Abstract
Oxidative stress related diseases often arise from over production of free radicals and reactive oxygen/nitrogen species. The prevention of these diseases could be possible with the use of natural antioxidant plants that could be promising as therapeutic candidates. Since antioxidant properties of a species could be stem from phenolic compounds, it is, therefore, important to evaluate antioxidant and total/individual phenolic and flavonoid content. For this purpose, we evaluated antioxidant properties of ginger (Zingiber officinale Rosc.) based on three parameters: the antioxidant capacity, total phenolic and flavonoid content as well as identification of phenolic acids of water extract (WEG) and ethanol extract (EEG) of ginger. For antioxidant capacity, we performed FRAP, CUPRAC assay, Fe2+ chelating ability, DPPH and DMPD radical scavenging activities. Also, total phenolic and flavonoid contents in both extracts were also measured via Folin Ciocalteu’s method. For identification of phenolic acids, HPLC-MS/MS method was performed. The results showed that EEG had generally better antioxidant activity than WEG in all assays. HPLC-MS/MS analysis showed that there are at least eight different phenolic acids found in ginger, among which pyrogallol p-hydroxybenzoic acid, ferulic acid and p-coumaric acid were more abundant in both extracts. This study clearly showed that ginger extracts demonstrated effective antioxidant properties and their consumption may reduce or delay the progression of diseases that oxidative stress take place due to lack of antioxidant supplementation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutritional plants have been used in medicine by having application in treating many diseases since ancient times. Ginger (Zingiber officinale Rosc.) is not only one of the frequently used species to enhance the taste and flavour of the food in many parts of the world [1], but also contains a numerous number of potential bioactive compounds that have biological and pharmaceutical effects. The rhizomes of ginger are one of the most widely used spice and condiment [2]. The consumption of ginger have been claimed to be useful in many oxidative stress related medical conditions, some of which include hypertension [3], diabetes-induced pancreatic and renal derangements [4], tumour progression [5] and Alzheimer diseases [6]. In traditional medicine it has been used for treating headaches, nausea, febrile conditions, colds, arthritis, rheumatic disorders and muscular discomfort [7]. It has been also suggested that ginger has anti-inflammatory, anticancer, hepatoprotective [8] and renoprotective [9] effects, androgenic properties [10], antiglycation potential [11] and antioxidant effects [11].
Antioxidant activity of a plant is important because of two reasons. First the consumption of a food rich in antioxidants has been suggested to prevent or delay oxidation of major biomolecules within the cell by chelating metals or scavenging free radicals that are produced as consequences of metabolism [12, 13]. Therefore antioxidants are necessary to prevent oxidative cell damages, which related many diseases and to maintain cell component in reduced state [14]. Antioxidant constituents can protect the human body from free radicals and reactive oxygen species (ROS) effects [15]. Reactive oxygen species (ROS) are continuously produced by the body’s normal use of oxygen such as respiration and some cell mediated immune functions. ROS include free radicals such as hydroxyl radicals (OH), superoxide anion radicals (O2 −), and non free radical species such as singlet oxygen (1O2) and hydrogen peroxide (H2O2). All organisms have antioxidant defences, including antioxidant enzymes and antioxidant constituents for removing or repairing of damaged molecules [16]. Secondly antioxidants are used in food preservation to prevent the food from oxidation, and increase their shelf life by retarding the process of lipid peroxidation, which is one of the major reasons for deterioration of food and pharmaceutical products during processing and storage [17]. Although synthetic antioxidant are strong, many of them have been criticized due to the toxic effects. Naturally occurring antioxidants could be safer in terms of human use. Therefore herbs and species have potential application to compensate the synthetic antioxidants [18, 19]. Hence, a need has appeared to identify alternative natural and safe sources of food antioxidants, and the search for natural antioxidants, especially of plant origin, has notably increased in recent years [20].
The antioxidant properties of plants may be stem from the polyphenolic compounds, which are secondary metabolites of a plant. Polyphenolic compounds have polyphenol structure where several hydroxyl groups (–OH) bonded to two or more benzene rings [21]. Polyphenolic compounds have the ability of delaying or inhibiting the initiation step or interrupting the propagation step of lipid peroxidation, therefore decreasing the amount of decomposition products [20, 22]. Thus considering both the global widespread incidence of oxidative stress related disorders such as diabetes, cancer and other complications in the recent times and need for inexpensive, nontoxic effective food preservatives, it is useful to evaluate the polyphenolic compounds of ginger to propose new potential antioxidant source [23].
The biological activity has often been presented in conjunction with antioxidant properties of which could be related to polyphenolic compounds of the plant material. In this context, in order to reveal the basis of scientific rationale of the therapeutic use of ginger, it is important to quantify, identify and evaluate antioxidant and polyphenolic properties [24]. Therefore the objectives of this study were three. First we aimed to analyse the antioxidant properties of water and ethanol extract of ginger in comparison with well-known synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), α-tocopherol and trolox [25–27]. The second objective was to quantitate total polyphenol and flavonoid contents. The third objective was to determine some acid-phenols such as ferulic, ellagic, gallic, caffeic, syringic acids using HPLC-MS/MS method, from water and ethanol extracts of ginger. As novelty, our study has focused on different antioxidant activities along with the separation and identification of major individual polyphenols from ginger that could be useful from the pharmacological point of view to reveal the connection between bioactive compounds and mechanism of action of ginger in different pathological conditions.
Materials and methods
Chemicals and instruments
In this study, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), the stable free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH·), linoleic acid, 3-(2-Pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine), 6-hydroxy- 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ethylendiamintetraacetic acid (EDTA), polyoxyethylene sorbitan monolaurate (Tween-20), 2′-bipyridine and trichloroacetic acid (TCA) were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany) and ammonium thiocyanate was purchased from Merck. The following compounds were used as standards in LC-MS/MS analysis: caffeic acid (98 %, Sigma-Aldrich), ferulic acid (98 % Sigma Aldrich), syringic acid (97 %, Fluka), ellagic acid (95 %, Fluka), quercetin (98 %, Sigma-Aldrich), α -tocopherol (98 %, Fluka), catechol (99 % Sigma Aldrich), pyrogallol (98 %, Sigma-Aldrich), p-hydroxybenzoic acid (99 %, Merck), vanillin (99 % Merck), p-coumaric acid (98 %, Sigma-Aldrich), gallic acid (98 %, Sigma-Aldrich) and ascorbic acid (99 %, Sigma-Aldrich). Stock solutions were prepared as 5 mg/L in ethanol, with the exception of catechol and ascorbic acid, which were prepared as 50 and 25 mg/L respectively, although in the same solvent. Curcumin (97 %) and HPLC grade methanol were purchased from Merck (Darmstadt, Germany). Calibration solutions were prepared in ethanol–water (50:50, v/v) in a linear range. Absorbance was measured with an UV-VIS spectrophotometer for antioxidant activity and total phenols content.
Plant samples and preparation of extracts
Dried ginger (Zingiber officinale Rosc.) was obtained from local market at Erzurum, Turkey. The plant materials were and authenticated by Dr. Mustafa Korkmaz, Department of Biology, Faculty of Science and Arts, Erzincan University (Erzincan University Herbarium—Herbarium No: 4327). An ethanol extract of sample was prepared by grounding 25 g the root of dried ginger in a mill, mixing ginger powder (Zingiber officinale Rosc.) with 100 mL ethanol on a magnetic stirrer for 1 h [28]. Ethanolic extract ginger (Zingiber officinale Rosc.) (EEG) was filtered and the mixture is then placed on the rotary evaporator at 50 °C to remove ethanol. For water extract preparation, 25 g of sample powder was mixed with 100 mL of distilled water by magnetic stirrer during 60 min. Then the extract was filtered over cheesecloth and Whatman No. 1 paper, respectively. The filtrates were frozen at −30 °C in low temperature freezer filtered, and lyophilized in a lyophilizator at 5 mm-Hg pressures at −50 °C. Samples then stored in a tightly caped plastic bottle at −20 °C until used for experimental studies [29].
Antioxidant assays
Total antioxidant activity determination
The total antioxidant activity of WEG, EEG and standard antioxidants is determined using the ferric thiocyanate method in linoleic acid emulsion [30] as described previously [31]. The stock solutions are prepared by 10 mg of WEG or EEG dissolved in 10 mL distilled water. The different concentrations of stock WEG or EEG solution samples (10–20 µg/mL) are prepared in 2.5 mL of potassium phosphate buffer solution (0.04 M, pH 7.0) and then these are added to 2.5 mL of linoleic acid emulsion in potassium phosphate buffer solution (0.04 M, pH 7.0). The final solutions are incubated at 37 °C. During the incubation periodically, a 0.1 mL aliquot of the mixture is diluted with 3.7 mL of ethanol, and then it is added to the mixture of 0.1 mL of 30 % ammonium thiocyanate and 0.1 mL of 20 mM ferrous chloride in hydrochloric acid (3.5 %). The absorbance is measured at 500 nm for the determination of the peroxide level. The peroxides formed during linoleic acid oxidation oxidize Fe2+ to Fe3+ and the latter ions form a complex with thiocyanate. The complex has a maximum absorbance at 500 nm [32]. The process is repeated every 6 h until the control reaches its maximum absorbance value. The amounts of inhibition are calculated by the following equation:
where Ac is the absorbance of the control reaction, which contains only linoleic acid emulsion and sodium phosphate buffer. As is the absorbance of the sample in the presence of WEG and EEG or other test compounds [33].
Fe3+ reducing power assay
The reducing capacity of WEG and EEG was determined by the method of Oyaizu [34]. Accordingly, 1 mL of the samples of WEG and EEG at different concentrations (10–30 µg/mL) in distilled water was mixed with 2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 mL potassium ferricyanide [K3Fe(CN)6] (1 %). The mixture was incubated at 50 °C for 20 min. Then, 2.5 mL trichloroacetic acid (10 %) and 0.5 mL of FeCl3 (0.1 %) were added to the reaction mixture. The absorbance was measured at 700 nm. An increase in the absorbance was considered as greater reducing capacity.
Cu2+ reducing power assay
The reducing capacity of WEG and EEG was also measured by CUPRAC method developed by Apak [35] as described previously [36]. In this assay, 0.25 mL of CuCl2 solution (0.01 M), 0.25 mL of ethanolic neocuproine solution (7.5 × 10−3 M) and 0.25 mL of CH3COONH4 buffer solution (1 M) were mixed and ginger extract at different concentrations (10–30 µg/mL) were added to this mixture. The final volumes were adjusted to 2 mL with distilled water and 30 min later absorbances were measured at 450 nm. Increased in absorbance were interpreted as increased reducing capacity.
Chelating activity on ferrous ion (Fe2+)
Ferrous ions (Fe2+) chelating activity of WEG and EEG were determined by according to the method of Re et al. [37]. Various concentrations of sample was mixed 0.25 mL FeSO4 solution (2 mM), 1 mL Tris–HCl buffer solution (pH 7.4), 1 mL of 2,2′-bipyridine solution (0.2 % in 0.2 M HCl) and 2.5 mL ethanol. The final volume was adjusted to 6 mL by addition of distilled water and thoroughly mixed. The absorbances of the samples were recorded spectrophotometrically at 562 nm. EDTA is used as a standard ferrous ions (Fe2+) chelator.
DPPH free radical scavenging activity
The radical scavenging property of WEG and EEG was evaluated by using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) as a reagent according to previous studies [38]. An ethanol solution of DPPH (1 mM) was prepared immediately before the assay and 1 mL of DPPH solution was mixed with 3 mL of ethanolic ginger extract at different concentrations (10–30 µg/mL). The mixture was incubated for 30 min and the absorbance measurements were spectrophotometrically made at 517 nm. Decreased absorbance of the sample indicates DPPH free radical scavenging capability.
DMPD free radical scavenging activity
Radical scavenging ability of WEG and EEG were determined by using DMPD radical scavenging activity assay with slight modifications [39]. This assay based on the inhibition of the DMPD·+ radical cation formation. For his propose, 105 mg of DMPD was dissolved in in 5 mL of distilled water and 1 mL of this solution was added to 100 mL of acetate buffer (0.1 M, pH 5.3). To this solution, 0.3 mL ferric chloride (0.05 M) was added to obtain DMPD·+. Different concentrations (10–30 μg/mL) of WEG and EEG or standard antioxidants were added in to the test tubes and total volume was adjusted with distilled water to 0.5 mL. One millilitre of DMPD·+ solution was directly added to the reaction mixture. These samples were vortexed and incubated in dark at room temperature for 15 min. Absorbance was measured at 505 nm. Buffer solution was used as blank and instead of sample distilled water was used for control.
Determination of total phenolic content
Polyphenols extracted from WEG and EEG reacts with specific Folin–Ciocalteu reagents and form a blue complex, which can be spectrophotometrically quantified at 760 nm [40]. 1 mL of WEG and EEG sample or standard solution was taken into test tube and 23 mL of final volume was achieved with distilled water. Then, 0.5 mL of Folin Ciocalteu’s reagent was added to test tube and after 5 min, 1.5 mL of Na2CO3 solution (2 %) was added. After being vortexed and keeping in room temperature in darkness for 30 min, the absorbance of the samples was spectrophotometrically measured at 760 nm. Various concentrations of gallic acid ranging from 0 to 500 μg were used as a standard along with the samples and the amount of total phenols were calculated by using gallic acids calibration curve (r2:0.9809).
Determination of total flavonoid content
Total flavonoid content of WEG and EEG was estimated by a colorimetric assay [41]. In this assay, 1 mg of ethanolic ginger extract was taken into a test tube and 0.1 mL CH3COOK (1 M), 0.1 mL of 10 % Al(NO3)3 in 4.3 mL of ethanol solution were added to the sample. Then the mixture was vortexed and kept at room temperature for 40 min. The absorbance of the sample was taken in triplicate at 415 nm by using a UV–VIS spectrophotometer. The standard curve of quercetin at different concentrations ranging from 0 to 100 μg were used for determination of flavonoid content of ginger in ethanol. The results are reported as μg quercetin equivalents per mg extract (r2:0.9609).
LC/MS method and quantification of some acid-phenols
HPLC-MS/MS analysis of EEG was performed using Zivak® HPLC and Zivak® Tandem Gold Triple quadrupole (Istanbul, Turkey) mass spectrometer equipped with a Macherey-Nagel Nucleoder C18 Gravity column (125 × 2 mm i.d., 5 μm particle size). HPLC separation of the samples was performed on Macherey-Nagel Nucleoder C18 Gravity column (125 × 2 mm i.d., 5 µm particle size). The mobile phase was composed of methanol (A, 0.5 % formic acid) in water (B, 0.5 % formic acid), the gradient programme of which was 0–1.00 min 50 % A and 50 % B, 1.01–30.00 min 100 % A and finally 30.01–35.00 50 % A and 50 % B. The injection volume of the sample was 10 µL and the flow rate of mobile phase was 300 µL/min. The chromatographic column was kept at 30 °C.
For MS analysis of samples, the following settings were used: CID gas pressure to 2.40 mTorr, EIS needle voltage to 5000 V, EIS shield voltage to 600 V, drying gas temperature to 300 °C and drying gas temperature 300 °C, API housing temperature to 50 °C, Nebulizer gas pressure to 55 psi and drying gas pressure to 40 psi.
The flavonoids and other phenolic compounds in EEG were identified based on the mass spectra and by comparison of the spectra of available standards. Experimental details regarding method optimization were provided previously [42].
Statistical analysis
The experiment regarding total antioxidant activity was carried out at duplicate, whereas the rest of the analysis was performed in triplicate. The values were expressed as Mean ± Standard deviation and analysed by SPSS (version 11.5 for Windows 98, SPSS Inc.). A one-way ANOVA was performed to determine the significance of difference. One-way analysis of variance was performed by ANOVA procedures. The significant differences between the means were determined by LSD tests. p < 0.05 was accepted as significant while p < 0.01 was regarded as being substantially significant.
Result and discussion
In this study, we have investigated three main properties of WEG and EEG including the antioxidant capacity, total phenolic and flavonoid content and using LC-MS techniques, the quantification of some acid-phenols of ethanolic and both ginger extract by HPLC-MS/MS.
Total antioxidant activity
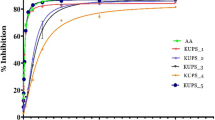
Total antioxidant activity of WEG and EEG was determined using ferric thiocyanate methods. The ferric thiocyanate method (FTM) measures the amount of peroxides produced during the initial stages of oxidation, which are the primary products of oxidation [43]. The autoxidation of linoleic acid emulsion without WEG and EEG or standard compounds was accompanied by a rapid increase in peroxides [41]. In this method, the peroxide levels were determined by reading the absorbance at 500 nm after reaction with FeCl2 and thiocyanate at intervals during incubation. The peroxides formed during linoleic acid peroxidation will oxidize Fe2+ to Fe3+, which forms a complex with rhodanide (SCN−) that has a maximum absorbance at 500 nm. The mechanistically approaches of the spectrophotometric analysis of lipid hydroperoxides based on the oxidation of Fe2+ to Fe3+ ions and following chelation of the latter by SCN− are thought. The FTM measures the amount of peroxide generated along the first steps of lipid peroxidation. In this test, hydroperoxides produced from linoleic acid supplement to the reaction solution, which has oxidized in air through the experimental period, were immediately evaluated. SCN− and FeCl2 react with one another to generate Fe(SCN−)2 by the agency of hydroperoxides [44]. Figure 1a shows the prevention of linoleic acid oxidation in the presence of WEG, EEG and other standards. WEG and EEG suppressed the linoleic acid peroxidation formation when compared to standards (α-tocopherol and trolox). WEG and EEG showed inhibition effect of 38.9 and 57.4 %, whereas α-tocopherol and trolox indicated 69.3 and 81.4 %, respectively. Using the same assay it has been shown that the essential oil extracted form ginger exhibited a better antioxidant activity than standard synthetic compound like BHT and BHA. It was reported that the putative active compounds in ginger are non-volatile pungent principles, namely gingerols, paradols, shogaols, and zingerone. Some of these compounds were the extensively studied phytochemicals and account for the antioxidant, antiemetic, anti-inflammatory, and gastroprotective activities [2, 45]. Also, it is known that the cancer protective effects of ginger are supposed to be mainly due to antioxidant pathways, free radical scavenging activity, induction of apoptosis, and alteration of gene expressions, all of which contribute towards decrease in tumour initiation, promotion, and progression [2]. The analysis of volatile oils of ginger showed camphene, p-cineole, alpha-terpineol, zingiberene and pentadecanoic acid as major components, while the major components in cumin volatile oil were cuminal, gamma-terpinene and pinocarveol [46].
Reducing powers
FRAP assay
Oxidation of biomolecules thought be related many diseases as well as aging. One of the ways that results in biomolecule oxidation is the reaction of metals with reactive oxygen species via Fenton reaction [47]. In Fenton reaction, H2O2 is converted to OH· in the presence of Fe2+ (or Cu+). OH· is more reactive than H2O2 and capable of oxidizing biologically important molecules such as carbohydrates or DNA [48]. Thus, Fe2+ or Cu+ reducing power of a food is of impotence. In this study, FRAP and CUPRAC assays were used to determine the reducing power of WEG and EEG. BHT, α-tocopherol and trolox were used as standards. FRAP assay measures the antioxidant effect of any substance in the reaction medium as reducing ability. The FRAP assay measures the ferric ions (Fe3+)–ferrous ions (Fe2+)-reducing ability of the substance and was initially proposed for the measurement of antioxidant capacity. The Fe3+-TPTZ reduction method measures the antioxidant effect of any molecule as reducing capability in the reaction. It was reported that the FRAP assay offers a well-known index of antioxidant, or reducing, potential of samples or pure compounds. At low pH values, FRAP assay measures the ability of antioxidants to reduce the ferric 2,4,6-tripyridyl-s-triazine complex [Fe3+-(TPTZ)2]3+ to the intensely blue coloured ferrous complex [Fe2+-(TPTZ)2]2+ which has maximum absorption at 593 nm with intense blue colour of reaction medium [49]. It was found that EEG (λ595: 1.122) had 3.38 fold higher Fe2+ reducing power than that of WEG (λ595: 0.332) at 30 µg/mL concentration. In this assay, FRAP reagent (TPTZ (2,4,6-tripyridyl-striazine) and FeCl3.6 H2O) is mixed with the antioxidant and results in ferrous ion formation which indicated absorbance at 595 nm. WEG and EEG showed that reducing power of EEG were comparable with the standards; WEG has shown the lower Fe3+ reducing power (Fig. 1b). The activity of EEG increased as concentration dependent manner, whereas WEG did not show significant increase in activity with increasing concentration.
CUPRAC assay
Cuprac assay is one of the methods used to measure reducing power of an antioxidant. CUPRAC assay is more suitable for measuring reducing capacity of water-soluble antioxidants. In this experiment, the decrease in the absorbance of extract due to Cu2+-neocuproine reduction in the presence of antioxidant is measured. This method can be used for the determination of the antioxidant capacity of food constituent by the Cu2+-neocuproine (Cu2+-Nc) reagent as the chromogenic oxidizing agent. The reduction of Cu2+ in the presence of neocuproine by a reducing agent yields a Cu+ complex with maximum absorption peak at 450 nm [50]. An increase in the absorbance indicates a higher antioxidant power. The result shows WEG and EEG had effective cupric ions (Cu2+) reducing activity. However, EEG indicated a higher reducing activity (λ450: 0.542) than α-tocopherol (λ450: 0.422) and trolox (λ450: 0.457) but close to BHA (λ450: 0.565) at 30 µg/mL concentration (Fig. 1c). The reducing activity of WEG was lowest among all. A recent study has shown that using CUPRAC assay, the menthol extract of fresh ginger has Cu2+ reducing activity, which was diminished by drying the samples [51]. The authors suggested that a reduction in the reducing activity could be due to reduced extraction efficacy or removal of phenolic contents by drying.
Metal chelation assay
Metal chelating activity of a sample appears to be particularly important for diseases in which the high levels of metal ions leads to oxidation of proteins and lipids. For example, increased levels of redox active Fe and Cu ions have been associated with various neurological diseases and iron chelation has been suggested as a potential therapy for retarding the progression of the disease [52]. In fact, it has been shown that incubation of rat brain in the presence of Fe caused significant increase in malondialdehyde (MDA) contents and lipid peroxidation in rat brain has been significantly reduced by water extract of red ginger [53]. The 2,2′-bipyridine method can detect iron up to nanomolar concentrations after preconcentration on resins. In the presence of chelating agents, complex formation is disrupted, resulting in a decreasing of the complex. Measurement of colour reduction therefore allows estimation of the metal-chelating activity of the coexisting chelator. Lower absorbance indicates higher metal-chelating activity. 2,2′-Bipyridine forms a complex with free Fe2+, but not with Fe2+ bound to other chelators; thus, a decrease in the amount of ferrozine-Fe2+ Complex formed after treatment indicates the presence of antioxidant chelators. The ferrozine-Fe2+ complex produced a red chromophore with absorbance that can be measured at 562 nm [54]. In this study, metal chelation effect of ginger was measured first time in both water and ethanol extracts (Fig. 1d). The result show that both extract had metal chelation activity but lower than standard control EDTA. WEG and EEG chelated 27.3 and 36.2 % ferrous ions (Fe2+) at 10 µg/mL concentration. However, at this concentration (10 µg/mL) EDTA chelated as 90.7 % Fe2+. As natural source of metal chelator, dietary intake of ginger could be potentially used to prevent neurodegenerative diseases.
Radical scavenging activities
Under normal physiological conditions formation of free radicals and reactive oxygen species can be occur. Free radicals have dual role in biological systems and in fact many free radicals-mediated responses protect cells from oxidative damage [55]. However when produced in excess, they cause oxidation of many macromolecules resulting in various diseases such as cardiovascular diseases, neuropsychiatric disorders and diabetes. Indeed, scavenging free radicals has been suggested to be used for therapeutic proposes. For example, edaravone as a free radical scavenger was used in treatment of cardiovascular diseases. Therefore in order to estimate the potential value of a food, evaluation of radical scavenging activity is important. The free-radical chain reaction is widely accepted as a common mechanism of lipid peroxidation. Radical scavengers may directly react with and scavenge peroxide radicals to terminate the peroxidation chain reactions and improve the quality and stability of food products. Assays based upon the use of DPPH· and DMPD·+ radicals are among the most popular spectrophotometric methods for determination of the antioxidant capacity of foods, beverbages and vegetable extracts. Both chromogens and radical compounds can directly react with antioxidants [40]. In this study, DPPH and DMPD radicals scavenging methods were studied to evaluate the radical scavenging activity of ginger extracts and compared with standards: BHA, BHT, α-Tocopherol and Trolox.
DPPH·scavenging assay
In DPPH assay, both extracts have shown lower activities than standard controls (Fig. 1e). Accordingly, WEG demonstrated weak activity for DPPH radical (Fig. 1e). DPPH scavenging activity of EEG was higher than WEG and its activity increased with higher concentrations. At the 30 µg/mL concentration, WEG and EEG scavenged DPPH free radicals as 16.2 and 43.8 %. However, at the same concentration, BHA, BHT, α-Tocopherol and Trolox scavenged DPPH free radicals as 74.3, 37.0, 78.8 and 77.1 %, respectively. Our study is in accordance with a previous study which shown that alcohol extract of air-dried ginger has significant effect on inhibiting DPPH radical [56]. Moreover methanol extract of these result shows that alcohol extracts of ginger acts a hydrogen donor [57]. DPPH· radical is one of the few stable organic nitrogen radicals, which bears a deep purple colour. It is commercially available. In DPPH assay, the antioxidants were able to reduce the stable radical DPPH to the yellow coloured diphenyl-picrylhydrazine (DPPH-H). This method is based on the reduction of DPPH in alcoholic solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H in the reaction [58].
In a recent study it was found that antiradical efficacy of ginger oleoresin measured by DPPH assay is similar to BHT, whereas ginger juice and oil had lower value [59]. Moreover dried ginger essential oil appeased to have higher antioxidant activity than fresh ginger oil, measured with DPPH assay [46]. In addition the leaves of ginger had higher antiradical activity than those of rhizomes and stems [60] and it appeared to be higher 8 weeks after of planting [61]. Overall these results indicates that antiradical activity of ginger is variable depending on the part of ginger used in the assay, dried or fresh and harvesting time.
DMPD·+ scavenging assay
Another assay that is very similar to the use of the DPPH· is the N,N-dimethyl-p-phenylenediamine or the DMPD·+ assay. In the presence of an oxidant or an acidic pH, the DMPD·+ is converted to a stable and coloured DMPD·+ radical cation (DMPD·+). The UV-visible spectrum of DMPD·+ shows a maximum absorbance at 505 nm. DMPD is only water soluble and particularly useful for measuring hydrophilic antioxidants. DMPD·+ assay is based on formation of radical cation of DMPD with FeCl3 at acidic pH [62]. This study is the first measuring antiradical activity of ginger based on DMPD radical scavenging activity. Both WEG and EEG have shown similar DMPD radical scavenging activity (Fig. 1f). They showed higher scavenging activity than BHT and lower than Trolox. The results showed that both WEG and EEG scavenged DMPD radical in a similar pattern and increasing the concentration the extracts did not further reduced the absorbance. DMPD assay was previously used to measure antiradical activity of propolis [63], olive oil [64], clove oil [19], the illyrian thistle [49] and cauliflower [65].
Total phenolic and flavonoid contents
Phenolic acids are considered as an important class of antioxidant due to their radical scavenging activity via hydrogen atom donation. Although less mentioned, they can also act through other mechanisms such as electron donation or singlet oxygen quenching. The aromatic ring and its substituents affect the stabilization of these phenolic acids, thus different acids have different stabilities. The potential beneficial effects of phenolic acids have been reported. For example, a recent study has revealed that caffeic acid phenethyl ester extracted form honeybee hive propolis effectively suppresses the proliferation, survival, and metastasis of oral cancer cells [66]. Also, this biological active substance had powerful antioxidant and antiradical activity. Similarly, ferulic acid found in plants to prevent lipid oxidation was suggested to have anti-carcinogenic action and high inhibitory activity on proliferating cells of liver, colon, tongue, breast and nervous system [67]. Therefore it is important to determine the amount of phenolic content of foods and medicinal plants.
In this study, Folin Ciocalteau reagent and the standard graph equation of gallic acid were used to estimate total phenolic compounds of WEG and EEG. The total amount of phenolic and flavonoid content of the WEG and EEG were shown in Table 1. Total phenolic contents of WEG and EEG were 52.8 and 137.5 µg/mg gallic acid equivalents, respectively. On the other hand, flavonoids are widely distributed in different parts of plants, fulfilling many functions. They make up one of the most widespread, groups of plant phenolics. Due to their importance in human health, it would be useful to have a better understanding of flavonoid concentration and biological activities that could indicate their potentials as therapeutic agents, and also for predicting and controlling the quality of food and medicinal plants [60, 61]. Total flavonoids contents of WEG and EEG were 3.9 and 25.1 µg/mg quercetin equivalents, respectively. They are the most important plant pigments for flower coloration, producing yellow, red or blue pigmentation in petals designed to attract pollinator animals [68]. Previously total phenolic content of ginger was found to be highest with 50 % aqueous ethanol extraction. Authors suggested that this is because 50 % aqueous ethanol has greater dielectric constant, thus yields the greater release of total phenolic content [69]. The amount of total phenols also appears to be locations dependent. It has been reported that reported that total phenolic content is higher in leaf than in rhizomes of different ginger species [70, 71]. Looking at overall data, it was clear that EEG showed superior antioxidant capacity compared to WEG (Fig. 1), which is consistent with the higher levels of total phenols and flavonoid contents in EEG (Table 1). Thus these results suggest that a better antioxidant activity of EEG could be due to considerable amount of polyphenols found in EEG.
The quantification of some acid-phenols
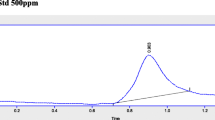
After determination of total phenolic and flavonoid contents, phenolic acids of WEG and EEG was determined by HPLC-MS/MS experiment. The mass spectra of samples were compared with those of authentic samples. Table 2 shows Collision energy (V) ESI mode (positive or negative) as well as parent ion, daughter ion and of the corresponding compounds. Standard chromatogram of antioxidant phenolic compounds by HPLC-MS/MS (mg/mL) was shown in Fig. 2.
As shown in Fig. 3a, b, HPLC-MS/MS profile of WEG and EEG showed similar phenolic profile and was resulted in identification of eight phenolic compounds. In the analysis, peak 1, with a negative molecular ion at [MS-H]+ at an m/z of 125 was identified as pyrogallol. The peak 2 has shown an m/z of 137, indicating that this compound is p-hydroxybenzoic acid. The peak 3 exhibited an m/z of 193, which corresponds to ferulic acid. The peak 4 had an m/z of 181 corresponding to vanillin. The peak 5 has shown an m/z of 163, which is the indication of P-coumaric acid. The peak 6 exhibited an m/z at 169 corresponding to gallic acid. The peak 7 has shown an m/z of 175. The corresponding compound was identified as Ascorbic acid. The peak 8 has indicated an m/z of 179, which corresponds to caffeic acid.
We also looked at other different authentic phenolic acids such as syringic, ellagic, p-coumaric acids, quercetin and catechol to compare with the samples, however but the detectible levels were not recorded for the samples (Table 2).
Conclusion
Antioxidant activity of different ginger species is known for long, however this study is the first providing evidence that antioxidant activity of ginger is due to high polyphenolic content which is variable depending on the extraction solution. The present study revealed that the different extracts of ginger have different antioxidant capacities and phenolic or flavonoid contents. We performed different antioxidant activity measurements to evaluate different antioxidant capabilities of the samples. Especially EEG has shown comparable metal reducing activity as shown by FRAP and CUPRAC assay. Metal chelating activity of samples, again especially for ethanolic extract, was better than standard metal chelator, EDTA. Radical scavenging activity as shown with DPPH and DMPD radical scavenging assay, ginger extracts generally showed results comparable to standard synthetic antioxidants. Furthermore phenolic and flavonoid content of the samples were determined and quantified with HPLC-MS/MS techniques. The result showed that ginger extracts have different phenolic acids, the majority of them being pyrogallol p-hydroxy benzoic acid, ferulic acid and p-coumaric acid. This study proposes that ginger might be used in diseases where the balance between free radicals and antioxidant imbalance is disrupted. In addition this medicinal plant could be used in different disease modals due to its rich phenolic content, however further studies are need to isolate its active compounds and investigate their mode of action.
References
K.V. Peter, Handbook of herbs and spices, Vol. 3 (Woodhead publishing, Cambridge, 2006)
M.S. Baliga, R. Haniadka, M.M. Pereira, J.J. D’Souza, P.L. Pallaty, H.P. Bhat, S. Popuri, Crit. Rev. Food. Sci. Nutr. 51, 499–523 (2011)
A.J. Akinyemi, A.O. Ademiluyi, G. Oboh, J. Med. Food 16, 641–646 (2013)
M.I. Kazeem, M.A. Akanji, M.T. Yakubu, Pathophysiology 22, 203–209 (2015)
Y.J. Surh, K.K. Park, K.S. Chun, L.J. Lee, E. Lee, S.S. Lee, J. Environ. Pathol. Toxicol. Oncol. 18, 131–139 (1999)
M. Mathew, S. Subramanian, Ind. J. Exp. Biol. 52, 606–612 (2014)
M.I. Kazeem, M.A. Akanji, R.M. Hafizur, M.I. Choudhary, Asian Pac. J. Trop. Biomed 2, 727–732 (2012)
O.I. Mohamed, A.F. El-Nahas, K.M. YS El-Sayed, Ashry. Pharm Biol 16, 1–9 (2015)
M.F. Mahmoud, A.A. Diaai, F. Ahmed, Ren. Fail. 34, 73–82 (2012)
Z. Ghlissi, R. Atheymen, M.A. Boujbiha, Z. Sahnoun, F. Makni Ayedi, K. Zeghal, A. El Feki, A. Hakim, Int. J. Food. Sci. Nutr. 64, 974–978 (2013)
K.L. Nagendra Chari, D. Manasa, P. Srinivas, H.B. Sowbhagya, Food Chem. 139, 509–514 (2013)
N. Öztaşkın, Y. Çetinkaya, P. Taslimi, S. Göksu, İ. Gülçin, Bioorg. Chem. 60, 49–57 (2015)
L.P. Köse, İ. A.C. Gülçin, Gören, J. Namiesnik, A.L. Martinez-Ayala, S. Gorinstein, Ind. Crop. Prod. 74, 712–721 (2015)
K. Aksu, F. Topal, I. Gülçin, F. Tümer, S. Göksu, Arch. Pharm. 348, 446–455 (2015)
J.H. Bae, Y.J. Park, J. Namiesnik, I. Gulcin, T.C. Kim, H.C. Kim, B.G. Heo, S. Gorinstein, Y.G. Ku, Int. J. Food Sci. Technol. 51, 1378–1385 (2016)
P. Kalın, İ. Gülçin, A.C. Gören, Rec. Nat. Prod. 9, 496–502 (2015)
M. Işık, M. Korkmaz, E. Bursal, I. Gülçin, E. Köksal, H. Tohma, Int. J. Pharmacol. 11, 366–371 (2015)
M.H. Sehitoglu, H. Han, P. Kalin, I. Gulcin, A. Ozkan, H.Y. Aboul-Enein, J. Enzyme Inhib. Med. Chem. 30, 264–269 (2015)
Y. Çetinkaya, H. Göçer, A. Menzek, I. Gülçin, Arch. Pharm. 345, 323–334 (2012)
I. Gülçin, M. Elmastaş, H.Y. Aboul-Enein, Arab. J. Chem. 5, 489–499 (2012)
H. Göçer, A. Akıncıoğlu, N. Öztaşkın, S. Göksu, I. Gülçin, Arch. Pharm. 346, 783–792 (2013)
I. Gülcin, S. Beydemir, Mini Rev. Med. Chem. 13, 408–430 (2013)
I. Gülçin, S. Beydemir, F. Topal, N. Gagua, A. Bakuridze, R. Bayram, A. Gepdiremen, J. Enzyme Inhib. Med. Chem. 27, 587–594 (2012)
I. Gülçin, F. Topal, R. Çakmakçı, M. Bilsel, A.C. Gören, U. Erdogan, J. Food Sci. 76, C585–C593 (2011)
E. Şıktar, D. Ekinci, E. Şıktar, Ş. Beydemir, I. Gülçin, M. Günay, Eur. J. Pharmacol. 668, 407–413 (2011)
I. Gülçin, F. Topal, S.B. Oztürk Sarikaya, E. Bursal, A.C. Gören, M. Bilsel, Rec. Nat. Prod. 5, 158–175 (2011)
E. Bursal, I. Gülçin, Food Res. Int. 44, 1482–1489 (2011)
H. Şerbetçi Tohma, I. Gülçin, Int. J. Food. Propert. 13, 657–671 (2010)
İ. Gülcin, M. Elmastas, H.Y. Aboul-Enein, Phytother. Res. 21, 354–361 (2007)
H. Mitsuda, K. Yuasumoto, K. Iwami, Eiyo to Shokuryo, 19, 210–214 (1996)
E. Köksal, E. Bursal, E. Dikici, F. Tozoğlu, İ. Gülçin, J. Med. Plants Res. 5, 217–222 (2011)
İ. Gülçin, Z. Huyut, M. Elmastaş, H.Y. Aboul-Enein, Arab. J. Chem. 3, 43–53 (2010)
İ. Gülçin, E. Kirecci, E. Akkemik, F. Topal, O. Hisar, Turk. J. Biol. 34, 175–188 (2010)
M. Oyaizu, Jpn. J. Nutr. 44, 307–315 (1986)
R. Apak, K. Guclu, B. Demirata, M. Ozyurek, S.E. Celik, B. Bektasoglu, K.I. Berker, D. Ozyurt, Molecules 12, 1496–1547 (2007)
İ. Gülçin, J. Enzyme Inhib. Med. Chem. 23, 871–876 (2008)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. 26, 1231–1237 (1999)
İ. Gülçin, Innov. Food Sci. Emerg. 11, 210–218 (2010)
İ. Gülçin, J. Med. Food 14, 975–985 (2011)
İ. Gülçin, A.Z. Tel, E. Kirecci, Int. J. Food Propert. 11, 450–471 (2008)
İ. Gülçin, R. Elias, A. Gepdiremen, A. Chea, F. Topal, J. Enzyme Inhib. Med. Chem. 25, 44–53 (2010)
E. Bursal, E. Köksal, İ. Gülçin, G. Bilsel, A.C. Gören, Food Res. Int. 51, 66–74 (2013)
H.T. Balaydın, İ. Gülçin, A. Menzek, S. Göksu, E. Şahin, J. Enzyme Inhib. Med. Chem. 25, 685–695 (2010)
O. Talaz, İ. S. Gülçin, Göksu, N. Saracoglu, Bioorg. Med. Chem. 17, 6583–6589 (2009)
T. Feng, J. Su, Z.H. Ding, Y.T. Zheng, Y. Li, Y. Leng, J.K. Liu, J. Agric. Food Chem. 59, 11690–11695 (2011)
A.H. El-Ghorab, M. Nauman, F.M. Anjum, S. Hussain, M. Nadeem, J. Agric. Food Chem. 58, 8231–8237 (2010)
İ. Gülçin, Chem. Biol. Interact. 179, 71–80 (2009)
M.E. Büyükokuroğlu, İ. Gülçin, Pharmacog. Mag. 4 (19), 189–195 (2009)
M. Topal, H. Gocer, F. Topal, P. Kalin, L. Polat Köse, İ. Gülçin, K.C. Çakmak, M. Küçük, L. Durmaz, A.C. Gören, S.H. Alwasel, J. Enzyme Inhib. Med. Chem. 31, 266–275 (2016)
İ. Gülçin, R. Elias, A. Gepdiremen, K. Taoubi, E. Köksal, Wood Sci. Technol. 43, 195–212 (2009)
Ö.A. Gümüşay, A.A. Borazan, N. Ercal, O. Demirkol, Food Chem. 173, 156–162 (2015)
E. Köksal, İ. Gülçin, S.B. Öztürk Sarıkaya, E. Bursa, J. Enzyme Inhib. Med. Chem. 24, 395–405 (2009)
G. Oboh, A.J. Akinyemi, A.O. Ademiluyi, Exp. Toxicol. Pathol. 64, 31–36 (2012)
F. Topal, M. Topal, H. Gocer, P. Kalın, U.M. Koçyiğit, İ. Gülçin, S.H. Alwasel, J. Enzyme Inhib. Med. Chem. 31, 674–683 (2016)
T. Ak, İ. Gülçin, Chem Biol. Interact. 174, 27–37(2008)
R.S. Policegoudra, K. Abiraj, D. Channe Gowda, S.M. Aradhya, J. Chromatogr. B. 852, 40–48 (2007)
I. Stoilova, A. Krastanov, A. Stoyanova, P. Denev, S. Gargova, Food Chem. 102, 764–770 (2007)
İ. Gülçin, R. Elias, A. Gepdiremen, L. Boyer, E. Köksal, Afr. J. Biotechnol. 6, 410–418 (2007)
E.S. Alinkina, T.A. Misharina, L.D. Fatkullina, E.B. Burlakova, Prikl. Biokhim. Mikrobiol. 48, 564–569 (2012)
A. Ghasemzadeh, H.Z. Jaafar, A. Rahmat, Molecules 15, 4324–4333 (2010)
A. Ghasemzadeh, H.Z. Jaafar, A. Rahmat, Molecules 15, 6231–6243 (2010)
İ. Gülcin, Arch. Toxicol. 86, 345–391 (2012)
I. Gülcin, E. Bursal, M.H. Sehitoglu, M. Bilsel, A.C. Goren, Food Chem. Toxicol. 48, 2227–2238 (2010)
F.J. Lara-Ortega, F.J. Sainz-Gonzalo, B. Gilbert-Lopez, J.F. Garcia-Reyes, A. Molina-Diaz, Talanta 147, 531–536 (2016)
E. Köksal, İ. Gülçin, Turk. J. Agric. For. 32, 65–78 (2008)
Y.Y. Kuo, W.T. Jim, L.C. Su, C.J. Chung, C.Y. Lin, C. Huo, J.C. Tseng, S.H. Huang, C.J. Lai, B.C. Chen, B.J. Wang, T.M. Chan, H.P. Lin, W.S.W. Chang, C.R. Chang, C.P. Chuu, Int. J. Mol. Sci. 16, 10748–10766 (2015)
H. Göçer, İ. Gülçin, Int. J. Food Sci. Nutr. 62, 821–825 (2011)
F. Galeotti, E. Barile, P. Curir, M. Dolci, V. Lanzotti, Phytochem. Lett. 1, 44–48 (2008)
R.K. Ismail, L. Jagan Mohan Rao, J. Appl. Chem. 1, 368–375 (2012)
E. Chan, Y.Y. Lim, L. Wong, F. Lianto, S. Wong, K. Lim, C. Joe, T. Lim, Food Chem. 109, 477–483 (2008)
S. Çakmakçı, E.F. Topdaş, P. Kalın, H. Han, P. Şekerci, L. Polat Köse, İ. Gülçin, Int. J. Food Sci. Technol. 50, 472–481 (2015)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Tohma, H., Gülçin, İ., Bursal, E. et al. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. Food Measure 11, 556–566 (2017). https://doi.org/10.1007/s11694-016-9423-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9423-z