Kinetic modelling of ultrasound-assisted extraction of phenolics from cereal brans. Ultrason. Sonochem. 79, 105761 (2021). https://doi.org/10.1016/j.ultsonch.2021.105761
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. RiceEvans, Antioxidant activity applying an improved ABTS•+ radical cation decolorization assay. Free Radical Biol. Med. 26, 1231–1237 (1999)
M.S.M. Rufino, R.E. Alves, E.S. Brito, S.M. Morais, C.G. Sampaio, J.P. Jimenez, F.D.S. Calixto, Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH•. Comunicado Técnico Embrapa. 127, 1–4 (2007)
M.M.R. do Socorro, R.E. Alves, E.S. de Brito, J. Pérez-Jiménez, F. Saura-Calixto, J. Mancini-Filho, Bioactive compounds and antioxidant capacities of non –traditional tropical fruits from Brazil. Food Chem. 121(4), 996–1002 (2010). https://doi.org/10.1016/j.foodchem.2010.01.037
I.F. Benzie, J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76 (1996). https://doi.org/10.1006/abio.1996.0292
S. Arslan-Tontul, Moisture sorption isotherm, isosteric heat and adsorption surface area of whole chia seeds. LWT-Food Sci. Technol. 119, 108859 (2020). https://doi.org/10.1016/j.lwt.2019.108859
J.V. García-Pérez, J.A. Cárcel, G. Clemente, A. Mulet, Water sorption isotherms for lemon peel at different temperatures and isosteric heats. LWT-Food Sci. Technol. 41(1), 18–25 (2008). https://doi.org/10.1016/j.lwt.2007.02.010
C.R. Oswin, The kinetics of package life. III. the isotherm. J Soc. Chem. Ind. 65(12), 419–421 (1946). https://doi.org/10.1002/jctb.5000651216
K.W. Lang, M.P. Steinberg, Predicting water activity from 0.30 to 0.95 of a multicomponent food formulation. J. Food Sci. 46(3), 670–672 (1981). https://doi.org/10.1111/j.1365-2621.1981.tb15320.x
N.C. Santos, R.L.J. Almeida, G.M. da Silva, S.S. Monteiro, V.H. de Alcântara Ribeiro, A.P. de França Silva, M.M. de Almeida Mota, Influence of high hydrostatic pressure (HHP) pretreatment on plum (Prunus salicina) drying: drying approach, physical, and morpho-structural properties of the powder and total phenolic compounds. J. Food Process. Preserv. (2022). https://doi.org/10.1111/jfpp.16968
W. Luo, S. Tappi, C. Wang, Y. Yu, S. Zhu, P. Rocculi, Study and optimization of high hydrostatic pressure (HHP) to improve mass transfer and quality characteristics of candied green plums (Prunus mume). J. Food Process. Preserv. 42(11), e13769 (2018). https://doi.org/10.1111/jfpp.13769
E.V. Silva Júnior, L.L. Melo, R.A.B. Medeiros, Z.M.P. Barros, P.M. Azoubel, Influence of ultrasound and vacuum assisted drying on papaya quality parameters. LWT Food Sci. Technol. 97, 317–322 (2018). https://doi.org/10.1016/j.lwt.2018.07.017
E.S. Silva, S.C.R. Brandão, A.L. da Silva, J.H.F. da Silva, A.C.D. Coêlho, P.M. Azoubel, Ultrasound-assisted vacuum drying of nectarine. J. Food Eng. 246, 119–124 (2019). https://doi.org/10.1016/j.jfoodeng.2018.11.013
N.C. Santos, R.L.J. Almeida, G.M. Silva, S.S. Monteiro, A.M.M.C.N. André, Effect of ultrasound pre-treatment on the kinetics and thermodynamic properties of guava slices drying process. Innov. Food Sci. Emerg. Technol. 66(12), 102507 (2020). https://doi.org/10.1016/j.ifset.2020.102507
N.C. Santos, R.L.J. Almeida, S.S. Monteiro, E.T. de Vilela Silva, V.M. de Alcântara Silva, A.M.M. André, V.H.A. Ribeiro, A.C.O. de Brito, Influence of ethanol and ultrasound on drying, bioactive compounds, and antioxidant activity of strawberries (Fragaria× ananassa). J. Indian Chem. Soc. 99(7), 100542 (2022). https://doi.org/10.1016/j.jics.2022.100542
D. Verma, N. Kaushik, P.S. Rao, Application of high hydrostatic pressure as a pretreatment for osmotic dehydration of banana slices (Musa cavendishii) finish-dried by dehumidified air drying. Food Bioprocess. Technol. 7(5), 1281–1297 (2014). https://doi.org/10.1007/s11947-013-1124-6
J.P. Ferreira, W.P. Silva, A.J. Queiroz, R.M. Figueirêdo, J.P. Gomes, B.A. Melo, A.G. Lima, Description of cumbeba (Tacinga inamoena) waste drying at different temperatures using diffusion models. Foods 9(12), 1818 (2020). https://doi.org/10.3390/foods9121818
J.P.D.L. Ferreira, A.J.D.M. Queiroz, R.M.F.D. Figueirêdo, W.P. Silva, J.P. Gomes, D.D.C. Santos, R.O.D. Andrade, Utilization of cumbeba (Tacinga inamoena) residue: drying kinetics and effect of process conditions on antioxidant bioactive compounds. Foods 10(4), 788 (2021). https://doi.org/10.3390/foods10040788
W.P. Silva, V.S.F. Oliveira, G.N. Araújo, A.G.B. Lima, Modeling of water transport in roof tiles by removal of moisture at isothermal conditions. Heat Mass Transf. 48(5), 809–821 (2012). https://doi.org/10.1007/s00231-011-0931-4
R.L.J. Almeida, N.C. Santos, C.E. Padilha, S.S. Monteiro, E.S. Santos, Impact of hydrothermal pretreatments on physicochemical characteristics and drying kinetics of starch from red rice (Oryza sativa L.). J. Food Proc. Preserv. 45, e.15448 (2021). https://doi.org/10.1111/jfpp.15448
M.J. Torres-Ossandón, L. Castillo, K.S. Ah-Hen, A. Vega-Gálvez, Effect of high hydrostatic pressure processing on phytochemicals, antioxidant activity, and behavior of Botrytis cinerea in white grape juice concentrate. J. Food Process. Preserv. 44(11), e14864 (2020). https://doi.org/10.1111/jfpp.14864
Y. Ma, J. Yi, J. Bi, Y. Zhao, X. Li, X. Wu, Q. Du, Effect of ultrasound on mass transfer kinetics and phenolic compounds of apple cubes during osmotic dehydration. LWT-Food Sci. Technol. 151, 112186 (2021). https://doi.org/10.1016/j.lwt.2021.112186
R.D. Khandare, P.D. Tomke, V.K. Rathod, Kinetic modeling and process intensification of ultrasound-assisted extraction of d-limonene using citrus industry waste. Chem. Eng. Proc.-Proc. Int. 159, 108181 (2021). https://doi.org/10.1016/j.cep.2020.108181
N. Kutlu, A. Isci, O. Sakiyan, A.E. Yilmaz, Effect of ohmic heating on ultrasound extraction of phenolic compounds from cornelian cherry (Cornus mas). J. Food Process. Preserv. 45(10), e15818 (2021). https://doi.org/10.1111/jfpp.15818
M. Babotă, O. Frumuzachi, A. Gâvan, C. Iacoviță, J. Pinela, L. Barros, A. Mocan, Optimized ultrasound-assisted extraction of phenolic compounds from Thymus comosus Heuff. ex Griseb. et Schenk (wild thyme) and their bioactive potential. Ultrason. Sonochem. 84, 105954 (2022). https://doi.org/10.1016/j.ultsonch.2022.105954
A.A. Casazza, B. Aliakbarian, E. Sannita, P. Perego, High-pressure high-temperature extraction of phenolic compounds from grape skins. Int. J. Food Sci. Technol. 47(2), 399–405 (2011). https://doi.org/10.1111/j.1365-2621.2011.02853.x
L. Eroman Unni, O.P. Chauhan, P.S. Raju, High pressure processing of garlic paste: effect on the quality attributes. Int. J. Food Sci. Technol. 49(6), 1579–1585 (2013). https://doi.org/10.1111/ijfs.12456
I. Park, J.U. Kim, H.M. Shahbaz, D. Jung, M. Jo, K.S. Lee, J. Park, High hydrostatic pressure treatment for manufacturing of garlic powder with improved microbial safety and antioxidant activity. Int. J. Food Sci. Technol. 54(2), 325–334 (2018). https://doi.org/10.1111/ijfs.13937
F.J. Barba, N.S. Terefe, R. Buckow, D. Knorr, V. Orlien, New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods a review. Food Res. Int. 77, 725–742 (2015). https://doi.org/10.1016/j.foodres.2015.05.015
G. Zhao, R. Zhang, M. Zhang, Effects of high hydrostatic pressure processing and subsequent storage on phenolic contents and antioxidant activity in fruit and vegetable products. Int. J. Food Sci. Technol. 52(1), 3–12 (2016). https://doi.org/10.1111/ijfs.13203
S. Dede, H. Alpas, A. Bayındırlı, High hydrostatic pressure treatment and storage of carrot and tomato juices: antioxidant activity and microbial safety. J. Sci. Food Agric. 87(5), 773–782 (2007). https://doi.org/10.1002/jsfa.2758
S. Zhang, Y. Zhao, X. Yao, Z. Zheng, C. Zheng, Z. Jiang, Effect of high hydrostatic pressure pretreatment on flavour and physicochemical properties of freeze-dried carambola slices. Int. J. Food Sci. Technol. 57(7), 1–10 (2022). https://doi.org/10.1111/ijfs.15748
G.A. Collazos-Escobar, N. Gutiérrez-Guzmán, H.A. Váquiro-Herrera, J. Bon, J.V. Garcia-Perez, Thermodynamic analysis and modeling of water vapor adsorption isotherms of roasted specialty coffee (Coffee arabica L. cv. Colombia). LWT-Food Sci. Technol. 160, 113335 (2022). https://doi.org/10.1016/j.lwt.2022.113335
P.P. Lewicki, The applicability of the GAB model to food water sorption isotherms. Int. J. Food Sci. Technol. 32(6), 553–557 (2008). https://doi.org/10.1111/j.1365-2621.1997.tb02131.x
R.F. Zabalaga, S.C. Carballo, Convective drying and water adsorption behavior of unripe banana: mathematical modeling. J. Food Process. Preserv. 39(6), 1334–1341 (2015). https://doi.org/10.1111/jfpp.12352
H. Al-Muhtaseb, M.A. Hararah, E.K. Megahey, W.A.M. McMinn, T.R.A. Magee, Moisture adsorption isotherms of microwave-baked Madeira cake. LWT-Food Sci. Technol. 43(7), 1042–1049 (2010). https://doi.org/10.1016/j.lwt.2010.01.003
S. Brunauer, L.S. Deming, W.E. Deming, E. Teller, On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 62(7), 1723–1732 (1940). https://doi.org/10.1021/ja01864a025
J.S. Zeymer, P.C. Corrêa, G.H. de Oliveira, F.M. Baptestini, R.C. Freitas, Desorption isotherms of Lactuca sativa seeds. Rev. Bras. de Eng Agríc e Ambient. 21, 568–572 (2017). https://doi.org/10.1590/1807-1929/agriambi.v21n8p568-572
K. Gościnna, J. Pobereżny, E. Wszelaczyńska, W. Szulc, B. Rutkowska, Effects of drying and extraction methods on bioactive properties of plums. Food Control 122, 107771 (2021). https://doi.org/10.1016/j.foodcont.2020.107771
Acknowledgements
NCS and RLJA were supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors are grateful to the Federal University of Rio Grande do Norte (UFRN), Federal University of Ceará (UFC), Federal University of Campina Grande (UFCG) and Federal Institute of Sertão Pernambucano (IFSertãoPE) for technical support.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
Ethics approval was not required for this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, N.C., Almeida, R.L.J., da Silva, G.M. et al. Impact of high hydrostatic pressure (HHP) pre-treatment drying cashew (Anacardium occidentale L.): drying behavior and kinetic of ultrasound-assisted extraction of total phenolics compounds. Food Measure 17, 1033–1045 (2023). https://doi.org/10.1007/s11694-022-01688-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01688-5