Abstract
This work aimed to evaluate the efficiency of subcritical ethanol extraction for recovering oil from coconut meal. Dried coconut meal with an oil content of 19.85% (w.b.) was treated with subcritical ethanol at various ethanol concentrations (80, 90, and 100% v/v), solvent to solid ratios (5:1, 8:1, 10:1, and 15:1 v/w), temperatures (80, 100, and 120 °C), and holding times (0, 15, 30, and 45 min) in a batch-type vessel. The highest oil recovery (81.49%), which was obtained from the extraction with 100% ethanol and solvent-solid ratio of 8:1 (v/w) at 100 °C for 45 min did not significantly differ from those obtained at 120 °C for 15–45 min. The chemical properties, including free fatty acid content, peroxide value, saponification value, unsaponifiable matter, and fatty acid profile of the oils conformed to the standard values for crude coconut oil. Gas chromatography showed the higher ratios of caprylic and capric acids in the subcritical ethanol-extracted oils than those in hexane extracted oil, approximately 1.05–1.4 times. Additionally, the total phenolic content and 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity of the oils extracted with the subcritical ethanol extraction were significantly higher (up to 3.5 and 6 times, respectively) than those of the oil Soxhlet-extracted with hexane. The present study showed that subcritical ethanol extraction is an effective method for extracting oil with good qualities and antioxidant properties from coconut meal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coconut oil is usually derived from the coconut kernel (meat) using various methods such as mechanical expression, fermentation, centrifugation, enzymatic, aqueous, and solvent extractions [1,2,3,4]. Coconut oil is widely used in food and cosmetic industries. It is high in medium chain fatty acids which make coconut oil a good carrier oil for vitamins, flavor, and color [5]. Moreover medium chain fatty acids also showed other functional properties such as anticancer, anti-hepatic steatosis, anti-diabetic, antioxidant, anti-inflammatory, anti-microbial, and moisturizing for skin [6].
Coconut meal is a residue obtained after the extraction of coconut milk from the grated fresh kernel. Our previous studies revealed that hydrolysis of coconut meal using subcritical water is an effective method for producing manno-oligosaccharides [7, 8]. However, since the meal still contains around 22% of oil [9, 10], extraction of the oil before hydrolysis process can add more value to the residue and might also improve the hydrolysis process. The most common solvent for edible oil extraction is hexane [11]. However, because hexane has been of concern with safety, health, and environmental issues [12]; therefore, various alternative solvents are often studied. Among them, ethanol is a promising candidate because of its lower toxicity, good operational safety, and legal permission for food extraction [13]. Extractions of oils using ethanol as solvent by different extraction methods such as Soxhlet, microwave-assisted, ultrasound-assisted, and pressurized extractions have been reported [14,15,16,17]. However, low selectivity, limited solubility capacity for oil at conventional extraction temperatures, and high regeneration cost limited the industrialization of oil extraction with ethanol [18]. Nevertheless, a recent study has suggested that the ethanol process for edible oil extraction possesses a lower carbon footprint and global warming potential than the conventional hexane method [19].
Subcritical fluid treatment is an emerging technique which employs liquid solvent at temperatures between its boiling and critical temperatures under pressurized conditions. Subcritical water treatment was widely investigated for valorization of agricultural by-products by its hydrolysis/extraction acceleration [20]. Previous studies reported the feasibility of subcritical ethanol extraction for extracting lipid from sesame pressed cake, Isochrysis biomass, cocoa shells, and soybean [21,22,23]. Castejón and Luna [24] showed that the echium oils extracted by pressurized ethanol extraction at 150 °C, 10 min and Soxhlet extraction using hexane for 8 h resulted in similar oil yields and fatty acid compositions. Subcritical ethanol extraction at 150 °C also increased the total lipid yields from microalgae compared to extraction at 50 °C [17]. Oliveira et al. [25] used the ethanol for extracting rice bran oil at temperatures varying from 60 to 90 °C. Results showed that ethanol extraction under subcritical condition at 82 °C with constant stirring for 3 h could recovered the oil of fresh rice bran up to 99.9%. The pressurized liquid extraction using ethanol was also effective for recovery of oil from favela seeds with lower solvent consumption and shorter extraction time than the Soxhlet method using ethanol [26]. Moreover, subcritical treatment using ethanol and aqueous ethanol have been showed the ability to achieve high total phenolic contents and antioxidant activity [27, 28].
Because of the high percentage of medium chain fatty acids, subcritical ethanol might be very effective for the extraction of oil from coconut meal. In addition, to add value to the oil, subcritical ethanol method might be able to improve the antioxidant properties. However, the process factors of the subcritical ethanol extraction affecting yield and properties of oil from coconut meal have never been reported in the literature. Therefore, the objectives of this study were to obtain oil from coconut meal using subcritical ethanol extraction and investigate the effect of ethanol concentration, solvent to solid ratio, temperature, and time on the extraction of coconut oil in terms of oil yield, chemical properties, fatty acid composition, total phenolic content, and antioxidant activity, in comparison with the oil obtained by Soxhlet extraction.
Materials and methods
Coconut meal
Freshly produced coconut meal was obtained from a local coconut milk processing factory (Vara Food and Drink Co. Ltd., Nakhon Pathom, Thailand). The received meal was dried in a hot-air oven at 60 °C for 12 h and then sieved to prepare the sample with a particle size in the range of 0.3–2.0 mm. The sample was vacuum packed and kept at −18 °C.
Proximate composition of coconut meal
A proximate analysis of the coconut meal was performed according to the AOAC methods [29]. Briefly, moisture content was determined by drying at 105 °C to a constant weight, and ash content was determined by dry ashing at 550 °C in a muffle furnace for 4 h. Protein content was determined by estimating the nitrogen content using the Kjeldalh method with a conversion factor of 6.25. Crude fat content was measured by Soxhlet extractor using petroleum ether as an extractant.
Subcritical ethanol extraction
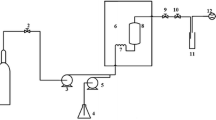
The dried coconut meal (5.3, 8.0, 10.0, and 16.0 g) was mixed with 80 mL of different concentrations of ethanol (80, 90, and 100% v/v) in a batch-type stainless steel vessel with a net volume of 120 mL (Taiatsu Techno Corporation, Osaka, Japan). The vessel was then tightly closed and heated using a pipe heating mantle (P5-2, 200 W, Heater Engineer, Tokyo, Japan). The extractions were performed at 80, 100, and 120 °C with a holding-time ranging from 0 to 45 min (after a heating-up time of approximately 15 min). The temperature inside the vessel was monitored using a type-K thermocouple (Fig. 1). After heating, the vessel was immediately cooled to stop the reaction in an ice-water bath. The treated mixture was filtered through a Whatman No. 1 paper. Crude oil was obtained after removal of the solvent by a rotary evaporation (RV 10 digital V, IKA, Königswinter, Germany). The extracted oil was weighed and stored at − 18 °C for further analysis. All extraction experiments were performed in triplicate. The recovery of the oil was calculated by the ratio of the mass of the extracted oil (M) to the mass of the crude fat content (MSoxhlet) according to Eq. (1).
Absolute ethanol (≥ 99.9%) was purchased from RCI Labscan, Thailand, and is referred to as 100% ethanol. The treatment that gave the highest oil recovery was selected for further study.
Soxhlet hexane extraction
Hexane extraction was performed using a Soxhlet apparatus as a control treatment. Ten grams of the dried coconut meal was placed in an extraction thimble. One hundred or one hundred fifty milliliters of hexane was added into a round bottom flask. Extraction was carried out for 15 or 45 min. Afterwards, the solvent was evaporated and the percentage of oil recovery was calculated according to Eq. (1). This extraction method is abbreviated as HS.
Physical and chemical properties of oil
Color determination
Color of the obtained coconut oil was measured using a ColorFlex EZ Spectrophotometer (HunterLab, Reston, VA, USA). Each oil sample was filled in a glass sample cup and measured for CIE L* (lightness), a* (redness/greenness), and b* (yellowness/blueness) values.
Chemical analysis
Peroxide value (Cd 8-53), free fatty acid content (Ca 5a-40), saponification value (Cd 3-25), and unsaponifiable matter (Ca 6a-40) of the extracted oil were determined according to methods of AOCS [30].
Fatty acid compositions
Fatty acid methyl ester (FAME) was prepared according to the method of AOAC [29]. Approximately 30 mg of coconut oil was dissolved in 3 mL of 0.5 M methanolic HCl. The mixture was thoroughly mixed using a vortex mixer, incubated at 50 °C for 5 h, and cooled to room temperature. FAME was purified by adding 3 mL of hexane and the clear upper layer containing FAME was then separated and dried with anhydrous Na2SO4.
The compositions of FAME were determined using a gas chromatograph (GC-2010 Plus, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector (FID). Separation was achieved on a capillary column (HP-5, 100 m × 0.25 mm, 0.25 μm film thickness, Agilent Technologies, Santa Clara, CA, USA) using an injection volume of 1 μL with a split ratio of 1:100. The temperature of an injection port was set at 240 °C, whereas the oven temperature was held at 80 °C for 1 min, then increased to 180 °C at a rate of 10 °C/min and kept for 5 min. The temperature was then further increased to 240 °C at a rate of 5 °C/min and kept at this final temperature for 10 min. Nitrogen was used as the carrier gas at a flow rate of 1 mL/min. Tridecanoic acid (C13:0) (Sigma-Aldrich, St. Louis, MO, USA) was used as an internal standard. Identification of FAMEs was performed by comparing their retention times with those of 9 reference standards (FAME Mix GCL-10 and FAME Mix GCL-30, Sigma-Aldrich). The fatty acid compositions were calculated from percentage area of each component to the total area of FAMEs.
Total phenolic content (TPC) and antioxidant activity
The extracted oil was separated into hydrophilic and lipophilic fractions by mixing 5.0 g of oil with 10 mL of hexane and 10 mL of 80% methanol using a vortex mixer for 2 min [31]. The mixture was then centrifuged at 10,200×g, 25 °C for 5 min (Sorvall RC6, Thermo Scientific, Waltham, MA, USA). The upper and lower phases were called lipophilic and hydrophilic fractions, respectively. One part of the hydrophilic fraction was used for determining total phenolic content. Another part of hydrophilic fraction and lipophilic fraction were dried using a vacuum oven at 45 °C, for 2 h before kept at –18 °C.
TPC of the coconut oil was estimated using Folin–Ciocalteau reagent according to method of Gutfinger [32] with some modifications. The hydrophilic fraction (0.3 mL) was mixed with 1.5 mL of tenfold diluted Folin‐Ciocalteau reagent. The solution was added with 1.3 mL of 7.5% sodium carbonate (Na2CO3) solution and allowed to stand in the dark at room temperature for 30 min. The absorbance of the solution at 725 nm was recorded using a spectrophotometer (Genesys 10 s, Thermo Scientific, Waltham, MA, USA). Gallic acid solutions at concentrations of 20–100 mg/L were used to prepare a calibration curve. TPC was expressed as µg gallic acid equivalent (GAE)/g coconut oil.
Antioxidant capacity of hydrophilic and lipophilic fractions was measured based on its 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical‐scavenging activity according to the method described by Ghani et al. [2] and Khuwijitjaru et al. [33] with some modifications. The dried hydrophilic fraction was dissolved in methanol while the lipophilic fraction was dissolved in ethyl acetate at the concentration of 5 mg/100 µL. One hundred microliters of each solution was mixed with 3.9 mL of 0.05 mM methanolic (hydrophilic fraction) and 0.05 mM ethyl acetate (lipophilic fraction) solutions of DPPH, respectively. The mixture was vigorously shaken and left to stand for 30 min in the dark, and then the absorbance was measured at 517 nm. DPPH inhibition activity was calculated according to Eq. 2.
where A0 is the absorbance of the DPPH solution and A1 is the absorbance of the DPPH solution with the sample.
Statistical analysis
All experiments were conducted in triplicate. Analysis of variance (ANOVA) and Duncan’s multiple range test were performed to determine significant differences between means at a significance level of 0.05. PASW Statistics for Windows version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results and discussion
Proximate composition of coconut meal
After drying and sieving, the dried coconut meal sample contained, on a wet basis, approximately 6.47% moisture content, 2.78% crude protein, 19.85% crude fat, 1.01% ash, and 69.89% total carbohydrate (calculated by difference). The lipid contents of coconut meal in the range of 22.6–23.6% were also reported by Sulaiman et al. [4], Khuwijitjaru et al. [9] and Klinchongkon et al. [8]. This indicated a relatively constant amount of lipid in the coconut meal obtained from the industrial coconut milk process and implied its potential as a source of coconut oil.
Oil recovery
The subcritical ethanol extraction performed in this study was a single batch extraction. The results indicated that subcritical ethanol extraction gave a variation in oil recovery from 10.38 to 83.55% depending on ethanol concentration, solvent to solid ratio, temperature, and holding time (Fig. 2). The effect of ethanol concentration at the solvent to solid ratio of 10:1 (v/w) and the temperature of 120 °C on the oil recovery is shown in Fig. 2A. Only approximately 10 and 15% of oil were recovered from the extraction using 80% aqueous ethanol after 15 and 45 min of holding times, respectively. Nevertheless, the extraction yields significantly increased with the increasing of ethanol concentration. As the concentration of ethanol was increased to 100%, the oil recoveries increased to 81.17 and 83.71% after 15 and 45 min, respectively. The influence of solvent to solid ratios from 5:1 to 15:1 was tested for the extraction with the absolute ethanol at 100 °C for 45 min (Fig. 2B). The oil recoveries were not significantly different (p > 0.05) when coconut meal was treated at the solvent to solid ratios of 15:1, 10:1, and 8:1. However, lowering the solvent to solid ratio to 5:1 significantly reduced the recovery to 59.34%, because the sample was not entirely immersed in the solvent at this ratio. Therefore, the ratio of 8:1, which was the least ratio that the solvent could cover all solid samples during the treatment, was chosen for further studies. The Soxhlet extractions using hexane gave only slightly higher yields.
Effects of the conditions for subcritical ethanol extraction on oil recovery: A Ethanol concentration at 120 °C, solvent to solid ratio 10:1; B solvent to solid ratio at 100% ethanol, 100 °C, 45 min; C temperature and time at 100% ethanol and solvent to solid ratio 8:1. The temperatures are (◊) 80, (□) 100, and (△) 120 °C; D dielectric constants of solvent under different conditions. Different letters indicate significant difference among oil recoveries when comparing all treatment (lowercase letters) and extraction time at each temperature (uppercase letters) (p ≤ 0.05, Duncan’s multiple range test). HS represents the Soxhlet extraction with hexane
Figure 2C shows the results of oil recoveries as a function of temperature and holding time using 100% ethanol and a solvent to solid ratio of 8:1 (v/w). The results showed that the recovery of coconut oil could be enhanced by increasing the process temperature and holding time. The interaction effect between the extraction temperature and the holding time contributed significantly to the extraction process (p ≤ 0.05). At 80 °C, the oil recovery gradually increased with an increase in the extraction time in which the maximum recovery of 71.65% was obtained at 45 min. At 100 °C, the oil recovery at 0 min of holding time was close to that of 80 °C and the value also significantly increased with the prolonging of extraction time and the maximum recovery of 81.49% was achieved at 45 min. Extraction at 120 °C gave higher oil recovery at 0 min of holding time than other temperatures and the value further increased after 15 min. However, further prolonging the extraction time (30 and 45 min) did not significantly affect (p > 0.05) oil recovery. The maximum oil recoveries were in the range of 82.21–83.54%, which were not significantly different from the maximum recovery at 100 °C (p > 0.05). From these results, the extraction conditions of 100 °C, 45 min as well as 120 °C, 15 min were considered as the best conditions for recovering oil from the coconut meal. The oils obtained under these conditions were further analyzed for their chemical characteristics and antioxidant activity. The control treatment, Soxhlet extraction using hexane at solvent to solid ratio 15:1 for 45 min gave higher recovery (89.07%) than all other tested subcritical ethanol extractions. However, considering that a comparable oil recovery from subcritical ethanol extraction could be achieved with less amount of solvent and shorter time without reflux, the subcritical ethanol extraction of coconut oil showed obvious advantage.
The results agreed with previous studies on oil extraction with ethanol from different raw materials. Sawada et al. [34] reported that the extraction yield of oil from soybean was strongly suppressed by the increasing water content of ethanol and absolute ethanol presented the highest capacity for oil extraction. Capellini et al. [21] indicated that the extraction of oil from sesame seed pressed cake using ethanol could be enhanced by increases in temperature. Sampaio Neto et al. [35] performed Brazil nut oil extraction with ethanol and concluded that water content and temperature also had a great influence on the solubility of the oil.
The efficiency of solvent oil extraction can be affected by several factors such as solvent to solid ratio, extraction temperature and time, solvent type, as well as particle size and shape. Generally, the less polar solvents are better for extracting lipids from plant matrices, while small and thin particles provide good solvent permeability and oil diffusion. In addition, high temperature increases solubility of solute and diffusion rate, thus improves extraction efficiency [36].
The mechanism of oil extraction from a solid matrix using a solvent relies on the diffusion of the solvent and extract inside and outside of the solid matrix, in which the diffusive steps depend on the physical properties of the solute and solvent [37]. In addition, the dielectric constant is usually employed as a parameter to determine the solute–solvent interaction [38]. In this study, the dielectric constants of subcritical ethanol at different ethanol concentrations and temperatures were estimated according to the equations proposed by Tir et al. [39] and Wohlfarth [40] to describe the effect of polarity on the oil recovery using the extraction yield data at the holding time of 45 min. A non-linear relationship between the dielectric constants of solvent and the oil recoveries is shown in Fig. 2D. Water is a very high polarity solvent with a dielectric constant of 78 and therefore the decrease in the water content resulted in lower dielectric constants. In addition, the dielectric constant of subcritical ethanol also decreased with increasing temperature and led to higher lipid solubility which explained the higher oil yields in absolute ethanol at higher temperatures.
Oil characterization
Characterizations of the oil were conducted for samples obtained from selected extraction conditions that provided high oil recoveries (i.e. > 80%), including subcritical extraction using absolute ethanol at 8:1, 100 °C, 45 min (abbreviated as 100-45) and 120 °C, 15 min (120-15), and Soxhlet extraction using hexane at 15:1, 45 min (HS).
Appearances and color parameters
Figure 3 shows photographs of the extracted oils and the coconut meal residues obtained from subcritical ethanol and Soxhlet hexane extractions. The subcritical ethanol extraction at higher temperatures and longer times resulted in slightly colored oils and residues. The oils and residues obtained at 80 and 100 °C were colorless and white, respectively similar to those from Soxhlet extraction, except the oil obtained at 100 °C, 45 min that was slightly yellow. The oil and residue obtained from subcritical ethanol extraction at 120 °C were light yellow to slight brownish-yellow. Results from the color measurement are expressed as L*, a* and b* values in Table 1. The extraction condition resulted in significant difference in color of the oil (p ≤ 0.05). The oil obtained from subcritical ethanol extraction at 100 °C, 45 min showed slightly lower lightness (96.62) and higher yellowness (3.05) whereas the increasing temperature of the subcritical ethanol extraction (120-15) resulted in the oil with even redder (− 2.46) and yellower (7.03) compared to the oil obtained from HS (L* = 97.89, a* = − 1.09 and b* = 1.32). The color of the oil from the subcritical ethanol extraction might be due to the products of browning reactions such as the Maillard reaction or caramelization of sugars because the coconut meal sample contained proteins and moisture and subcritical extraction was performed at high temperature. High amount of 5-hydroxymethyl-2-furaldehyde, an intermediate product from Maillard reaction was found in subcritical water treatment of coconut meal as shown in a previous report [8].
Chemical properties
Peroxide value (PV), saponification value (SV), and unsaponifiable matter (USM) of the three oils were in the ranges of 0.99–2.97 meq O2/kg, 251–258 mg KOH/g oil, and 0.17–0.28%, respectively, which were within the standard recommendation for crude coconut oil (Table 1). Peroxide value is an indicator of primary lipid oxidation. Generally, coconut oil has a low peroxide value because it consists a high percentage of saturated fatty acids which can withstand autoxidation. Nevertheless, it was found that shorter extraction time of subcritical ethanol extraction (120-15) resulted in significantly lower peroxide value. The free fatty acid contents (FFA) as lauric acid obtained from oils were in the range of 0.69–1.72% which are much below the standard of crude coconut oil. This might be ascribed to the high quality of coconut meal used in this study. Subcritical ethanol extraction resulted in significantly higher FFA than hexane extraction and the FFA increased with increasing extraction temperature (p ≤ 0.05). This might be because small amount of water in the raw material promoted the hydrolysis reaction of triacylglycerols into free fatty acids at higher temperatures of subcritical ethanol extraction. The extracted oil should be further refined to conform to the refined, bleached and deodorized (RBD) coconut oil standard, particularly by removing of free fatty acid which should be lower than 0.1% according to the Asian and Pacific Coconut Community (APCC) standard.
Fatty acid compositions
The fatty acid compositions of oil extracted under different conditions are shown in Table 2. Eight different fatty acids were identified. The contents in these extracted oils were not different (p > 0.05) and they conformed to the Codex standard (CODEX-STAN 210-1999). The results from previous studies [41, 42] also showed that the fatty acid compositions of the passion fruit seed and foxtail millet bran oils from subcritical propane extraction were similar to those of oil extracted with hexane. Approximately 93% of fatty acids of the extracted oil were saturated ones.
Main fatty acids in the coconut oil were medium chain fatty acids (MCFA), including caprylic, capric, and lauric acids (59.81–62.04%). Moreover, the oil extracted with subcritical ethanol extraction tended to consist of higher amount of shorter chain fatty acids than the Soxhlet extracted oil.
The ratios of each fatty acid content obtained from the subcritical ethanol extractions to that from Soxhlet hexane extraction are shown in Fig. 4. It can be seen that the subcritical ethanol extraction enhanced the extraction efficiency of shorter-chain fatty acids, especially caprylic and capric acids while it gave lower percentages of long chain fatty acids. The ratios of caprylic, capric, palmitic, stearic, oleic and linoleic acids in the subcritical ethanol extracted oil at 100 °C, 45 min were approximately 1.2, 1.12, 0.93, 0.88, 0.93, and 0.95 times of that in hexane extracted oil. The subcritical ethanol extraction at 120 °C, 15 min gave the caprylic and capric acids approximately 1.4 and 1.05 times, while other fatty acids were similar to those of the hexane extracted oil. The different solvents dissolved different kinds of lipids depending on their properties, such as polarity and chain length of molecules [43]. Mansour et al. [44] reported that microalgae lipids recovered with hexane: isopropanol solvent mixture contained higher amount of longer-chain length fatty acids whereas ethanol gave higher amount of shorter-chain length fatty acids. This might be also an advantage of subcritical ethanol extraction of coconut oil because caprylic and capric acids exhibit anti-inflammatory and antibacterial properties [45, 46].
The ratio of fatty acid of oil from subcritical ethanol extraction (FASEE) to that from Soxhlet extraction using hexane (FAHS). 100-45 and 120-15 are explained in Table 1
Total phenolic content and antioxidant activity
TPC of the dried coconut sample, which was determined according to Mahayothee et al. [47] using 1:30 w/v of 80% methanol at 35 °C (3 times extraction, 3 h each), was 67.58 µg GAE/g. This value was about half of that reported for coconut meat (139–141 µg GAE/g) [47] which was reasonable because a part of phenolic compounds must be extracted with coconut milk. TPC of the oils was evaluated from the methanolic extracts (hydrophilic fraction). As shown in Fig. 5A, the oil extraction condition affected the TPC values (p ≤ 0.05). The oils contained TPC in the range of 13.67–47.80 µg GAE/g oil. The highest TPC value was obtained from the oil extracted by subcritical ethanol at 100 °C, 45 min, approximately 3.5 times higher than that in the oil from Soxhlet extraction using hexane. These results indicated that the subcritical ethanol extraction could increase phenolic compounds in the extracted oil. Generally, alcoholic extraction is effective for extracting phenolic compounds from plant materials [48] and the main phenolic components of coconut meat were gallic, caffeic, salicylic, and p-coumaric acids [47] which can be easily dissolved in ethanol.
Total phenolic content of the coconut oil (A) and DPPH radical-scavenging activity of the hydrophilic and lipophilic oil fractions at 5 mg extract/100 µL (B), different letters indicate significant difference (p ≤ 0.05, Duncan’s multiple range test.). 100-45, 120-15, and HS are explained in Table 1
The antioxidant activities of the hydrophilic and lipophilic fractions of oils determined by DPPH radical-scavenging activity assay are shown in Fig. 5B. The hydrophilic and lipophilic fractions (5 mg extract/100 µL) of the oil obtained from subcritical ethanol extraction at 100-45 inhibited DPPH radical by 51.6% and 5.64%, respectively which are higher than those of oil obtained at 120-15 (33.15%, 3.58%) and hexane extraction (8.46%, 2.58%). The results of hydrophilic fractions agreed with the TPC values of the oils. Seneviratne et al. [49] reported that the coconut oils extracted directly from coconut milk by wet process under hot conditions (100–120 °C) gave higher TPC and antioxidant capacities than those of the coconut oil extracted under cold condition (10 °C). However, at condition of 120-15, shorter extraction time would yield lower TPC and some TPC possibly destroyed at extraction temperature of 120 °C [50], therefore; oil extracted by 120-15 possessed lower antioxidant capacities. It should be noted that, previous studies reported that coconut oil contained only a trace amount of tocopherols [51, 52].
Conclusions
In this study, oil extraction from coconut meal, a by-product from coconut milk process, by subcritical ethanol was investigated. The highest oil recovery rate obtained from subcritical ethanol extraction was 81.49%. The peroxide value, free fatty acid content, saponification value, unsaponifiable matter and fatty acid profile of the oil obtained from subcritical ethanol extraction conformed to the standard of conventional coconut oil and showed higher antioxidant activity compared to the oil extracted with hexane. It can be concluded that subcritical ethanol extraction of oil from coconut meal is feasible. The high oil recovery could be achieved with less amount of solvent and shorter time without reflux. The obtained crude oil has good qualities and good antioxidant properties which is suitable for use in the food and pharmaceutical industries.
Data availability
Research data are available from the corresponding author upon requested.
References
P.P. Soo, Y. Ali, O.M. Lai, C.H. Kuan, T.K. Tang, Y.Y. Lee, E.T. Phuah, Eur. J. Lipid Sci. Technol. 125(5), 1900220 (2020)
N.A.A. Ghani, A.A. Channip, P. ChokHwee Hwa, F. Ja’afar, H.M. Yasin, A. Usman, Food Sci. Nutr 6(5), 1298–1306 (2018)
N.F.M. Idrus, N.A. Febrianto, W. Zzaman, T.E. Cuang, T.A. Yang, Food Sci. Technol. Res. 19(5), 729–737 (2013)
S. Sulaiman, A.R. Abdul Aziz, M. KheireddineAroua, J. Food Eng. 114(2), 228–234 (2013)
L.J. Pham, in Industrial Oil Crops. ed. by T.A. McKeon, D.G. Hayes, D.F. Hildebrand, R.J. Weselake (AOCS Press, Urbana, 2016), pp. 231–242
A. Deen, R. Visvanathan, D. Wickramarachchi, N. Marikkar, S. Nammi, B.C. Jayawardana, R. Liyanage, J. Sci. Food Agric. 101(6), 2182–2193 (2021)
P. Khuwijitjaru, A. Pokpong, K. Klinchongkon, S. Adachi, Int. J. Food Sci. 49(8), 1946–1952 (2014)
K. Klinchongkon, T. Bunyakiat, P. Khuwijitjaru, S. Adachi, Food Bioprocess Tech. 12(7), 1197–1204 (2019)
P. Khuwijitjaru, A. Pokpong, K. Klinchongkon, S. Adachi, Int. J. Food Sci. Technol. 49(8), 1946–1952 (2014)
P. Khuwijitjaru, K. Watsanit, S. Adachi, J. Ind. Eng. Chem. 18(1), 225–229 (2012)
R. O’Brien, in Handbook of food science, technology, and engineering. ed. by Y.H. Hui, F. Sherkat (CRC, Boca Raton, 2006)
R.C. de Oliveira, S.T. Davantel de Barros, M.L. Gimenes, J. Food Eng. 117(4), 458–463 (2013)
A. Perrier, C. Delsart, N. Boussetta, N. Grimi, M. Citeau, E. Vorobiev, Ultrason. Sonochem. 39, 58–65 (2017)
M.G. Pereira, G.M. Maciel, C.W.I. Haminiuk, F. Bach, F. Hamerski, A. de Paula Scheer, M.L. Corazza, Waste Biomass Valoriz. 10(9), 2611–2625 (2019)
A.E. Kate, A. Singh, N.C. Shahi, J.P. Pandey, T.P. Singh, O. Prakash, J. Food Meas. Charact. 11(1), 272–280 (2017)
E. Rojo-Gutiérrez, O. Carrasco-Molinar, J.M. Tirado-Gallegos, A. Levario-Gómez, M.L. Chávez-González, R. Baeza-Jiménez, J.J. Buenrostro-Figueroa, J. Food Meas. Charact. 101, 1–10 (2021)
F. Derwenskus, F. Metz, A. Gille, U. Schmid-Staiger, K. Briviba, U. Schließmann, T. Hirth, GCB Bioenergy 11(1), 335–344 (2019)
M. Citeau, S. AlbeSlabi, F. Joffre, P. Carré, OCL 25(2), D207 (2018)
E. Potrich, S.C. Miyoshi, P.F.S. Machado, F.F. Furlan, M.P.A. Ribeiro, P.W. Tardioli, R.L.C. Giordano, A.J.G. Cruz, R.C. Giordano, J. Clean. Prod. 244, 2118660 (2020)
P. Khuwijitjaru, Japan. J. Food Eng. 17(2), 33–39 (2016)
M.C. Capellini, L. Chiavoloni, V. Giacomini, C.E.C. Rodrigues, J. Food Eng. 240, 145–152 (2019)
Y. He, Z. Huang, C. Zhong, Z. Guo, B. Chen, Bioresour. Technol. 293, 122049 (2019)
D.C.G. Okiyama, I.D. Soares, T.A. Toda, A.L. Oliveira, C.E.C. Rodrigues, Ind. Crops Prod. 130, 96–103 (2019)
N. Castejón, P. Luna, F.J. Señoráns, Food Chem. 244, 75–82 (2018)
R. Oliveira, V. Oliveira, K.K. Aracava, C.E.D.C. Rodrigues, Food Bioprod. Process. 90(1), 22–31 (2012)
K.A. Santos, C.M. de Aguiar, E.A. da Silva, C. da Silva, J. Supercrit. Fluids 169, 105125 (2021)
A.R.C. de Souza, S. Stefanov, M.C.M. Bombardelli, M.L. Corazza, R.P. Stateva, J. Supercrit. Fluids 152, 104573 (2019)
B. Tangkhavanich, T. Kobayashi, S. Adachi, J. Ind. Eng. Chem. 20(4), 2610–2614 (2014)
D.W. Horwitz, Official Methods of Analysis of AOAC International (AOAC International, Maryland, 2000)
AOCS, Official Methods And Recommended Practices of the American Oil Chemists’society (American Oil Chemists’Society, Champaign, 1998)
D.F. Ferreira, J.S. Barin, A. Binello, V.V. Veselov, G. Cravotto, Food Bioprod. Process. 117, 224–230 (2019)
T. Gutfinger, J. Am. Oil Chem. Soc. 1, 966–968 (1981)
P. Khuwijitjaru, T. Yuenyong, R. Pongsawatmanit, S. Adachi, J. Oleo Sci. 58(10), 491–497 (2009)
M.M. Sawada, L.L. Venâncio, T.A. Toda, C.E.C. Rodrigues, Food Res. Int. 62, 662–670 (2014)
O.Z. Sampaio Neto, E.A.C. Batista, A.J.A. Meirelles, J. Clean. Prod. 180, 866–875 (2018)
J.R. Lajara, in Edible Fats and Oils Processing: Basic Principles and Modern Practices. ed. by D.R. Erickson (The American Oil Chemists Society, Champaign, 1990)
V.S. Kislik, Solvent Extraction (Elsevier, Amsterdam, 2012), pp. 3–67
J.D. Lamb, R.M. Izatt, J.J. Christensen, in Progress Macrocyclic Chemistry. ed. by R.M. Izatt, J.J. Christensen (Wiley, New York, 1981), pp. 41–90
R. Tir, P.C. Dutta, A.Y. Badjah-Hadj-Ahmed, Eur. J. Lipid Sci. Technol. 114(12), 1427–1438 (2012)
C. Wohlfarth, in Handbook of Solvents, 2nd edn., ed. by G. Wypych (ChemTec Publishing, Oxford, 2014), pp. 167–264
M.G. Pereira, F. Hamerski, E.F. Andrade, Ad.P. Scheer, M.L. Corazza, J. Supercrit. Fluids 128, 338–348 (2017)
Y. Shi, Y. Ma, R. Zhang, H. Ma, B. Liu, J. Food Sci. Technol. 52(5), 3099–3104 (2015)
C. Wang, L. Chen, B. Rakesh, Y. Qin, R. Lv, Front. Energy 6(3), 266–274 (2012)
E.A. Mansour, S.A. Abo El-Enin, A.S. Hamouda, H.M. Mahmoud, Environ Nanotechnol. Monit. Manag. 12, 100271 (2019)
M.K.M. Nair, J. Joy, P. Vasudevan, L. Hinckley, T.A. Hoagland, K.S. Venkitanarayanan, J. Dairy Sci. 88(10), 3488–3495 (2005)
W.C. Huang, T.H. Tsai, L.T. Chuang, Y.Y. Li, C.C. Zouboulis, P.J. Tsai, J. Dermatol. Sci. 73(3), 232–240 (2014)
B. Mahayothee, I. Koomyart, P. Khuwijitjaru, P. Siriwongwilaichat, M. Nagle, J. Müller, Int. J. Food Prop. 19(9), 2041–2051 (2016)
C.W.I. Haminiuk, M.S.V. Plata-Oviedo, G. de Mattos, S.T. Carpes, I.G. Branco, J. Food Sci. Technol. 51(10), 2862–2866 (2014)
K.N. Seneviratne, C.D. HapuarachchI, S. Ekanayake, Food Chem. 114(4), 1444–1449 (2009)
Z. Ju, L.R. Howard, J. Food Sci. 70(4), S270–S276 (2005)
D.A. Ananth, G. Deviram, V. Mahalakshmi, T. Sivasudha, Z. Tietel, Biocatal. Agric. Biotechnol. 17, 416–421 (2019)
I.D. Desai, H. Bhagavan, R. Salkeld, J.E. de Dutra Oliveira, J. Food Compos. Anal. 1(3), 231–238 (1988)
Funding
This work was funded by the Royal Golden Jubilee Ph.D. program (PHD/0190/2561) and the Mid-Career Research Grant (NRCT5-RSA63021-01) from the National Research Council of Thailand (NRCT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Plangklang, T., Khuwijitjaru, P., Klinchongkon, K. et al. Chemical composition and antioxidant activity of oil obtained from coconut meal by subcritical ethanol extraction. Food Measure 15, 4128–4137 (2021). https://doi.org/10.1007/s11694-021-00989-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00989-5