Abstract
Chilean papaya slices were dried using different technologies to evaluate the effect of the different technologies on drying kinetics, bioactive compounds and biological activities. Five techniques were used: freeze drying (FD), vacuum drying (VD), solar drying (SD), convective drying (CD) and infrared drying (IRD). Fresh and dried samples were evaluated in terms of proximate composition, phenolic profiles, total phenolic and flavonoid contents, β-carotene, vitamin C, and antioxidant and α-glucosidase activities. CD-papaya showed lower processing time, requiring 270 min to reach the dynamic equilibrium condition, while SD-papaya required 870 min. The five drying technologies were found to have variable effects on proximate composition. VD-samples showed the lowest loss of individual phenolic compounds, total phenolic content and vitamin C while IRD- and CD-samples showed lower total flavonoids (42%) and β-carotene (32%) loss after processing, respectively. With respect to biological activities, all samples possessed enzymatic activity in a dose-dependent manner (0–2.0 mg ml−1), being IRD-sample the most effective in inhibiting α-glucosidase (IC50 = 13 mg ml−1). Also, the highest antioxidant capacity measured by DPPH and ORAC was obtained for the papaya samples dried using a vacuum drier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among a wide array of tropical fruits, the papaya is deemed to be one of the most economical fruits, which not only is cultivated roughly in 60 countries but also is marketed worldwide [1]. Papaya fruit has experienced an increase in demand due to its delicious taste and mouthfeel, nutritional value, and health applications [2]. The fruit contains an important amount of vitamins (A, B1, B2, C), minerals (calcium, iron, potassium, sodium) and carotenoids (lycopene, β-carotene, β-cryptoxanthin) while being low in sodium, fats, and calories [3, 4]. Concerning to health applications, papaya has been used as topical dressings for ulcer and dermatitis treatment, gastrointestinal uses such as antihelminthic and antibacterial activity treatments, and traditional uses for fertility control [5]. This tropical fruit is a member of the Caricaceae family, it is native from the tropical America and has been disseminated around the world [6]. The species cultivated in Chile, known as mountain papaya, is Vasconcellea pubescens [7] and is distributed in South America from Panama to Bolivia [8]. This fruit has a juicy yellow flesh with an intense and aromatic flavor, characterized by a high content of the proteolytic enzyme papain, keeping a great value as an added ingredient in the pharmaceutical, cosmetics, and food industries [8, 9]. In addition to its striking aroma and high vitamin content, the fruit of V. pubescens is attractive to consumers because it contains numerous phenolic compounds, specifically hydroxycinnamic acid and quercetin glycoside derivatives [8], which play an important role on the anti-hyperglycemic, antioxidant and insulin stimulating activities [10].
In nature, ripened papaya is a perishable product due to high moisture content and easily prone to microbial spoilage at room temperature [11]. It can be maintained intact only up to 3 weeks under storage conditions (10 °C at 90–95% RH; [12]). Therefore, this is a major hindrance considering the export market demands worldwide. Dehydration is one of preservation methods to extend the storability of papaya [3]. It is a method where water removal halts the growth of spoilage microorganisms, as well as the occurrence of enzymatic or non-enzymatic browning reaction in the food matrix, thus preserving the structure, sensorial characteristics and nutritional value of the starting material [13]. However, the final quality product will highly depend upon the drying technology and drying conditions. The effect of convective drying, heat pump drying, spray drying, solar drying, vacuum drying and freeze-drying [11, 12, 14,15,16], as well as alternative drying processes, e.g. explosion puffing drying [17, 18] and ultrasound and vacuum assisted drying [19] were widely investigated in Carica papaya and in other exotic tropical fruits. These studies demonstrated that several drying techniques can preserve the overall nutritional quality and retain the concentration of bioactive compounds. Nevertheless, information of the effect of different drying processes on the bioactive components and biological activity of V. pubescens specie is scarce. To our knowledge, the suitability of using different drying technologies to preserve papaya V. pubescens has not been evaluated before. Thus, the aim of this study was to evaluate the drying kinetics, chemical properties and bioactive components of papaya (V. pubescens) submitted to different drying technologies (freeze drying, vacuum drying, solar drying, convective drying and infrared drying) as well as the influence of these technologies on the antioxidant capacity and α-glucosidase inhibition of the dried fruit.
Materials and methods
Raw material
Chilean papayas (V. pubescens) of similar ripeness were purchased at a local market (Elqui Valley, Coquimbo, Chile). Fruits were selected in order to obtain samples of uniform shape, size and ripening grade, based on skin color (80–90% of yellowness). The selected fruits were washed and peeled using a boiling solution of NaOH (10%) and fastpeel additive (1%; Quimica Norte Verde Ltda., La Serena, Chile) and washed immediately with cold water in order to remove skin remains. The peeled fruits were cut into slices (9.0 × 1.5 cm) with 0.4 cm of thickness after seeds and mucilage removing. Papaya slices were divided into five batches for freeze-, vacuum-, solar-, convective- and infrared-drying.
Drying
Freeze drying (FD)
Fresh papaya slices (1.05 kg) were first frozen at − 80 °C for 24 h. The sample was then separate horizontally onto three metallic drying trays with a charge density of 4.25 kg m−2 per tray and dried in a freeze dryer (VirTis Wizard 2.0 Advantage Plus XL-70, Gardiner, NY, USA) for 73 h in two stages: primary drying was programmed with 8 ramps temperature from − 40 to 15 °C and the secondary drying at 20 °C under 0.027 kPa vacuum pressure.
Vacuum drying (VD)
Fresh papaya slices (0.60 kg) were uniformly distributed over two specialized stainless steel trays with a charge density of 2.48 kg m−2 per tray and then dried in a vacuum oven (Memmert, model VO 400, Schwabach, Germany) at 70 °C and 15 kPa pressure for 480 min. The drying temperature was selected based on previous studies from Sehrawat et al. [20].
Solar drying (SD)
The solar-dryer was designed and constructed at the Department of Food Engineering of University of La Serena, Chile and drying was installed at the Elqui valley (20 km from La Serena city). Fresh papaya slices (4.00 kg) were spread onto two stainless-steel trays with a charge density of 4.13 kg m−2 per tray and placed on the solar-dryer chamber at ambient air heated by a solar collector through recirculation of the drying air. The collector used a cupper plate to absorb the incident solar radiation and a glass sheet as the transparent cover. The drying conditions were variable during daylight and recorded with a data logger (lascar EL-USB-2, Whiteparish, England). The temperature ranged from 31.0 to 49.9 °C and air humidity ranged from 20 to 45%. Samples were dried for 870 min.
Convective drying (CD)
Fresh papaya slices (1.44 kg) was evenly spaced on two stainless steel trays with a load density of 3.77 kg m−2 per tray and then dehydrated using a convective drier designed and built at the Department of Food Engineering of Universidad de La Serena at 70 °C for 270 min and an air flow rate of 1.5 m s−1. The drying conditions were selected based on previous work from Lyu et al. [17].
Infrared drying (IRD)
The IR dryer was designed and constructed at the Department of Food Engineering of University of La Serena, Chile with two IR incandescent lamps of 175 W (Philips, PAR38 IR 175W E27 240V CL 1CT/12) installed inside the oven as radiation sources. Fresh papaya slices (0.255 kg) were placed on a holding tray (21 × 30 cm) facing the IR lamps. The distance between the lamps and the samples was set at 20 cm. Drying was carried at a temperature fixed at 70 °C for 390 min. All different dehydrated samples were powdered using a basic analytical mill (IKA A-11, USA) and passed through a 35-mesh sieve of 500 µm (U.S. Standard Sieve Series, Dual Manufacturing Co., USA). The powdered samples were sealed and stored in plastic bags at 5 °C until further analysis.
Drying characteristics and drying rate curve
The characteristic drying curves were obtained to describe the effects of different drying conditions on papaya drying kinetics. Moisture ratio of samples during drying can be expressed by:
where MR is moisture ratio (dimensionless); Mt [kg water kg−1 dry matter (D.M)] is moisture content at any time t (min), Mi (kg water kg−1 D.M) is initial moisture content [20].
In addition, drying rate (DR) refers to moisture loss per unit time, which can be calculated according to Eq. (2) where t1 and t2 are the drying time, min, Xwt1 and Xwt2 are the moisture contents on dry basis, g g−1, at time t1 and t2, respectively.
Proximate composition and dietary fiber
The moisture content (AOAC no. 934.06), fat (AOAC no. 960.39), crude fiber (AOAC no. 962.09) and crude ash (AOAC no. 923.03) were determined according to AOAC methods [21]. Total protein content was determinate by Dumas Combustion methodology (AOAC no. 99033; Elementar, rapid N exceed, Donaustrasse, Germany). The water activity (aw) of samples was measured at 25 °C using an AQUA LAB equipment (4 TE, Pullman, WA, USA).
All papaya samples were analyzed for soluble (SDF) and insoluble dietary fiber (IDF) fractions according to a gravimetric enzymatic method (AOAC no. 991.43). A total dietary fiber assay kit (TDF100A; Sigma-Aldrich, St. Louis, MO, USA), an enzymatic digestion unit and a filtration system (VELP Scientifica, GDE—CSF6, Usmate, Italy) were used. The total dietary fiber (TDF) was calculated as the sum of soluble and insoluble dietary fibers, and expressed as g 100 g−1 dry matter (D.M). All measurements were performed in triplicate.
Identification and quantification of phenolic acids
Preparation of extracts
Powdered dried papaya (5.0 g) were homogenized in 15 ml of 69% acetone, using an orbital shaker (Boeco, OS 20, Hamburg, Germany) at 200 rpm for 30 min at room temperature, centrifuged at 4193×g for 5 min in an Hettich® centrifuge model Universal 320 R (Tuttlingen, Germany), the supernatant was removed and extraction was repeated three times in a similar way. The combined supernatants were evaporated in a rotary evaporator (Buchi RE12, Flawil, Switzerland) at 37 °C and then the dried residue resuspended in 5 ml of methanol–formic acid (99:1) to be directly analyzed in a HPLC/UV-DAD system.
High-performance liquid chromatography (HPLC)
Samples containing phenols were injected automatically into an Agilent 1200 series HPLC system (Santa Clara, Agilent 1200 series, CA, USA) equipped with a diode array detector (DAD). Absorption spectra for the main peaks were recorded at 280 and 310 nm. The HPLC system was equipped with a Kromasil 100-5C18 column (250 × 4.6 mm; Eka Chemical, Bohus, Sweden) with a spherical particle size of 5 µm, which was kept at 25 °C. The mobile phase was composed of 0.1% formic acid, pH 3 (A) and 100% acetonitrile (B), and the elution gradient was initially set at 87% A and 13% B from time 0 to 16 min; 45% A and 55% B from time 16 to 23 min; 60% A and 40% B from time 23 to 25 min; 87% A and 13% B from time 25 to 30 min and then returned to the initial conditions within 4 min at a flow rate of 0.7 ml min−1 and 25 °C. The injection volume was 10 µl.
Determination of bioactive compounds content
Preparation of extracts
Extracts of fresh papaya were prepared from 10.0 g milled frozen fruit (− 80 °C) with a basic analytical mill (IKA A-11) or 5.0 g powdered dried papaya mixed in a 1:4 ratio with 80% aqueous methanol, according to the method described by Uribe et al. [9] but slightly modified. The mixture was homogenized under continuous stirring for 30 min at 200 rpm using an orbital shaker (OS-20, Boeco) and then centrifuged at 4193×g for 3 min (Hettich Universal 320 R). The supernatant was removed and the extraction was repeated one more time in a similar way. The combined supernatants were evaporated in a multivapor (Büchi, P-6, Flawil, Switzerland) at 37 °C and resuspended in 10 ml of methanol–formic acid (99:1). Extractions were performed in triplicate and were used for analysis of total phenolic and flavonoid contents, and antioxidant activity.
Determination of total phenolic contents (TPC)
The TPC were evaluated using the Folin–Ciocalteu assay based on the method of Chuah et al. [22] with slight modifications. A total of 0.5 ml of extracted sample was mixed with 0.5 ml of Folin–Ciocalteu reagent in a test tube. The mixture was shaken and allowed to react for 5 min. Subsequently, the sample was mixed with 2.0 ml of sodium carbonate (200 mg ml−1) solution and incubated at ambient temperature in the dark for 15 min. After incubation, 10 ml of ultra-pure water was added and the samples were centrifuged at 4193×g for 5 min. The TPC was measured at 725 nm using a VIS spectrophotometer (Spectronic R20 GenesysTM131, Illinois, USA). A standard calibration curve was plotted using gallic acid. The TPC was expressed as mg gallic acid equivalent (GAE) per g D.M of sample.
Determination of total flavonoid contents (TFC)
The TFC were evaluated using a colorimetric assay developed by Dini et al. [23] with slight modification. A total of 0.5 ml of extracted sample was mixed with 2.0 ml of ultra-pure water on a volumetric flask followed by 0.15 ml 5% NaNO2. The mixture was shaken and allowed to react for 5 min. Subsequently, 0.15 ml 10% AlCl3·6H2O were added to the reaction and incubated at ambient temperature in the dark for 6 min. To end the reaction, 1 ml 1 M NaOH was added followed by 1.2 ml ultra-pure water and then thoroughly mixed. A standard calibration curve was plotted using quercetin. The TFC was expressed as mg quercetin equivalent (QE) per g D.M of sample.
Determination of β-carotene
Extracts of fresh papaya were prepared from 3.5 g milled frozen fruit (− 80 °C) or 1.5 g powdered dry papaya mixed in 25 ml of a solvent mixture hexane/acetone/ethanol (50/25/25 v/v/v) under continuous stirring for 30 min at 200 rpm using an orbital shaker (OS-20, Boeco). The extracts were centrifugated at 4193×g for 5 min to separate to the supernatant. The residue was re-extracted until it became colourless. After extraction, mixture was filtered using Whatman filter paper N #1 and the filtrate was dried at 37 °C on a multivapor (Büchi, P-6). The extracted was resuspended to a final volume of 5 ml in ethyl acetate and then was filtrated through 0.45 µm membrane filters. Ten microliters were used to HPLC analysis.
A Kromasil 100-5C18, 250 × 4.6 mm, connected with a Kromasil guard column was used. The column temperature was controlled at 30 °C during the HPLC runs. Data were processed by using the Agilent ChemStation software. The flow rate was set at 1 ml min−1 and the mobile phases were (A) acetonitrile/H2O/trimethylamine (900/99/1, v/v/v), adjusted with a phosphoric acid solution 1 M at pH 8.5, and ethyl acetate (B). The gradient elution program was: 0–5 min, 100–75% A; 5–10 min, 75–30% A; 10–14 min, 30–0% A; 14–15 min, 0–100% A; 15–20 min, 100% A. The absorbance was read at 450 nm, β-carotene was identified based on the retention time and peak areas compared to authentic standards. The calibration curve was prepared between 2 and 50 µg of β-carotene ml−1 in ethyl acetate. Results were expressed as µg β-carotene 100 g−1 D.M.
Determination of vitamin C
The vitamin C content was extracted from 1.0 g milled frozen fruit (− 80 °C) or 0.25 g powdered dry papaya with 15 ml of 5% metaphosphoric acid (MPA) and then mixed for 20 min. The samples were centrifuged at 4193×g for 15 min at 10 °C, and 10 µl of the supernatant was injected into HPLC. A Zorbax Eclipse XDB-C18, 4.6 × 150 mm, 5 µm reversed-phase column was used, a mobile phase containing 5 mM cetyltrimethylammonium bromide as the ion-pairing agent and 50 mM potassium dihydrogen phosphate as buffer, at pH 3.9 and filtered through a 0.22 µm filter. All measurements were done at 20 °C with a flow-rate of 0.7 ml min−1, isocratic elution and detection at 254 nm. A calibration curve was prepared in 5% MPA. Total ascorbic acid (vitamin C) was estimated after 120 min of dehydroascorbic acid (DHA) reduction with DTT (1,4-dithiothreitol) phosphine hydrochloride and the samples were injected into HPLC. Results were expressed as mg vitamin C g−1 D.M.
Determination of antioxidant capacity
2,2-Diphenyl-1-picryl-hydrazyl (DPPH) assay
The DPPH assay was performed by preparing a solution of 50 µM DPPH in methanol formic (99:1). A total of 100 µl papaya extract obtained as described in “Preparation of extracts” section was allowed to react with 3.9 ml of the DPPH solution for 30 min under dark conditions. The absorbance was read at 517 nm and the result is expressed in µmol trolox equivalent (TE) per g D.M of sample.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was carried out according to Zhang et al. [24] in a Multilabel Plate Reader (Perkin–Elmer, VictorTM χ3, Turku, Finland). To each well of a polystyrene 96-well microplate (OptiPlateTM-96 F HB, Perkin–Elmer, Turku, Finland), 40 µl of papaya extract also obtained as described in “Preparation of extracts” section were mixed with 200 µl of fluorescein (100 µmol l−1) prepared in phosphate buffer (75 mmol l−1, pH 7.4) and incubated for 20 min at 37 °C. Then, 35 µl of AAPH (2,2′-Azobis(2-methylpropionamidine) dihydrochloride; 0.36 mol l−1) were added to start the reaction. Fluorescence was read every 60 s with an excitation wavelength of 485 nm and an emission filter of 535 nm, until fluorescence reading had declined to less than 5% of the initial value. Inhibition capacity was expressed as trolox equivalents (TE, µmol g−1 D.M), and was quantified by the difference between the sample and blank areas under the kinetic fluorescein decay curve and a calibration curve of Trolox (0–1 mM).
Determination of α-glucosidase activity
The α-glucosidase enzymatic activity was determined as described by Unnikrishnan et al. [25] with some modifications. A total of 50 µl papaya diluted extract obtained as described in “Preparation of extracts ” section with varying concentrations (0–2.0 mg ml−1) and 100 µl α-glucosidase from saccharomyces cerevisiae (Sigma G5003, USA) solution (0.5 U ml−1) was added in phosphate buffer 0.1 M (pH 6.9) and incubated into well plates at 25 °C for 10 min. After a preincubation, 50 µl of 4-nitrophenyl α-d-glucopyranoside (Sigma N1377, Switzertand) in 0.1 M phosphate buffer (pH 6.9) was added. Then, the absorbance readings were recorded at 405 nm using a Victor™ χ3 Multilabel Plate Reader and compared to the control (50 µl of buffer solution) corresponding to the highest enzymatic activity. The measurement was read every 30 s by 10 min. The experiments were performed in triplicates for each sample of each concentration, and α-glucosidase activity was calculated as the percentage obtained through the slope of each exponential curve.
Statistical analysis
An analysis of variance (ANOVA) was performed using Statgraphics Centurion XVI (Statistical Graphics Corp., Herdon, USA) to determine significant differences among the treatments. Means were compared by least significant difference (LSD) test at p < 0.05. In addition, the multiple range test (MRT) included in the statistical program was used to demonstrate the existence of homogeneous groups within each of the parameters. Data were averaged from three treatment replicates and reported as mean ± standard deviation.
Results and discussion
The drying characteristics
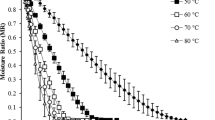
Comparison of the drying behavior and drying rate of papaya slices dried by four different drying techniques are shown in Fig. 1. It is observed that moisture ratios decreased continuously with drying time (Fig. 1a). The time required to decrease the moisture ratio to 0.01 was 270 min for convective drying (CD), 390 min for infrared drying (IRD), 480 min for vacuum drying (VD) and 870 min for sun drying (SD). Comprehensively, the time was longer for SD due to the fluctuating sunlight (ambient temperature) and laminar air flow over the product during the drying period [26]. However, the drying time for CD was considerably shortened when compared with the other drying technologies. The continuous hot air circulation inside the oven removed fast the surface water from the product exposed with a large transfer area to hot dry air. This effect causes a rapid drying rate period followed by a short falling rate period. A similar behavior was also noted by Lemus-Mondaca et al. [27, 28] in papaya slices dried by CD under similar conditions. However, Vieira da Silva Júnior et al. [19] reported higher drying rates in papaya slices when drying was performed with the use of ultrasound and/or vacuum than with those of the convective drying.
a Drying behavior and b drying rate of V. pubescens at different drying techniques. Vacuum drying at 70 °C (filled triangle), solar drying (open triangle), convective drying at 70 °C (open circle), and infrared drying at 70 °C (filled circle). Values are averages (n = 3), error bars are standard deviation
As shown in Fig. 1b, in all drying rate curves, the three typical phases of drying described by Fox et al. [29] can be distinguished; i.e. an initial adjustment period, a constant rate period and a falling rate period, including a 1st and a 2nd falling drying rate phase [30]. In the initial stage, the material adapts quickly to the drying conditions [27]. The effects of the initial adjustment period is less pronounced for the SD samples because the equilibrium air temperature was similar to the product temperature. The second phase (constant rate period) occurred between moisture ratios of 0.5–0.8. During this period, intense heat transfer evaporates free water from the sample [31]. Conversely, during the third and final drying phase, drying rate decreases because the water is trapped inside the food matrix and slowly diffuses to the surface through capillary action [29]. Diffusion is also altered by the tortuosity of the material [32].
As expected, the four drying technologies displayed different drying rate patterns due to differences in dryer designs, mechanisms of heat input, operating temperatures and pressure condition, among others.
Proximate composition
The proximate composition of fresh and dried papaya obtained by five different drying techniques is shown in Table 1. The composition for fresh papaya was 93.85 g 100 g−1 moisture, 0.18 g 100 g−1 fat, 0.74 g 100 g−1 ash, 0.59 g 100 g−1 protein, and 0.85 g 100 g− 1 fiber. All values were expressed in wet basis. These values were within the ranges found in the literature [2, 33,34,35,36]. The different values for the proximate composition of fresh papaya found in the literature can be explained by differences in cultivar variation, growing location, sunlight exposure, agricultural practices, stage of ripeness and postharvest handling [5].
To ensure products are microbiologically stable, the water activity in dried fruits should be lower than 0.600 [37]. Depending on the drying method, the water activity varied between 0.318 and 0.631 and the moisture content for the dried papaya varied between 7.25 (freeze drying) and 21.57 g 100 g−1 D.M (solar drying). The different operating modes of the dryers explain the different moisture contents and water activities in the dried samples. All drying methods caused a 13–51% decrease of fat content with respect to fresh papaya. Moreover, SD- and IRD-samples showed a slight increase in the ash content when compared with fresh sample, though this increase was not statistically significant while FD-, VD- and CD-samples showed a significant decrease in ash content. Some authors attributed the changes in fat and ash contents during drying to leakage or volatilization related to water loss [38, 39]. For all the samples except the IR-sample, drying caused a slight increase in protein content probably because protein nitrogen was not lost during drying [38]. The significant decrease in protein content observed for the IR-sample might be related to protein denaturation and/or browning reactions [40].
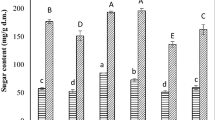
The TDF in fresh papaya was 48.88 g 100 g−1 D.M, predominated by IDF (36.20 g 100 g−1 D.M), which constituted 74% of the TDF. The ratio of IDF:SDF obtained was 1:2.9. Drying caused a significant loss (12–23%) in TDF. The ratio IDF:SDF was dependent on the drying method. The IDF/SDF values were 3.2, 2.2, 2.7, 5.2 and 2.5 for FD, VD, SD, CD and IRD samples, respectively. Calvache et al. [41] reported SDF:IDF values of 2.0 and 6.1 for papaya pulp and peel respectively. Talens et al. [42] reported that SDF:IDF ratio close to 1:2 are suitable fiber sources for use as food ingredient.
Phenolic compounds
Nine phenolic compounds were identified for fresh papaya (Table 2). However, only gallic acid was quantified (2.41 mg 100 g−1 D.M). Several authors reported low amounts or traces of phenolic compounds in papaya fruit and only identified compounds such as coumaric acid, ferulic acid, quercetin, apigenin, kaempherol and myricetin without quantifying them [8, 9, 43, 44]. After drying, it was possible to identified and quantified 6 phenolic compounds (Table 2), most probably due to the structural modifications that occurred during drying, which would facilitate the liberation of the phenolic compounds.
The drying method significantly (p < 0.05) affected the content of individual phenolic compounds. FD samples showed the lowest values for p-coumaric and trans-ferulic acids, when compared with the other drying technologies. A similar effect was observed by Gomes et al. [11] who obtained even lower concentrations than the method detectable limit for several phenolic acids and naringenin, when compared with the fresh papaya and spray dried product. The authors explained that enzymatic oxidation by polyphenol oxidase and peroxidase might have occurred during FD, also the cells might be injured by ice crystals formation leading to cell rupture and exposure of phenolics to the aforementioned oxidative conditions [45]. In contrast, VD and IRD papayas exhibited larger amounts of phenolic compounds when compared with other drying technologies, being the most abundant gallic acid, chlorogenic acid, tyrosol and naringin for VD sample, and p-coumaric and trans-ferulic acids for IRD sample (Table 2). Oxygen deficient environment would have prevented the aerobic degradation of phenolic compounds for VD samples, whereas during IRD the fruit cell walls might be destroyed facilitating the liberation of the phenolic compounds [20, 46]. Finally, it is worth mentioning that the main phenolic compound present in all the assessed dried papaya samples was tyrosol, it was present in concentrations of about 8.55–21.01 mg 100 g−1 (Table 2). Tyrosol has many beneficial properties, such as antioxidant, antimicrobial and anti-inflammatory activities. This natural antioxidant may contribute to the prevention of chronic inflammatory conditions [47].
Bioactive compound content and antioxidant capacity
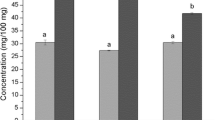
Table 3 presents the bioactive compounds and antioxidant capacity of papaya fruits as affected by different drying technologies. The TPC value for fresh papaya was 7.02 mg GAE g−1 D.M or 0.432 mg GAE g−1 F.W. The obtained value is similar to previously reported values by Patthamakanokporn et al. [48] (0.54 mg GAE g−1 F.W); Shofian et al. [15] (0.68 mg GAE g−1 F.W) and Abrol et al. [16] (0.56 mg GAE g−1 F.W). Freeze-, convective- and infrared-drying did not change significantly the TPC of the fruit (p > 0.05). This is different from several previous studies that have reported that CD, FD and combined methods may promote a decrease in TPC of different tropical fruits such as mango, pitaya, starfruit, muskmelon, watermelon and papaya [15, 18]. In the current study, only SD caused a statistically significant decrease (p < 0.05) in TPC of papaya. The SD-papaya was submitted to the longest processing time. It was necessary 870 min to reach the dynamic equilibrium condition at fluctuating temperatures between 30 and 50 °C. The longer exposure time of the fruit at a temperature close to 40 °C which is the optimum temperature of the polyphenol oxidase [37], might have caused the observed large loss of phenolic compounds of SD-samples. In contrast, other authors have reported that CD and FD processes cause an increase in TPC in papaya as compared to fresh ones which they attributed to the release of polyphenolic compounds from the food matrix during drying [12]. In our study, only VD technology caused an increase in TPC (Table 3). During VD, rapid heating of samples due to the presence of an electric heater in an oxygen free environment might have caused inactivation of oxidative enzymes and contribute to a better protection of phenolic compounds [20, 37].
In fresh papaya, the TFC was 3.33 mg g−1 D.M (0.226 mg g−1 F.W) quercetin equivalent. This value was similar to the results reported by Spínola et al. [44]. All drying methods caused 42–65% decrease in TFC, with a final content of 1.17–1.93 mg g−1 D.M quercetin equivalent. Infrared drying caused the lowest loss in TFC in the resultant dried papaya (42%). Far infrared may have the capability to break down covalent bonds and thus facilitate the liberation of biocompounds such as flavonoids from repeating polymers [49], resulting in higher quantification of flavonoids. In contrast, FD caused the largest loss (65%), which might be due to the disruption of fruit cells cause by ice crystals and the concomitant decompartmentalization of enzymes, substrates and activators. The increased enzyme activity upon thawing might have led to a greater degradation of some biocompounds [15]. Annegowda et al. [12] also observed a 50% loss of TFC in aqueous extracts obtained from FD-papaya, but for ethanolic extracts, TFC was statistically equivalent to that of the fresh fruits. Yi et al. [18] reported approximately 30% reduction in the content of TFC. However, they reported that FD was the best technology to preserve the TFC in papaya fruit chips compared with CD and combined drying methods.
β-carotene is found in all types of papaya cultivars leading to yellow and orange hues in the flesh [5]. In the present study, the content of β-carotene in fresh fruit was 2595 µg 100 g−1 D.M (162.9 µg 100 g−1 F.W), which is within the reported range values of 110.0–410.3 µg 100 g−1 F.W for papaya from different cultivars [3, 15, 50,51,52]. These differences in literature were due not only to different cultivars and production areas, but also stage of ripeness [53]. All drying technologies caused a decrease in β-carotene when compared with fresh papaya (Table 3). Interestingly, CD allowed the better percentage retention of β-carotene (68%). This is different from several previous studies that reported that CD caused the largest decrease in total carotenoids when compared to other drying technologies (vacuum-, freeze-drying and combined methods) [17,18,19]. The differences observed in CD samples in this study are most likely related to the inactivation of oxidative enzymes during the fast sample heating.
SD-papaya showed the lowest decrease in β-carotene when compared with dried papaya using other drying technologies (Table 3). This can be attributed to two reasons, the photosensitive and epoxide forming nature of carotenoids and the enzymatic degradation by lipoxygenase [20] due to the longer residence time of the fruit at a low and fluctuating temperature.
The initial vitamin C content of raw papayas (7.27 mg g−1 D.M or 0.495 mg g−1 F.W) is within the ranges reported previously in the literature (0.434 mg to 0.713 mg g−1 F.W; [4, 19, 35, 36, 50, 51]). All processing methods resulted in a significant decrease in vitamin C (p < 0.05). The greatest retention of vitamin C was obtained for the VD-papaya (5.39 mg g−1 D.M), followed by FD-papaya (4.95 mg g−1 D.M). Oxygen deficient environment would have prevented the aerobic degradation of vitamin C in VD and FD as compared to the other drying processes (SD, CD and IRD). This finding is similar to previous studies. Hawlader et al. [14] showed that FD and VD processes can retained up to 88 and 86% of vitamin C, respectively. Vieira da Silva Júnior et al. [19] reported that papaya samples with lower losses of vitamin C were those dried with the use of vacuum (vacuum drying and ultrasound and vacuum assisted drying). Sehrawat et al. [20] observed that vitamin C of mango is better retained when using low-pressure superheated drying and vacuum drying than when using convective drying. These authors explained that the vacuum employed prevented the contact of the ascorbic acid from the sample with the free oxygen, thus preventing its oxidation. Also, Shofian et al. [15] observed no significant differences between vitamin C content of FD-papaya and fresh sample; whereas, Yi et al. [18] obtained a better retention of vitamin C in FD-papaya as compared to CD and combined methods.
In the current study, SD-papaya showed the greatest loss in vitamin C (71%). The slow drying at fluctuating temperatures (between 30 and 50 °C) might not be effective enough to inactivate the enzymes responsible for vitamin C degradation [20]. This finding is similar to previous studies that also reported significant decrease in vitamin C after solar drying (60 ± 2 °C for 6 h), with up to 78, 75 and 77% vitamin C loss for mango, banana and papaya, respectively [16].
Antioxidant activity of papaya was evaluated using DPPH and ORAC assay (Table 3). Since each assay is based on a different chemical system and/or reaction, different antioxidant activity results could be expected depending on the specific analysis performed. The antioxidant activity in fresh fruit was 81.26 µmol TE g−1 D.M (5.00 µmol TE g−1 F.W) determined using the DPPH assay and 55.20 µmol TE g−1 D.M (3.39 µmol TE g−1 F.W) determined using the ORAC assay. Similar data were found in the studies of Udomkun et al. [3], Patthamakanokporn et al. [48] and Isabelle et al. [54] for fresh papaya. The drying process significantly affected the reduction in antioxidant activity of papaya with values between 25.64 and 34.51 µmol TE g−1 D.M when using the DPPH assay. This can be attributed to thermal degradation of the compounds responsible for the anti-oxidative properties such as flavonoids, carotenoids and vitamin C (Table 3). On the other hand, the antioxidant capacity values, using the ORAC assay, significantly increased for the dried samples except for the FD samples which decreased with respect to the fresh sample. A possible explanation for the increase in antioxidant activity is the formation of novel compounds with antioxidant activity such as Maillard reaction-derived melanoidins, responsible for color change during the thermal drying process [55]. However, during freeze-drying browning reactions are enhanced [15] decreasing the antioxidant activity. Shofian et al. [15] also reported that freeze-drying resulted in a decrease in the antioxidant activity of starfruit and mango compared to those of fresh samples; although FD-papaya, FD-muskmelon and FD-watermelon showed no significant difference when were measured by DPPH and FRAP assays.
VD papaya showed the highest antioxidant capacity measured by DPPH and ORAC (Table 3), which could be due to a better retention of vitamin C and TPC in these samples. Thus, drying at 70 °C in oxygen free environment is a better process to retain higher antioxidant activity and some bioactive compounds of dried papayas.
α-glucosidase activity
The enzyme α-glucosidase catalyzes the final step in the digestion and breakdown of carbohydrates, so its inhibition can be effective for the regulation of Type II diabetes, by controlling glucose absorption [56]. Figure 2 shows α-glucosidase activity as a function of the sample concentration of fresh and dried papaya using different technologies. The results revealed that all the samples possessed enzymatic activity in a dose-dependent manner (0–2.0 mg ml−1). The highest enzymatic activity was observed for the fresh papaya; high concentration of sample was needed to completely suppress the enzyme (2.0 mg ml−1). Instead, the enzymatic activity was totally suppressed when dried papaya concentration was about 0.5 mg ml−1, regardless of the drying technology. The IC50 values were calculated in order to compare the effect of the different drying technologies on half-maximal inhibitory concentration of papayas. Under this approach, the IC50 value of fresh sample was found to be 312 mg ml−1, whereas the values of FD-, VD-, SD-, CD- and IRD-samples were 24, 29, 46, 22 and 13 mg ml−1, respectively. Therefore, IRD-sample was the most effective in inhibiting α-glucosidase, being even 24-fold more effective than fresh sample in inhibiting the enzyme. This can be attributed to the best retention of TPC (Table 3) and individual phenolic acids such as chlorogenic, p-coumaric and trans-ferulic acids (Table 2) in IRD-samples. Oboh et al. [57] concluded that the inhibition of α-glucosidase activity in various parts of unripe papaya may be due to phenolic constituents such as sinapinic acid, 2-phenyl-6I-O-β-d-glucoside, o-coumaric acid, epicatechin, quercetin, luteolin, ferulic acid, kaempferol and caffeic acid, which may be exhibiting either an additive or a synergistic effect with the nonphenolic constituent such as carpaine. Gironés-Vilaplana et al. [56] found that lyophilized papaya was effective against α-glucosidase (IC50 = 1.58 mg ml−1), probably due to mangiferin and its derivatives (mangiferin gallate and isomangiferin gallate), which are associated with a strong antidiabetic effect.
The anti-hyperglycemic effect of papaya is thought to target pancreatic beta cells by boosting their sensitivity to insulin at the same time inhibiting α-amylase and α-glucosidase [10]. Thus, the inhibition of α-glucosidase activity slows down the breakdown of disaccharide to simple glucose and by doing so reduces the amount of glucose absorbed in the blood [57]. The results of the current study demonstrate the efficacy of dried papaya in inhibiting α-glucosidase in vitro. However, papaya fruit is not yet recognized asset in dietary management of diabetes since further evaluations at the molecular level are necessary [10].
Conclusions
The phytochemical composition of dried Chilean papaya was dependent on the drying method. Papaya slices dried in a vacuum drier retained the highest amount of ascorbic acid (75%) and antioxidant activity (43%) due to the absence of oxygen in the environment. The samples also retained 39% of flavonoids and 57% of β-carotene. In addition, this sample showed an increase in TPC and high amounts of individual phenolic acids. On the other hand, solar drying caused the largest loss in phenolic compounds (8%), individual phenolic acids, antioxidant activity (68%), ascorbic acid (71%) and β-carotene (73%) due to the extended drying times and high-water content. The highest retention in flavonoids was observed for the infrared dried sample (58%) while the highest retention in β-carotene was observed for the convective dried sample (68%).
In this study the α-glucosidase activity was totally suppressed when dried papaya concentration was about 0.5 mg ml−1, regardless of the drying technology, though the most effective against α-glucosidase was the infrared dried sample (IC50 = 13 mg ml−1).
References
H.V. Annegowda, R. Bhat, in Nutritional Composition of Fruit Cultivars, ed. by M.S.J. Simmonds, V.R. Preedy (Academic Press, London, 2016), pp. 497–516
T. Vij, Y. Prashar, Asian Pac. J. Trop. Dis. 5(1)), 1–6 (2015)
P. Udomkun, M. Nagle, B. Mahayothee, D. Nohr, A. Koza, J. Müller, LWT—Food Sci. Technol. 60(2), 914–922 (2015)
I. Hewajulige, S.A. Dhekney, in Encyclopedia of Food and Health, ed. by B. Caballero, P.M. Finglas, F. Toldrá (Academic Press, Amsterdam, 2016), pp. 209–212
E.H.K. Ikram, R. Stanley, M. Netzel, K. Fanning, J. Food Compost. Anal. 41, 201–211 (2015)
W. Zhang, G. Zeng, Y. Pan, W. Chen, W. Huang, H. Chen, Y. Li, Carbohydr. Polym. 172, 102–112 (2017)
M.A. Moya-León, M. Moya, R. Herrera, Postharvest Biol. Technol. 34, 211–218 (2004)
M.J. Simirgiotis, P.D.S. Caligari, G. Schmeda-Hirschmann, Food Chem. 115, 775–784 (2009)
E. Uribe, A. Delgadillo, C. Giovagnoli-Vicuña, I. Quispe-Fuentes, L. Zura-Bravo, J. Chem. 2015, 1–8 (2015)
O.I. Aruoma, J. Somanah, E. Bourdon, P. Rondeau, T. Bahorun, Mutat. Res. 768, 60–68 (2014)
W.F. Gomes, F.R.M. Franca, M. Denadai, J.K.S. Andrade, E.M. da Silva Oliveira, E. Sousa de Brito, S. Rodrigues, N. Narain, J. Food Sci. Technol. 55(6), 2095–2102 (2018)
H.V. Annegowda, R. Bhat, K.J. Yeong, M.-T. Liong, A.A. Karim, S.M. Mansor, Int. J. Food Prop. 17, 283–292 (2014)
M.C. Karam, J. Petit, D. Zimmer, E.B. Djantou, J. Scher, J. Food Eng. 188, 32–49 (2016)
M.N.A. Hawlader, C.O. Perera, M. Tian, K.L. Yeo, Drying Technol. 24, 77–87 (2006)
N.M. Shofian, A.A. Hamid, A. Osman, N. Saari, F. Anwar, M.S.P. Dek, M.R. Hairuddin, Int. J. Mol. Sci. 12, 4678–4692 (2011)
G.S. Abrol, D. Vaidya, A. Sharma, S. Sharma, Natl. Acad. Sci. Lett. 37(1), 51–57 (2014)
J. Lyu, J. Yi, J.F. Bi, H. Gao, M. Zhou, X. Liu, Int. J. Food Eng. 13(2), 20160250 (2016)
J.Y. Yi, J. Lyu, J.F. Bi, L.Y. Zhou, M. Zhou, J. Food Process. Preserv. 41, e13300 (2017)
E. Vieira da Silva, L. Júnior, Lins de Melo, R.A., Batista de Medeiros, Z.M.P., P.M. Barros, Azoubel, LWT—Food Sci. Technol. 97, 317–322 (2018)
R. Sehrawat, P.K. Nema, B.P. Kaur, LWT—Food Sci. Technol. 92, 548–555 (2018)
AOAC, Official Methods of Analysis of AOAC International, 15th edn. (AOAC, International, Arlington, 1990)
A.M. Chuah, Y.C. Lee, T. Yamaguchi, H. Takamura, L.J. Yin, T. Matoba, Food Chem. 111(1), 20–28 (2008)
I. Dini, G.C. Tenore, A. Dini, LWT—Food Sci. Technol. 43(3), 447–451 (2010)
L. Zhang, J. Li, S. Hogan, H. Chung, G.E. Welbaum, K. Zhou, Food Chem. 119(2), 592–599 (2010)
P.S. Unnikrishnan, K. Suthindhiran, M.A. Jayasri, Pharmacogn. Mag. 11(44), S511–S515 (2015)
A.K. Babu, G. Kumaresan, V.A.A. Raj, R. Velraj, Renew. Sust. Energ. Rev. 90, 536–556 (2018)
R. Lemus-Mondaca, N. Betoret, A. Vega-Galvez, E. Lara-Aravena, J. Food Process. Eng. 32, 645–663 (2009)
R. Lemus-Mondaca, C.E. Zambra, A. Vega-Galvez, N.O. Moraga, J. Food Eng. 116, 109–117 (2013)
B. Fox, G. Bellini, L. Pellegrini, in Fermentation and Biochemical Engineering Handbook, 3rd edn, ed. by C.M. Tadaro, H.C. Vogel (Springer, New York, 2014), pp. 295–305
D.I. Onwude, N. Hashim, R.B. Janius, N.M. Nawi, K. Abdan, Compr. Rev. Food Sci. Food Saf. 15, 599–618 (2016)
P. Zhao, L. Zhong, R. Zhu, Y. Zhao, Z. Luo, X. Yang, Energy Convers. Manag. 120, 330–337 (2016)
O.-V. Nistor, L. Seremet, D.G. Andronoiu, L. Rudi, E. Botez, Food Chem. 236, 59–67 (2017)
L. Bari, P. Hassan, N. Absar, M.E. Haque, M.I.I.E. Khuda, M.M. Pervin, S. Khatun, M.I. Hossain, Pak. J. Biol. Sci. 9, 137–140 (2006)
O.C. Othman, Tanzan. J. Sci. 35, 47–55 (2009)
S. Tripathi, J.Y. Suzuki, J.B. Carr, G.T. McQuate, S.A. Ferreira, R.M. Manshardt, K.Y. Pitz, M.M. Wall, D. Gonsalves, J. Food Compos. Anal. 24(2), 140–147 (2011)
G.E. Nwofia, P. Ojimelukw, C. Eji, Int. J. Med. Aromat. Plants 2, 200–206 (2012)
J. Samoticha, A. Wojdyło, K. Lech, LWT—Food Sci. Technol. 66, 484–489 (2016)
T. Wu, L. Mao, Food Chem. 110, 647–653 (2008)
M.G. Liman, A.S. Abdullahi, A.L. Maigoro, K.J. Umar, IOSR J. Appl. Chem. 7(1), 38–42 (2014)
S. Lenaerts, M. Van Der Borght, A. Callens, L. Van Campenhout, Food Chem. 254, 129–136 (2018)
J.N. Calvache, M. Cueto, A. Farroni, M. de Escalada Pla, L.N. Gerschenson, J. Funct. Foods 27, 319–328 (2016)
C. Talens, J.C. Arboleya, M. Castro-Giraldez, P.J. Fito, LWT—Food Sci. Technol. 77, 110–118 (2017)
D.M. Rivera-Pastrana, E.M. Yahia, G. González-Aguilar, J. Sci. Food Agric. 90, 2358–2365 (2010)
V. Spínola, J. Pinto, P.C. Castilho, Food Chem. 173, 14–30 (2015)
D.S. Sogi, M. Siddiq, K.D. Dolan, LWT—Food Sci. Technol. 62, 564–568 (2015)
N. Boudhrioua, N. Bahloul, I.B. Slimen, N. Kechaou, Ind. Crops Prod. 29, 412–419 (2009)
I.C. Vlachogianni, E. Fragopoulou, I.K. Kostakis, S. Antonopoulou, Food Chem. 177, 165–173 (2015)
O. Patthamakanokporn, P. Puwastien, A. Nitithamyong, P.P. Sirichakwal, J. Food Compos. Anal. 21, 241–248 (2008)
K. An, D. Zhao, Z. Wang, J. Wu, Y. Xu, G. Xiao, Food Chem. 197, 1292–1300 (2016)
M.M. Wall, J. Food Compos. Anal. 19, 434–445 (2006)
M. Wall, K. Nishijima, M. Fitch, W. Nishijima, J. Food Qual. 33, 131–149 (2010)
Z. Jiao, J. Deng, G. Li, Z. Zhang, Z. Cai, J. Food Compos. Anal. 23, 640–647 (2010)
L.E. Gayosso-Garcia Sancho, E.M. Yahia, G.A. González-Aguilar, Food Res. Int. 44, 1284–1291 (2011)
M. Isabelle, B.L. Lee, M.T. Lim, W.-P. Koh, D. Huang, C.N. Ong, Food Chem. 123, 77–84 (2010)
P. Udomkun, M. Nagle, D. Argyropoulos, B. Mahayothee, S. Latif, J. Muller, Food Chem. 196, 712–719 (2016)
A. Gironés-Vilaplana, N. Baenas, D. Villaño, H. Speisky, C. García-Viguera, D.A. Moreno, J. Funct. Foods 7, 599–608 (2014)
G. Oboh, A.A. Olabiyi, A.J. Akinyemi, A.O. Ademiluyi, J. Basic Clin. Physiol. Pharmacol. (2013). https://doi.org/10.1515/jbcpp-2013-0002
Acknowledgements
The authors gratefully acknowledge the Project FONDECYT 1170601 for providing financial support for the publication of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vega-Gálvez, A., Poblete, J., Quispe-Fuentes, I. et al. Chemical and bioactive characterization of papaya (Vasconcellea pubescens) under different drying technologies: evaluation of antioxidant and antidiabetic potential. Food Measure 13, 1980–1990 (2019). https://doi.org/10.1007/s11694-019-00117-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00117-4