Abstract
The effect of different drying methods on sugar content and amino acid content, color and non-enzymatic browning of Chilean papaya (Vasconcellea pubescens) slices was investigated. The obtained fruit extracts were tested in cell viability assays on human endothelial ECV-304 cells. Drying techniques included freeze-, vacuum-, solar-, convective- and infrared-drying. Consistently, infrared-dried papaya had a lower sugar content and a higher non-enzymatic browning intensity than papaya dehydrated by the other methods. All dried samples were lighter in color with a lower yellow intensity compared to fresh papaya. The amino acid lysine was the most abundant in the infrared-dried sample. On the other side, the methods that employed vacuum, increased their cellular viability. Based on these results, operational parameters during drying processes should be considered to preserve, on one hand, product quality attributes, while, on the other hand, increasing cell viability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chilean papaya (Vasconcellea pubescens) is well known for its nutritional value, mostly due to its elevated sugar content and many bioactive components with high antioxidant activity related to potential functional values [1,2,3,4]. In ripe state, V. pubescens is bright yellow, with obloid–ovoid form 5–6 cm in diameter and 6–14 cm long, five crests or lobules and average weight of 200 g [2, 3]. It has black, bright and spicy seeds inside, a juicy yellow pulp with levels of vitamin C upon 74 mg/100 g fresh weight [4], and characterized by the presence of several phenolic acids and flavonoids [1, 3, 4], and aliphatic 3-hydroxyesters as the volatile constituents responsible for the aroma [5]. Like in many fruits and vegetables, the antioxidant components of V. pubescens are suspected to play an important role against proliferation of cancer cells. For instance, several studies have demonstrated in vitro, cytotoxic effects of tropical papaya (Carica papaya) leaves, juice and seed extracts against a number of cell lines, including SCC25 (tongue cancer), HepG2 cell (liver cancer) and PC-3 cell (prostate cancer) [6,7,8].
Because raw V. pubescens fruit is characterized by a high papain content, it is consumed cooked or processed into jams, preserves, juice and sweets [3, 9]. Papaya fruits may also be dried to obtain snacks or additives for preparation of functional foods. However, if the drying process is not handled effectively, product quality can be adversely affected resulting in the loss of antioxidant compounds and organoleptic properties such as visual appearance [10]. Previous studies have showed color changes in Carica papaya during convective drying, as a consequence of pigments formation via enzymatic browning, Maillard reactions, vitamin C oxidation and carotenoid degradation [10, 11]. Vacuum based drying methodologies have been shown to aid in color preservation, possibly due to vacuum application reduces oxygen thus protecting vitamin C from oxidative damage and ascorbic acid from the degradation to the substances responsible for darkening [12]. Consequently, Vega-Gálvez et al. [1] reported a higher retention of vitamin C in vacuum-dried V. pubescens in comparison to other drying methods. Gomes et al. [13] found that spray dried papayas provided the highest retention of phenolic and flavonoid compounds, but freeze-dried fruit possessed better vitamin C and sugars content. Yi et al. [11] combined, on one hand, the hot air and explosion puffing drying (AD-EPD), while, on the other hand, freeze drying with explosion puffing drying (FD-EPD) to be applied to papaya. They found that FD-EPD improved of texture, color and integrated quality of papaya; besides would retained better the ascorbic acid, total phenolics, total carotenoids and total flavonoids of the fruit. Vieira da Silva Junior et al. [12] has demonstrated in the study of different methods for papaya drying that when the sample is dehydrated with combination of ultrasound and vacuum, it resulted in a minor nutritional loss, as well as resulting in dehydrated fruits with better color and texture characteristics.
To our knowledge, no comprehensive study has simultaneously investigated the effect of different drying methods on the sugars, color, non-enzymatic browning and amino acids of papaya (Vasconcellea pubescens). In addition, the effect of papaya extracts obtained from dehydrated fruit by different methods on human endothelial cell has not been investigated. Therefore, this study investigated the influence of different drying methods (namely, freeze drying, vacuum drying, solar drying, convective drying and infrared drying) applied to papaya on a cytotoxicity assay for screening the effect of its extracts on the endothelial cell line ECV304 while considering the conservation of papaya quality attributes.
Materials and methods
Sample preparation and drying conditions
Papaya slices of 9.0 × 1.5 cm and 0.4 cm thickness were prepared and dehydrated as previously described [1]. Five different drying methods were used: freeze drying (FD), vacuum drying (VD), solar drying (SD), convective drying (CD) and infrared drying (IRD). Vacuum drying was carried out under two conditions, VD1 was described in a previous study and was carried out at 70 °C, a vacuum pressure of 15 kPa and at a 480 min period [1], and VD2, designed in this study and consisting in a drying temperature at 40 °C, a vacuum pressure of 1 kPa and a drying time for 1170 min. The final moisture values were 7.25, 14.65, 21.57, 10.03, 11.07 and 12.69% on the wet basis for FD, VD1, SD, CD, IRD and VD2, respectively. Further details of the drying experiments can be found in our latest published work [1].
Sugars content determination
Separated extractions of glucose, fructose and sucrose were performed according to the method proposed by Djendoubi-Mrad et al. [14] with slight modifications. Briefly, 1 g of ground papaya was dissolved in 6 mL 80% methanol. The mixture was agitated on an orbital shaker (Boeco, 0S-20, Hamburg, Germany) at 200 rpm for 30 min and then centrifuged at 4193×g for 3 min (Hettinch®, model Universal 320 R, Tuttlingen, Germany). For quantification, supernatants were filtered through a 0.45 µm pore size membrane filter and injected into an HPLC system (Flexar LC model; Perkin Elmer, Shelton, Washington, USA) equipped with a Flexar binary LC pump system, a refractive index detector (RID), a Flexar LC autosampler and Flexar column oven. Sugars were analyzed on a Phenomenex Luna NH2 100A, 5 µm (25 cm × 4.6 mm) column (Phenomenex, CA, USA) and kept at 25 °C. The analytical conditions were as follows: mobile phase acetonitrile:water (82.5:17.5), flow 1 mL/min and isocratic elution. The chromatographic peak corresponding to each sugar was identified by comparing the retention time with that of a standard. A calibration curve was prepared using standards to determine the relationship between peak area and concentration. Data were processed using the TotalChrom software version 6.2 (Perkin Elmer, WA, USA).
Color analysis
Color measurements were determined by means of a colorimeter MiniScan™ XE Plus (HunterLab, Reston, USA) using D65 illuminant and an observation angle of 10º in terms of CIE units as L* (lightness and darkness), a* (redness and greenness) and b* (yellowness and blueness). The instrument was calibrated against standard white and black reference tiles. A Petri dish containing the sample (fresh or dried sample) was placed above the light source and covered with a black cap to prevent light from scattering away. Total color difference (∆E) was calculated using Eq. (1), where L0, a0 and b0 are the control values measured in the fresh sample, whereas he parameters a* and b* were used to compute the Chroma (Eq. (2)) and hue angle (Eq. (3)).
Non-enzymatic browning (NEB) analysis by UV–VIS spectroscopy
Papaya browning was evaluated spectrophotometrically as previously described [15]. Briefly, dried papaya slices were rehydrated with 50 mL distilled water for 24 h at room temperature. Rehydration water was centrifuged (Hettich® Universal 320 R) at 4193×g for 10 min. The supernatant was diluted 1:2 with 95% ethanol, thoroughly mixed and centrifuged at 4193×g for 10 min. Absorbance of supernatants were read using an UV–VIS Spectrophotometer (HALO SB-10, Dynamica, Australia) in triplicate in 10 mm quartz cuvettes at 420 nm.
Amino acid profile
The amino acids composition was performed by the method of White et al. [16]. Samples (200 mg) were hydrolyzed in 25 mL airtight glass ampoules with 6 eq/L HCl containing 0.1% phenol at 110 °C for 24 h. Hydrolyzed samples were reconstituted with sodium citrate buffer (pH 2.2) and filtered using 0.22 μm membrane filters. Derivatization was done using phenylisothiocyanate (PITC). Amino acid analysis was performed on a Merck-Hitachi L-6200 HPLC system coupled with a Tunable UV–Vis detector (Merck-Hitachi L-4250) with a Kromasil KR100-C18 (250 × 4.6 mm; particle size, 5 μm) column. The instrumental parameters were as follows: column temperature = 60 °C, injection volume = 20 μL, UV detection = 254 nm and flow rate = 1.0 mL/min. Only tryptophan was not determined in the samples. Amino acids were identified based on a comparison of their retention times with those of known standards.
Evaluation of ECV-304 cell viability
ECV-304 is an endothelial cell line derived from human umbilical cord [17]. ECV-304 cells were maintained in M199 growth medium supplemented with 20% fetal bovine serum (FBS). One hundred microliters of cells were deposited in 96 well plates with an initial concentration of 4 × 105 cells/well. The plates were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 24 h to allow cell-adherence to the bottom of the plate. Growth medium was removed and replaced with 100 µL of M199 medium supplemented with 2% FBS. Cells were incubated for 4 h before the assay was carried out. To study the effect of papaya extracts on ECV-304 cell viability, cells were incubated with papaya extracts at 0.005, 0.01, 0.05, 0.1, 0.5 and 1.0 mg/mL for 24 h at 37 °C. Cell viability was determined by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. To this end, growth medium was replaced with 90 µL fresh medium, 10 µL of MTT were added to each well, and cells were incubated for 4 h at 37 °C. Metabolically active cells reduced MTT to blue formazan crystals, which were dissolved in dimethylsulfoxide (DMSO). Absorbance was read at 540 nm. Cell viability (%) was calculated as the ratio between the absorbance obtained at a given concentration normalized by the absorbance obtained by control cells and expressed as percentage.
Statistical analysis
The data were analyzed using Statgraphics-Centurion XVI software (Statistical Graphics Corp., USA) with ANOVA at 95% confidence, followed by Least Significant Difference test, and represent mean ± standard deviation for three replicates, except for color parameter that was for six replicates.
Results and discussion
Effect on sugar content
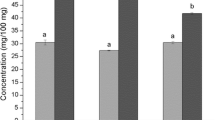
The three sugars found in papaya extracts obtained by the different dehydration methods, fructose and glucose were detected in all samples at a concentration range of 52.62–195.63 mg/g d.m. (dry matter), while sucrose was absent in all processed samples (Fig. 1). Udomkun, Argyropoulos et al. [18] and Gomes et al. [13] also reported the lack of sucrose in Carica papaya. This could be explained by the enzymatic activity of invertase, which hydrolyzes sucrose into glucose and fructose [18]. Both SD- and CD-samples showed a higher glucose and fructose retention while the efficacy of IRD was lower. This is probably a result of the intense heat irradiated inside the papaya during IRD, which provides more opportunities for the breakage of glucosidic bonds in polysaccharide chain and accelerating the degradation of monosaccharides [19]. Instead, a relatively short exposure time at 70 °C as it occurred during CD and lower fluctuating temperatures (between 30 and 50 °C) during SD would lead to a minor conversion of polysaccharide into other compounds, retaining thus its content to the utmost. These results were consistent with previous reports showing that SD retained better fructose and glucose contents in Jujube (Ziziphus mauritiana) fruit in contrast to freeze-, microwave- and oven-drying [20]. However, markedly higher levels of glucose and fructose were observed by Gomes et al. [13] in freeze-dried papaya fruits (cv. “Sunrise Solo”) than spray-dried–fruits at 150 °C, perhaps owing to the used temperature since sugars are prone to chemical conversion at elevated temperatures [21].
Effect of different drying methods on sugar content of papaya.  Fructose;
Fructose;  Glucose. Values are averages (n = 3), error bars are standard deviation. Different letters (lowercase and uppercase letters) on the bars represent significant differences (p < 0.05). FD freeze drying; VD1 vacuum drying at 70 °C and 15 kPa; SD solar drying; CD convective drying; IRD infrared drying; VD2 vacuum drying at 40 °C and 1 kPa
Glucose. Values are averages (n = 3), error bars are standard deviation. Different letters (lowercase and uppercase letters) on the bars represent significant differences (p < 0.05). FD freeze drying; VD1 vacuum drying at 70 °C and 15 kPa; SD solar drying; CD convective drying; IRD infrared drying; VD2 vacuum drying at 40 °C and 1 kPa
Effect on color
Color changes and NEB intensity of papaya slices dehydrated by different drying methods are listed in Table 1. As expected, fresh papaya was characterized by a high luminosity with a tendency toward bright yellow (L* = 62.1; hº = 82.8; C* = 43.2). However, after all drying processes, the increase of L* values and reduction of b* and C* values reflected the lighter color with a lower yellow intensity compared to the fresh samples. The reduction in yellow color is probably a consequence of the degradation of the yellow carotenoids present in V. pubescens which is supported by our previous findings on β-carotene degradation in dehydrated papaya fruits [1]. Amongst dehydrated samples, FD-papaya slices obtained the lightest values of L* and presented red (a*) and yellow (b*) components reduced. Other authors have reported that FD papaya were the brightest in comparison to fresh sample and dried samples by other methods [11, 14, 22, 23]. These changes in brightness might be the result of removal of internal moisture of food by sublimation after FD, which forms small pores and open networks in the food matrix. A pore network scatters more light than the large pores formed by other drying methods [24]. Conversely, IRD produced an increase in a* value compared to fresh papaya, probably due to isomerization or the increase in the concentration of some types of red carotenoids as lycopene (which has an intensely red tonality) because of heat irradiated and reduction of moisture content [12]. However, the darker color observed in the IRD sample may not only be explained by the concentration of these pigments but also by the formation of dark compounds from browning reactions. Based on these considerations, non-enzymatic browning (NEB) reactions were measured in fresh and dried papaya. As reported in Table 1, IRD-samples presented the strongest browning with NEB values 64-fold higher than fresh papaya. The intense heat produced at the food surface during IRD is transferred to the interior by conduction, concentrating the thermal load in the interior of the food matrix. This may cause the degradation of phenolic compounds and vitamin C, which in turns, favors NEB reactions [12, 25]. Our latest work showed the degradation of vitamin C and of some phenolic compounds after IRD of papaya [1]. The high NEB values seen in IRD-prepared papaya may also be attributed to the consumption of sugars during Maillard reaction [26]. This proposition is consistent with our findings where lower glucose and fructose contents in IRD papaya (Fig. 1) were obtained.
The maximum ΔE value was obtained after FD and the minimum after IRD. These results indicate that papaya dehydrated by IRD is more similar in color to the fresh sample than samples dried by the other methods. However, FD samples showed the highest hº value among all samples, which might be an indication of a more attractive and appealing appearance to consumers [27].
Effect on amino acids
The amino acid composition of papaya slices dehydrated by the different drying methods is reported in Table 2. Based on previous studies, changes in amino acid composition will depend on the drying methods used, food matrix and amino acid type [13, 28, 29]. Total amino acid content was significantly different in all dried papaya samples (p < 0.05) and ranged between 50.46 and 88.33 g/100 g of protein. The highest values of almost all amino acids, except for glycine, were obtained in IRD papaya (Table 2). The high amount of amino acids present in this sample might owe to modifications to the proteins secondary structure induced by far-infrared radiation during IRD [28]. Lysine was the most abundant amino acid in dried papaya, while alanine was the least abundant. It is known that lysine is the most reactive aminoacid in Maillard reactions [30], nonetheless, it appears that lysine was not degraded during IRD. Therefore, it seems plausible that the generation of non-enzymatic browning compounds in IRD-samples (Table 1) involves only sugars (Fig. 1) and not amino acids.
Effect on ECV-304 cell viability
To assess the effect of papaya extracts on the viability of the human endothelial cell line ECV-304, MTT assays were performed with extracts prepared with papaya dried by different methods at concentrations ranging between 0.005 and 1.0 mg/mL.
Papaya extracts decreased or increased ECV-304 cellular viability depending on the method used to dehydrate the samples and, on the concentration (Table 3). Statistical analyses showed that cell viability was not affected (p > 0.05) when cells were treated with papaya extracts obtained by FD at all concentrations tested (Table 3). Nevertheless, the SD-, VD1- and CD-extracts at 0.05, 1.0 and 0.005 mg/mL respectively, induced significant differences (p < 0.05) in cell viability compared to the control as well as the IRD- and VD2 extracts at most concentrations tested. When comparing the effect of the different drying methods on cell viability at a given concentration, extracts obtained by the VD2 and FD at 0.5 and 1.0 mg/mL, respectively, increased the viability of ECV-304 cells in over 30% (Fig. 2). Vacuum and freeze dying processes occur in the complete absence of oxygen, which would minimize degradation reactions, yielding high contents of antioxidant compounds which alleviate the stress of cells when they are cultured in vitro. Such conditions would explain the increased viability of ECV-304 cells treated with VD2 and FD papaya extracts. Cell culturing induces oxidative stress, where the balance of intracellular reactive oxygen species (ROS) levels and the cellular antioxidant defense mechanisms is shifted towards higher ROS levels [31]. Yusa et al. [32] stated that cellular ROS production is limited at normal O2 cellular levels and thus will increase if O2 levels are raised. Therefore, more ROS will be produced in cells in culture. Cellular oxidative stress can cause senescence, cell death, or adaptation [33]. Cells that fail to adapt may not divide or may die [34]. Following this scenario, the antioxidant compounds present in papaya extracts will likely contribute to ECV-304 cell adaptation increasing their viability. Our latest work showed the behavior of antioxidant components of V. pubescens after drying [1]. Nonetheless, future studies should focus on the individual constituents responsible for such activities.
Effect of 0.5 and 1.0 mg/mL papaya extract concentrations on the endothelial cell viability of the line ECV-304. Filled circle 0.0 mg/mL; filled triangle 0.5 mg/mL; open square 1.0 mg/mL. Values are averages (n = 3), error bars are standard deviation. FD freeze drying; VD1 vacuum drying at 70 °C and 15 kPa; SD solar drying; CD convective drying; IRD infrared drying; VD2 vacuum drying at 40 °C and 1 kPa
Conclusion
In this work is shown that quality parameters of Chilean papaya (V. pubescens) can be preserved by dehydration. Favorable dehydration conditions that led to a higher retention of bioactive compounds such as antioxidants increased the viability of human endothelial ECV-304 cells. The fructose and glucose contents of dehydrated papaya were higher when SD and CD methods were applied. Color properties of the dried products were acceptable owing to their high lightness and low Maillard reactions. Changes of individual amino acid composition depended on amino acid type and drying methods applied. Most drying methods (IRD, VD2, CD and FD) did not affect viability of human endothelial ECV-304 cells in concentrations of 0.5 and 1.0 mg/mL and thus not presenting possible adverse effects on ECV-304 cells. Future studies could focus on the potential action of Chilean papaya extracts on the viability of other cellular lines. Nevertheless, the results of this study are relevant on the optimization of drying processes and to improve both cell viability and final dried papaya quality.
References
A. Vega-Gálvez, J. Poblete, I. Quispe-Fuentes, E. Uribe, C. Bilbao-Sainz, A. Pastén, J. Food Meas. Charact. 13, 1980–1990 (2019)
C. Gaete-Eastman, C.R. Figueroa, C. Balbontín, M. Moya, R.G. Atkinson, R. Herrera, M.A. Moya-León, Postharvest Biol. Technol. 53, 58–65 (2009)
M.J. Simirgiotis, P.D.S. Caligari, G. Schmeda-Hirschmann, Food Chem. 115, 775–784 (2009)
E. Uribe, A. Delgadillo, C. Giovagnoli-Vicuña, I. Quispe-Fuentes, L. Zura-Bravo, J. Chem. 2015, 1–8 (2015)
C. Balbontín, C. Gaete-Eastman, M. Vergara, R. Herrera, M.A. Moya-León, Postharvest Biol. Technol. 43, 67–77 (2007)
A. Rahmat, R. Rosli, W. Zain, S. Endrini, H. Sani, J. Med. Sci. 2, 55–58 (2002)
T. Nguyen, M.-O. Parat, P. Shaw, A. Hewavitharana, M. Hodson, PLoS ONE 11, e0147956 (2016)
K.S. Alotaibi, H. Li, R. Rafi, R.A. Siddiqui, J. Cancer Metastasis Treat. 3, 161–168 (2017)
M.A. Moya-León, M. Moya, R. Herrera, Postharvest Biol. Technol. 34, 211–218 (2004)
P. Udomkun, M. Nagle, D. Argyropoulos, A.N. Wiredu, B. Mahayothee, J. Müller, J. Food Meas. Charact. 11, 2142–2150 (2017)
J.-Y. Yi, J. Lyu, J.-F. Bi, L.-Y. Zhou, M. Zhou, J. Food Process. Preserv. 41, e13300 (2017)
E. Vieira da Silva Junior, L. Lins de Melo, R.A. Batista de Medeiros, Z.M. Pimienra-Barros, P. Moreira-Azoubel, LWT-Food Sci. Technol. 97, 317–322 (2018)
W.F. Gomes, F.R.M. Franca, M. Denadai, J.K.S. Andrade, E.M. da Silva Oliveira, E. Sousa de Brito, S. Rodrigues, N. Narain, J. Food Sci. Technol. 55, 2095–2102 (2018)
N. Djendoubi Mrad, N. Boudhrioua, N. Kechaou, F. Courtois, C. Bonazzi, Food Bioprod. Process. 90, 433–441 (2012)
S. Meydav, J. Agric. Food Chem. 25, 602–604 (1977)
J.A. White, R.J. Hart, J.C. Fry, J. Automat. Chem. 8, 170–177 (1986)
N.M. Yartseva, R.F. Fedortseva, Cell Tissue Biol. 2, 428–435 (2008)
P. Udomkun, D. Argyropoulos, M. Nagle, B. Mahayothee, J. Müller, J. Food Eng. 157, 14–23 (2015)
Q. Wang, S. Li, X. Han, Y. Ni, D. Zhao, J. Hao, LWT-Food Sci. Technol. 107, 236–242 (2019)
Q.H. Gao, C.S. Wu, M. Wang, B.N. Xu, L.J. Du, J. Agric. Food Chem. 60, 9642–9648 (2012)
S. Kayacan, S. Karasu, P.K. Akman, H. Goktas, I. Doymaz, O. Sagdic, LWT-Food Sci. Technol. 118, 108830 (2020). https://doi.org/10.1016/j.lwt.2019.108830
J. Lyu, J. Yi, J.F. Bi, H. Gao, M. Zhou, X. Liu, Int. J. Food Eng. 13, 20160250 (2016)
P. Udomkun, D. Argyropoulos, M. Nagle, B. Mahayothee, A.E. Oladeji, J. Müller, J. Food Meas. Charact. 12, 1028–1037 (2018)
A.M. Ceballos, G.I. Giraldo, C.E. Orrego, J. Food Eng. 111, 360–365 (2012)
M.C. Karam, J. Petit, D. Zimmer, E.B. Djantou, J. Scher, J. Food Eng. 188, 32–49 (2016)
M. Alongi, G. Verardo, A. Gorassini, M. Anese, LWT-Food Sci. Technol. 98, 366–371 (2018)
L. Seremet (Ceclu), O.-V. Nistor, D.G. Andronoiu, G.D. Mocanu, V.V. Barbu, A. Maidan, L. Rudi, E. Botez, Food Chem. 310, 125637 (2020). https://doi.org/10.1016/j.foodchem.2019.125637
Y. Deng, Y. Wang, J. Yue, Z. Liu, Y. Zheng, B. Qian, Y. Zhong, Y. Zhao, Food Control 36, 102–110 (2014)
Y. Deng, Y. Luo, Y. Wang, Y. Zhao, Food Chem. 171, 168–176 (2015)
D. Virág, A. Kiss, P. Forgó, C. Csutorás, S. Molnár, Microchem. J. 107, 172–177 (2013)
B. Halliwell, FEBS Lett. 540, 3–6 (2003)
T. Yusa, J.D. Crapo, B.A. Freeman, Biochim. Biophys. Acta 798, 167–174 (1984)
B. Halliwell, Nutr. Rev. 57, 104–113 (1999)
B. Halliwell, Lancet 355, 1179–1180 (2000)
Acknowledgements
The authors gratefully acknowledge the Project FONDECYT 1170601 and DIDULS PT17331 (Dirección de Investigación y Desarrollo de la Universidad de La Serena) for providing financial support for the publication of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vega-Gálvez, A., Uribe, E., Poblete, J. et al. Comparative study of dehydrated papaya (Vasconcellea pubescens) by different drying methods: quality attributes and effects on cells viability. Food Measure 15, 2524–2530 (2021). https://doi.org/10.1007/s11694-021-00845-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00845-6