Abstract
Recently, a number of studies on health benefits associated with natural compounds have been demonstrated. Phenolics in fruits, vegetables, herbs and spices possess potent antioxidant, anti-inflammatory, antimutagenic and anticarcinogenic activities. The fringe tree (Chionanthus virginicus) is used as a raw material by pharmaceutical industries for the preparation of homeopathy tinctures. The potential antioxidant activities of two secoiridoids from root bark of fringe tree (Chionanthus virginicus L.) were investigated to evaluate their potential value as the natural products for foods or cosmetic applications. In this study, antioxidant activities were measured by 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging, 1,1-diphenyl-2-picryl-hydrazyl free radical (DPPH·) scavenging, superoxide anion (O ·−2 ) radical scavenging, total antioxidant activity, reducing activity, hydrogen peroxide (H2O2) scavenging and ferrous metal chelating activity assays. These secoiridoids, as antioxidants neutralized the activities of radicals and inhibited the peroxidation reactions of linoleic acid emulsion. Total antioxidant activity was measured according to ferric thiocyanate method. Butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), α-tocopherol and trolox, a water-soluble analog of tocopherol, were used as the reference antioxidant compounds. Ligustroside (3.70 × 10−3 M) and oleuropein (3.80 × 10−3 M) showed 71.9, 82.4, 80.7 and 90.4% inhibition on lipid peroxidation of linoleic acid emulsion, at the concentrations of 10 and 20 μg/mL. On the other hand, 20 μg/mL of standard antioxidant such as α-tocopherol (4.64 × 10−3 M), trolox (7.98 × 10−3 M), BHA (10.08 × 10−3 M) and BHT (9.06 × 10−3 M) exhibited 61.5, 29.8, 74.4 and 71.2% inhibition on peroxidation of linoleic acid emulsion, respectively. In addition, ligustroside and oleuropein had effective DPPH·, ABTS·+ and superoxide anion radicals scavenging, hydrogen peroxide scavenging, total reducing power and metal chelating on ferrous ions activities. Also, those various antioxidant activities were compared to BHA and BHT, α-tocopherol and trolox that are references antioxidants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidation processes are very important for the living organism. Uncontrolled production of reactive oxygen species (ROS) and the unbalanced mechanism of antioxidant protection result in onset of many diseases and accelerate aging. ROS are a class of highly reactive molecules formed during aerobic life in living organisms and include superoxide anion radicals (O2·−), hydroxyl radicals (OH·) and non free-radical species such as H2O2 and singlet oxygen (1O2) (Halliwell and Gutteridge 1989; Gülçin et al. 2002a, b). There is a balance between the generation of ROS and inactivation of ROS by the antioxidant systems in organisms. ROS leads to oxidative modification in cellular membrane or intracellular molecules if there is no balance between ROS and antioxidant defence mechanisms (Duh et al. 1999; Büyükokuroğlu et al. 2001; Gülçin et al. 2003a). In addition, under pathological conditions or oxidative stress, ROS are overproduced and result in peroxidation of membrane lipids, leading to the accumulation of lipid peroxides. However, they are removed by antioxidant defence mechanisms. Antioxidants are considered as possible protection agents to reduce oxidative damage of human body from ROS and retard the progress of many chronic diseases as well as lipid peroxidation (Pryor 1991; Kinsella et al. 1993; Lai et al. 2001; Gülçin et al. 2003a). Therefore, there is a growing interest in the substances exhibiting antioxidant properties that are supplied to human and animal organisms as food components or as specific pharmaceutics. Antioxidants may be defined as compounds that inhibit or delay the oxidation of other molecules by inhibiting the initiation or propagation of oxidizing chain reactions (Velioglu et al. 1998; Gülçin et al. 2006a).
BHA, BHT, propylgallate and tert-butylhydroquinone are the most commonly used antioxidants. However, their safety is questioned due to their toxicity and possible carcinogenicity, liver damage and carcinogenesis (Wichi 1988; Sherwin 1990; Sun and Fukuhara 1997; Gülçin et al. 2003a). Hence, in the recent years, the restriction in the use of synthetic antioxidants, such as BHA and BHT, has caused an increased interest towards natural antioxidant substances (Baardseth 1989; Oktay et al. 2003; Gülçin et al. 2005). Therefore, much attention has been focused on natural antioxidants. These antioxidants occur in all higher plants and in all parts of the plant, such as wood, bark, stems, pods, leaves, fruits, roots, flowers and seeds (Al-Ismail and Aburjai 1989). Thus, development of safer natural antioxidants that can replace synthetic ones has been of interest (Liyana-Pathirana and Shahidi 2006).
Chionanthus virginicus L., fringe tree, is a shrub of the eastern America. This Oleaceae is used in folk medicine as cholagogue, diuretic and tonic (Duke and Wain 1981). The leaves contain flavonoids, such as rutin, kaempferol-3-glucoside, kaempferol-3-rutinoside, quercetin triglycosides (Harborne and Green 1980) and triterpenoid compounds such as ursolic acid (Pourra et al. 1954). Stem and root barks contain a lignin, phillyrin (Steinegger and Jacober 1959). Nowadays, root bark is used in homeopathy for hepatitis (Guermonprez et al. 1997).
Oleuropein, and ligustroside are the most abundant secoiridoid glucosides. Oleuropein, a non-toxic secoiridoid, is a powerful antioxidant and antiangiogenic agent with anticancer effect (Hamdi et al. 2003) that actively scavenges reactive oxygen (Manna et al. 2002) and nitrogen species (De la Puerta et al. 2001), inducing the production of nitric oxide in macrophages (Visioli et al. 1998). Oleuropein had a potent antitumor agent with direct effects against tumor cells (Hamdi and Castellon 2005). Two secoiridoid glucosides, ligustroside and oleuropein were isolated from the fruits of Ligustrum lucidum and their structures were elucidated by spectroscopic methods (He et al. 2001).
As mentioned above ligustroside and oleuropein have been isolated from different plant species, but no information has been found about in vitro antioxidant and antiradical activities of ligustroside and oleuropein from root bark of fringe tree (Chionanthus virginicus L.). The main objectives of the present study were to assess the antioxidant potential of ligustroside and oleuropein from root bark of fringe tree (Chionanthus virginicus L.) in different in vitro antioxidant assays including total antioxidant activity determination, DPPH free radical scavenging, ABTS radical scavenging, reducing power, superoxide anion radical scavenging, hydrogen peroxide scavenging and metal chelating activities.
Materials and methods
Chemicals
2,2-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), nicotinamide adenine dinucleotide (NADH), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), nitroblue tetrazolium (NBT), phenazine methosulphate (PMS), the stable free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH·), linoleic acid, 3-(2-Pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ethylenediaminetetraacetic acid (EDTA), α-tocopherol, polyoxyethylenesorbitan monolaurate (Tween-20), and trichloroacetic acid (TCA) were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). Ammonium thiocyanate was purchased from Merck. All other chemicals used were of analytical grade and were obtained from either Sigma-Aldrich or Merck.
Plant materials
Samples of dried root bark of Chionanthus virginicus were provided by BOIRON laboratories (No: 97070214, 97070425 and 97030136). They were collected in south-east of USA and dried naturally on site. Root bark was powdered and stored in a dry place and protected from light until used.
Extraction, isolation and identification of secoiridoids
Powdered root bark of Chionanthus virginicus (100 g) were extracted with 300 ml of MeOH twice under reflux during 30 min. The extract was concentrated in rotary evaporator. The residue was dissolved in 300 mL of distilled water and was successively extracted with 4 × 100 mL of AcOEt. Organic phase was evaporated in rotary evaporator to give residues of 4.4 g. This residue was subjected to LPLC (low pressure liquid chromatography) on Chromatospac Prwep 10 (Jobin Yvon) with a 40 × 500 mm column filled with Lichroprep RP-18 (25–40 μm, 200 g, Merck). The gradient solvent system was MeOH/H2O (v/v): 30/70 (700 mL); 40/60 (500 mL); 50/50 (500 mL); 60/40 (500 mL); 70/30 (500 mL); 80/20 (500 mL); 90/10 (500 mL); 100/0 (1000 mL). Collected fractions (100 mL) were examined by TLC; it was performed on silica gel 60F254 (Merck) with EtOAc/HCOOH/HOAc/H2O (100:11:11:27). Plates were examined under UV light at 254 and 366 nm; then they sprayed with H2SO4 solution (20% MeOH).
Two main compounds, oleuropein (MeOH 45%; 1230.9 mg) and ligustroside (MeOH 50%; 297.5 mg) were isolated from AcOEt extract. Chemical structures of both the secoiridoids are shown in Fig. 1. Structure elucidation was carried out using spectroscopic methods: LC-UV, LC-MS (mass spectrophotometer with negative ion detection), 1H NMR (DRX Brüker 500 spectrophotometer operating at 500 MHz), and 13C NMR (DRX Brüker 500 spectrophotometer operating at 125 MHz). NMR measurements were performed in CD3OD or in DMSO-d6. HPLC-MS, HPLC analysis and identification of secoiridoids compounds have been described in previous report (Boyer et al. 2005).
Total antioxidant activity: ferric thiocyanate method
The antioxidant activity of ligustroside, oleuropein and standards was determined according to the ferric thiocyanate method in linoleic acid emulsion (Mitsuda et al. 1996). A stock solution contained 10 mg of ligustroside and oleuropein dissolved in 10 mL deionized water. Different concentrations of stock ligustroside, and oleuropein solution samples (10–20 μg/mL) were prepared by diluting the stock solution in 2.5 mL of potassium phosphate buffer (0.04 M, pH 7.0) and these were added to 2.5 mL of linoleic acid emulsion in potassium phosphate buffer (0.04 M, pH 7.0). The mixed solution (5 mL) was incubated at 37°C in glass flask. At regular intervals during incubation, 0.1 ml aliquot of the mixture was diluted with 3.7 ml of ethanol, followed by the addition of 0.1 ml of 30% ammonium thiocyanate and 0.1 ml of 20 mM ferrous chloride in 3.5% hydrochloric acid. The peroxide level was determined by reading the absorbance at 500 nm in a spectrophotometer (CHEBIOS s.r.l. UV-VIS). During linoleic acid oxidation, peroxides that oxidize Fe2+ to Fe3+ are formed. The latter ions form a complex with thiocyanate and this complex has a maximum absorbance at 500 nm. These steps were repeated every 12 h until the control reached its maximum absorbance value. Therefore, high absorbance indicates high linoleic acid emulsion oxidation. Solutions without added samples were used as blanks. All data on total antioxidant activity are the average of duplicate experiments. The percentage of inhibition of lipid peroxidation in linoleic acid emulsion was calculated by following equation:
where A c is the absorbance of the control reaction and A s is the absorbance in the presence of the sample of ligustroside, oleuropein or other test compounds. In the control, the sample was replaced with an equal volume of ethanol (Gülçin et al. 2004a; Gülçin 2006a).
Total reduction activity by Fe3+–Fe2+ transformation
The samples prepared for ferric thiocyanate method was used for the present and other antioxidant assays. The reducing activities of ligustroside and oleuropein were determined by the method of Oyaizu (1986). The capacity of ligustroside and oleuropein to reduce the ferric–ferricyanide complex to the ferrous–ferricyanide complex of Prussian blue was determined by recording the absorbance at 700 nm after incubation. Simply, different concentrations of ligustroside and oleuropein (10–20 μg/mL) in 1 mL of distilled water were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. Aliquots (2.5 mL) of trichloroacetic acid (10%) were added to the mixture, which was then centrifuged for 10 min at 1000×g (MSE Mistral 2000, UK). The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm in a spectrophotometer. In the control, the sample was replaced with an equal volume of ethanol. Increased absorbance of the reaction mixture indicates grater reduction capability (Elmastaş et al. 2006a).
Metal chelating activity on ferrous ion
Ferrous ion (Fe2+) chelation by ligustroside, oleuropein and standards was estimated by the Ferrozine assay (Dinis et al. 1994). Briefly, 0.4 mL of ligustroside and oleuropein (10 μg/mL) was added to a solution of 2 mM FeCl2 (0.05 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL) and the total volume was adjusted to 4 mL with ethanol. Then, the mixture was shaken vigorously and left for waiting at room temperature for 10 min. After the mixture had reached the equilibrium, the absorbance of the solution was then measured spectrophotometrically at 562 nm. The results were expressed as percentage of inhibition of the ferrozine–Fe2+complex formation. The percentage of inhibition of ferrozine–Fe2+ complex formation was calculated using the formula given below:
where A C is the absorbance of the ferrozine–Fe2+ complex and A S is the absorbance in the presence of the sample of ligustroside and oleuropein or standards (Gülçin et al. 2004b; Elmastas et al. 2006b).
Hydrogen peroxide scavenging activity
The hydrogen peroxide scavenging ability of ligustroside and oleuropein was determined according to the method of Ruch et al. (1989). A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). Ligustroside and oleuropein at the 10 μg/mL concentration in 3.4 mL phosphate buffer was added to a H2O2 solution (0.6 mL, 40 mM). The absorbance value of the reaction mixture was recorded at 230 nm. A blank solution contained the phosphate buffer without H2O2. The percentages of H2O2 scavenging of ligustroside, oleuropein and standard compounds were calculated as:
where A C is the absorbance of the control, and A S is the absorbance in the presence of the sample of ligustroside and oleuropein or standards (Elmastas et al. 2006c; Gülçin et al. 2006a).
ABTS radical cation decolorization assay
The spectrophotometric analysis of ABTS·+ radical scavenging activity was determined according to Re et al. (1999). The ABTS·+ cation radical was produced by the reaction between 7 mM ABTS in H2O and 2.45 mM potassium persulfate, stored in the dark at room temperature for 12 h. Before usage, the ABTS·+ solution was diluted to get an absorbance of 0.700 ± 0.025 at 734 nm with phosphate buffer (0.1 M, pH 7.4). As mentioned above, the ABTS·+ was generated by incubating ABTS with potassium persulfate. Chemical compounds that inhibit the potassium persulfate activity may reduce the production of ABTS·+. This reduction results in a decrease of the total ABTS·+ in the system and contributes to the total ABTS·+ scavenging capacity.
For stock solutions of 10 mg of ligustroside and oleuropein was dissolved in 10 ml distilled water. Then, 1 ml of ABTS·+ solution was added to 3 mL of ligustroside and oleuropein solution in ethanol at different concentrations (10–20 μg/mL). Thirty minutes later, the percentage of inhibition in the absorbance at 734 nm was calculated for each concentration relative to a blank absorbance (ethanol). All determinations were carried out at least three times, and in triplicate. The ABTS·+ concentration (mM) in the reaction medium was calculated from the following calibration curve, determined by linear regression (R 2: 0.9922):
The capability to scavenge the ABTS·+ radical was calculated using the following equation:
where A C is the initial concentration of the ABTS·+ and A S is absorbance of the remaining concentration of ABTS·+ in the presence of scavengers (Gülçin et al. 2006b; Gülçin 2006b).
1, 1-Diphenyl-2-picryl-hydrazil free radical scavenging activity
1, 1-Diphenyl-2-picryl-hydrazil (DPPH) free radical scavenging activities for ligustroside and oleuropein were measured from the bleaching of purple colored methanol solution of the stable DPPH radical, following the method described by Blois (1958). The capacity of ligustroside and oleuropein to scavenge the lipid-soluble DPPH radical, which results in the bleaching of the purple color exhibited by the stable DPPH radical, is monitored at an absorbance of 517 nm. Basically, 0.1 mM ethanolic solution of DPPH· was prepared daily. Then, 1 ml of this solution was added to 3 mL of ligustroside and oleuropein solution in ethanol at different concentrations (10–20 μg/mL). Thirty minutes later, the absorbance was measured at 517 nm. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The DPPH· concentration (mM) in the reaction medium was calculated from the following calibration curve, determined by linear regression (R 2: 0.9974):
The capability to scavenge the DPPH· radical was calculated using the following equation:
where A C is the initial concentration of the stable DPPH radical without the test compound and A S is absorbance of the remaining concentration of DPPH· in the presence of ligustroside and oleuropein (Gülçin et al. 2004c, 2007; Cristiane de Souza et al. 2004).
Superoxide anion radical scavenging activity in PMS-NADH/NBT systems
Measurement of superoxide anion scavenging activity of ligustroside and oleuropein was based on the method described by Liu et al. (1991). Superoxide radicals are generated in PMS-NADH systems by oxidation of NADH and assayed by the reduction of NBT. In these experiments, the superoxide radicals were generated in 3 mL of Tris–HCl buffer (16 mM, pH 8.0) containing 1 mL of NBT (50 μM) solution, 1 mL NADH (78 μM) solution and sample solution of ligustroside and oleuropein (30 μg/mL) in water. The reaction was started by adding 1 mL of PMS solution (10 μM) to the mixture. The reaction mixture was incubated at 25°C for 5 min and the absorbance was measured against blank samples at 560 nm. l-Ascorbic acid was used as a positive control. Decreased absorbance of the reaction mixture indicates increased superoxide anion radical scavenging activity. The percentage inhibition of superoxide anion generation was calculated using the following formula:
where A C is the absorbance of the l-ascorbic acid, and A S is the absorbance of ligustroside and oleuropein or standards (Gülçin et al. 2004d; Gülçin and Daştan 2007).
Statistical analysis
All the analyses on total antioxidant activity were done in duplicate sets. The other analyses were performed in triplicate. The data were recorded as mean ± standard deviation and analyzed by SPSS (version 11.5 for Windows 98, SPSS Inc.). One-way analysis of variance was performed by ANOVA procedures. Significant differences between means were determined by LSD tests. P values <0.05 were regarded as significant and p values <0.01 very significant.
Results and discussion
Natural antioxidants have biofunctionalities such as the reduction of chronic diseases like DNA damage, mutagenesis, carcinogenesis, etc., and inhibitions of pathogenic bacteria growth, which are often associated with the termination of free radical propagation in biological systems (Zhu et al. 2002). Thus, for medicinal bioactive components, antioxidant capacity is widely used as a parameter. A number of assays have been introduced to measure the total antioxidant activity of pure compounds (Miller et al. 1996).
In this study, the antioxidant activity of the ligustroside and oleuropein were compared to BHA, BHT, α-tocopherol and its water-soluble analog trolox. The antioxidant activity of the ligustroside, oleuropein, α-tocopherol, trolox, BHA and BHT was also evaluated in a series of the following in vitro tests: DPPH free radical, ABTS radical and superoxide anion radicals scavenging, total antioxidant activity by ferric thiocyanate method, reducing activity, hydrogen peroxide scavenging activity and metal chelating activity.
Total antioxidant activity determination in linoleic acid emulsion system by ferric thiocyanate method
The effects of various concentrations of ligustroside and oleuropein (from 10 to 20 μg/mL) on lipid peroxidation of linoleic acid emulsion are shown in Fig. 2 and were found to be 71.9 and 82.4 (for ligustroside), 80.7 and 90.4% (for oleuropein), respectively. On the other hand, α-tocopherol (4.64 × 10−3 M), trolox (7.98 × 10−3 M), BHA (10.08 × 10−3 M) and BHT (9.06 × 10−3 M) exhibited 61.5, 29.8, 74.4 and 71.2% inhibition on peroxidation of linoleic acid emulsion, respectively, at the 20 μg/mL concentration. The results clearly showed that ligustroside and oleuropein had higher total antioxidant activity than α-tocopherol, trolox, BHA and BHT at the same concentration (20 μg/mL). In the present study, we determined the antioxidant activity of the ligustroside, and oleuropein in a concentration-dependent manner. In a recent study, the antioxidant activity of different lignans was evaluated in a time, temperature and concentration-dependent manner. Also, antioxidant activity of the lignans had been investigated in a structure–activity relationship study (Eklund et al. 2005).
Total reductive capability using the potassium ferricyanide reduction method
Figure 3 depicts the reducing activity of the ligustroside, oleuropein and standards (BHA, BHT, α-tocopherol and trolox) using the potassium ferricyanide reduction method. For the measurements of the reductive activity, the Fe3+–Fe2+ transformation was investigated in the presence of ligustroside and oleuropein using the method of Oyaizu (1986). The reducing activity of ligustroside, oleuropein, α-tocopherol and trolox increased with increasing concentration of samples. As can be seen in the Fig. 3, ligustroside and oleuropein showed more effective reducing activity than control at different concentrations (R 2 = 9962, R 2 = 9950). These differences were statistically significant (p < 0.01). Reducing power of ligustroside, oleuropein and standard compounds are as follows: BHA > BHT > oleuropein > α-tocopherol > ligustroside > trolox.
Ferrous ions chelating capacity
Transition metals have a major role in the generation of free oxygen radicals in living organisms. Iron exists in two distinct oxidation states; ferrous (Fe2+) or ferric ions (Fe3+). The ferric ion (Fe3+) is the relatively biologically inactive form of iron. However, it can be reduced to the active Fe2+, depending on the conditions, particularly pH (Strlic et al. 2002), and oxidized back through Fenton type reactions, with production of hydroxyl radicals; or Haber–Weiss reactions with superoxide anions (Kehrer 2000; Wong and Kitts 2001). The production of these radicals can lead to lipid peroxidation, protein modification and DNA damage. Chelating agents may inactivate metal ions and potentially inhibit the metal-dependent processes (Finefrock et al. 2003).
Phenolic compounds are one of many natural chelating agents in fresh foods, in addition to ascorbic acid, phosphorylated compounds and proteins (Gülçin et al. 2003b; Wong and Kitts 2001). Also, the production of highly ROS, such as superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals is also catalyzed by free iron through Haber and Weiss (1934). Free iron is known to have low solubility and a chelated iron (i.e., iron-ligant) complex, such as EDTA–Fe, and has greater solubility in solution, which can be contributed solely from the ligant. Furthermore, chelated iron, such as EDTA–Fe, is also known to be active, since it can participate in iron-catalyzed reactions (Wong and Kitts 2001).
Ferrous ion chelating activities of ligustroside, oleuropein, α-tocopherol, trolox, BHA and BHT are shown in Fig. 4. The chelating effect of ferrous ions by ligustroside, oleuropein and standards was determined according to the method of Dinis et al. (1994). Among the transition metals, iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. The ferrous state of iron accelerates lipid oxidation by breaking down hydrogen and lipid peroxides to reactive free radicals via the Fenton reaction (Fe2+ + H2O2 → Fe3+ + OH− + OH·). Fe3+ ion also produces radicals from peroxides, although the rate is tenfold less than that of Fe2+ ion (Miller 1996). Fe2+ ion is the most powerful pro-oxidant among various species of metal ions (Halliwell and Gutteridge 1984).
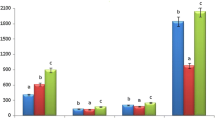
Comparison of hydrogen peroxide scavenging, superoxide anion radical scavenging and ferrous ions chelating activity of ligustroside (1.85 × 10−3 M) and oleuropein (1.90 × 10−3 M) from root bark of fringe tree (Chionanthus virginicus L.), BHA (5.54 × 10−3 M), BHT (4.53 × 10−3 M), α-tocopherol (2.32 × 10−3 M) and trolox (3.99 × 10−3 M) at the same concentration (10 μg/mL) (BHA butylated hydroxyanisole, BHT butylated hydroxytoluene)
The chelating of ferrous ions by ligustroside and oleuropein was estimated by the ferrozine assay. Ferrozine can quantitatively form complexes with Fe2+. In the presence of chelating agents, the complex formation is inhibited and the red color of the complex fades. By measuring the color reduction, therefore, it is possible to estimate the chelating activity of the co-existing chelator (Yamaguchi et al. 2000). In this assay, the natural compound interfered with the formation of the ferrozine–Fe2+ complex, suggesting that it has chelating activity and captures ferrous ions before ferrozine.
In fact, as shown in Fig. 4, ligustroside and oleuropein disrupted the Fe2+–ferrozine complex at 10 μg/mL concentration. The difference among all ligustroside and oleuropein concentrations and the control was statistically significant (p < 0.01). In addition, ligustroside (1.85 × 10−3 M) and oleuropein (1.90 × 10−3 M) exhibited, respectively, 62 and 50% chelation of ferrous ion at the above concentration. On the other hand, at the 10 μg/mL concentration, the percentages of metal chelating capacity of BHA (5.54 × 10−3 M), BHT (4.53 × 10−3 M), α-tocopherol (2.32 × 10−3 M) and trolox (3.99 × 10−3 M) were found to be 72, 64 and 22 and 49%, respectively. The metal scavenging effects of those samples decreased in the order of BHA > BHT > ligustroside > oleuropein > trolox > α-tocopherol. These results showed that trolox, which is a water-soluble synthetic analog of α-tocopherol, did not show any metal chelation activity.
Metal chelating capacity was significant, since it reduced the concentration of the catalyzing transition metal in lipid peroxidation. The data obtained from Fig. 4 reveal that ligustroside and oleuropein demonstrate a marked capacity for iron binding, suggesting that their main action as peroxidation protector may be related to its iron binding capacity.
Hydrogen peroxide scavenging activity
Hydrogen peroxide can be formed in vivo by many oxidizing enzymes such as superoxide dismutase. It can cross membranes and may slowly oxidize a number of compounds. The ability of ligustroside and oleuropein to scavenge hydrogen peroxide, determined according to the method of Ruch et al. (1989) is shown in Fig. 4 and is compared with that of α-tocopherol and trolox as standards. Ligustroside (1.85 × 10−3 M) and oleuropein (1.90 × 10−3 M) exhibited 83 and 81% scavenging effect of hydrogen peroxide at the 10 μg/mL concentration. On the other hand, BHA (5.54 × 10−3 M), BHT (4.53 × 10−3 M), α-tocopherol (2.32 × 10−3 M) and trolox (3.99 × 10−3 M) exhibited, respectively, 71, 67, 70 and 51% hydrogen peroxide scavenging activity at the same concentration (10 μg/mL). These results showed that ligustroside and oleuropein have effective hydrogen peroxide scavenging activity. At the above concentration, the hydrogen peroxide scavenging effect of ligustroside, oleuropein and four standards decreased in the order of ligustroside > oleuropein > BHA > α-tocopherol > BHT > trolox. Hydrogen peroxide itself is not very reactive; however, it can at times be toxic to cell because it may give rise to hydroxyl radical in the cells. Addition of hydrogen peroxide to cells in culture can lead to transition metal ion-dependent OH radicals mediated oxidative DNA damage. Thus, removing hydrogen peroxide as well as superoxide anion is very important for protection of pharmaceutical and food systems.
ABTS·+ radical scavenging activity
Radical scavenging activities are very important due to the deleterious role of free radicals in foods and biological systems. Excessive formation of free radicals accelerates the oxidation of lipids in foods and decreases food quality and consumer acceptance (Min 1998).
The improved technique for the generation of ABTS·+ described here involves the direct production of the blue/green ABTS·+ chromophore through reaction between ABTS and potassium persulfate. As shown in Table 1, ligustroside and oleuropein had ABTS·+ radical scavenging activity in a concentration-dependent manner (10–20 μg/mL). There is a significant decrease (p < 0.01) in the concentration of ABTS·+ due to the scavenging capacity of ligustroside, oleuropein and standards. In addition, the scavenging effect of ligustroside (3.70 × 10−3 M), oleuropein (3.80 × 10−3 M) and standards on the ABTS·+ decreased in the order: BHA > oleuropein > BHT > trolox > α-tocopherol > ligustroside, which were 98.0, 97.2, 96.2, 94.8, 89.2 and 51.8%, respectively, at the 20 μg/mL concentration.
DPPH· radical scavenging activity
1,1-Diphenyl-2-picryl-hydrazyl free radical (DPPH·) has been widely used to test the free radical-scavenging ability of various dietary antioxidants. Antioxidants react with DPPH·, which is a stable free radical, and convert it to 1,1-diphenyl-2-picryl hydrazine. The degree of discoloration indicates the radical-scavenging potential of the antioxidant (Singh et al. 2002). Swertiamarin and sweroside, secoiridoid glycosides, have been isolated from the aerial parts of Centaurium erythraea and tested for DPPH free radical scavenging activities. However, neither of the two secoiridoid glycosides showed any antioxidant activity in this assay (Kumarasamy et al. 2003). In another work, it was found that the ethanol extract of the fruits of Ligustrum lucidum showed significant inhibitory effects on free radical-induced hemolysis of red blood cells. Ligustroside and oleuropein were isolated from the active fraction of the fruits of Ligustrum lucidum (He et al. 2001). In this study, antioxidant activities of ligustroside, oleuropein and standard antioxidants such as α-tocopherol and trolox were determined using a DPPH· method. Since the DPPH· assay can accommodate a large number of samples in a short period and is sensitive enough to detect natural compounds at low concentrations, it was used in the present study for a primary screening of the ligustroside and oleuropein free radical-scavenging activity. This assay provides information on the reactivity of test compounds with a stable free radical. DPPH· gives a strong absorption band at 517 nm in visible spectroscopy because of its odd electron. As this electron becomes paired off in the presence of a free radical scavenger, the absorption vanishes, and the resulting decolorization is stoichiometric with respect to the number of electrons taken up. Ligustroside and oleuropein exhibited marked DPPH free radical scavenging activity in a concentration-dependent manner. Table 1 illustrates a significant decrease (p < 0.05) in the concentration of DPPH radical due to the scavenging ability of ligustroside, oleuropein and standards. BHA, BHT, α-tocopherol and trolox were used as references radical scavengers. The scavenging effect of ligustroside (3.70 × 10−3 M), oleuropein (3.80 × 10−3 M) and standards on the DPPH radical decreased in that order: BHT > BHA > α-tocopherol > oleuropein > ligustroside > trolox, which were 100, 86, 85, 84, 33 and 14%, respectively, at the 20 μg/mL concentration.
Superoxide anion radical scavenging activity
Superoxide anion radical included in free radical species is a factor that can induce aging and destruct the cell membrane and it can be generated by oxidative stress. They are produced in vivo by electron leakage from the mitochondrial electron transport chain, by activated phagocytes (Halliwell 1991) and in the conversion of xanthenes to uric acid (Bast et al. 1991). Superoxide anions are precursor to active free radicals that have the potential to react with biological macromolecules, thereby inducing tissue damage (Halliwell and Gutteridge 1984). Also, it has been implicated in several pathophysiological processes due to its transformation into more reactive species, such as hydroxyl radical that initiate lipid peroxidation. Superoxide has also been observed to directly initiate lipid peroxidation (Wickens 2001). It has also been reported that antioxidant properties of some flavonoids are effective, mainly via scavenging of superoxide anion radical (Yen and Duh 1994). Superoxide anion plays an important role in formation of other ROS such as hydrogen peroxide, hydroxyl radical, and singlet oxygen, which induce oxidative damage in lipids, proteins, and DNA (Pietta 2000). Also, superoxide anion is an oxygen-centered radical with selective reactivity. This species is produced by a number of enzyme systems in auto-oxidation reactions and by non-enzymatic electron transfers that univalently reduce molecular oxygen. It can also reduce certain iron complex such as cytochrome c.
In this method, superoxide anion derived from dissolved oxygen by PMS–NADH coupling reaction reduces the yellow dye (NBT2+) to produce the blue formazan, which is measured spectrophotometrically at 560 nm. Antioxidants are able to inhibit the blue NBT formation (Cos et al. 1998; Parejo et al. 2002). The decrease of absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion in the reaction mixture. Figure 4 shows that the percentage inhibition of superoxide radical generation by 10 μg/mL concentration of ligustroside; oleuropein and standards were found to be statistically similar. As can be seen in Fig. 4, the percentage inhibition of superoxide anion radical generation by 10 μg/mL concentration of ligustroside (1.85 × 10−3 M) and oleuropein (1.90 × 10−3 M) was found to be 69 and 34%. On the other hand, at the same concentration, BHA (5.54 × 10−3 M), BHT (4.53 × 10−3 M), α-tocopherol (2.32 × 10−3 M) and trolox (3.99 × 10−3 M) exhibited 76, 47, 71 and 78% superoxide anion radical scavenging activity, respectively.
Conclusion
This study demonstrated the potential antioxidant properties of two secoiridoids, ligustroside and oleuropein, from fringe tree (Chionanthus virginicus). Oleuropein has an extra phenolic hydroxyl group compared to ligustroside. Because of this phenolic hydroxyl group, it was respected that oleuropein has higher antioxidant activity than ligustroside. We found similar results in another study. In that study, it was found that l-dopa had higher antioxidant activity than l-tyrosine because of extra hydroxyl group (Gülçin 2007). According to data of the present study, ligustroside and oleuropein were found to be effective antioxidants in different in vitro assays including ferric thiocyanate method, reducing power, DPPH· scavenging, ABTS·+ scavenging and superoxide anion radical scavenging, hydrogen peroxide scavenging and metal chelating activities when compared to standard antioxidant compounds, such as synthetic antioxidants (BHA, BHT), α-tocopherol, a natural antioxidant, and trolox, which is a water-soluble analog of tocopherol.
References
Al-Ismail KM, Aburjai T (1989) Antioxidant activity of water and alcohol extracts of chamomile flowers, anise seeds and dill seeds. J Sci Food Agric 84:173–178
Baardseth P (1989) Effect of selected antioxidants on the stability of dehydrated mashed potatoes. Food Addit Contam 6:201–207
Bast A, Haenen GRMM, Doelman CJA (1991) Oxidants and antioxidants: state of the art. Am J Med 91:2–13
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 26:1199–1200
Boyer L, Elias R, Taoubi K, Debrauwer L, Faure R, Baghdikian B, Balansard G (2005) Lignans and secoiridoids from root bark of Chionanthus virginicus L.: isolation, identification and HPLC analysis. Phytochem Anal 16:380–387
Büyükokuroğlu ME, Gülçin İ, Oktay M, Küfrevioğlu Öİ (2001) In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 44:491–495
Cos P, Ying LY, Calomme M, Hu JH, Cimanga K, Van Poel B, Pieters L, Vlietinck AJ, Berghe DV (1998) Structure activity relationships and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 61:71–76
Cristiane de Souza L, Soares de Araujo SM, Imbroisi DO (2004) Determination of the free radical scavenging activity of dihydropyran-2,4-diones. Bioorg Med Chem Lett 14:5859–5861
De la Puerta R, Martinez Dominguez ME, Ruiz-Gutierrez V, Flavill JA, Hoult JR (2001) Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci 69:1213–1222
Dinis TCP, Madeira VMC, Almeida LM (1994) Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Duh PD, Tu YY, Yen GC (1999) Antioxidant activity of water extract of harng jyur (Chrysanthemum morifolium Ramat). Lebensm Wiss Technol 32:269–277
Duke JA, Wain KK (1981) In medicinal plants of world. Computer index with more than 85000 entries, 3 vols. p 1654
Eklund PC, Langvik OK, Wärna JP, Salmi TP, Willför SM, Sjöholm RE (2005) Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org Biomol Chem 3:3336–3347
Elmastaş M, Gülçin İ, Beydemir Ş, Küfrevioğlu Öİ, Aboul-Enein HY (2006a) A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) seeds extracts. Anal Lett 39:47–65
Elmastaş M, Gülçin İ, Işıldak Ö, Küfrevioğlu Öİ, İbaoğlu K, Aboul-Enein HY (2006b) Antioxidant capacity of bay (Laurus nobilis L.) leave e extracts. J Iran Chem Soc 3:258–266
Elmastaş M, Türkekul İ, Öztürk L, Gülçin İ, Işıldak Ö, Aboul-Enein HY (2006c) The antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta). Comb Chem High T Scr 9:443–448
Finefrock AE, Bush AI, Doraiswamy PM (2003) Current status of metals as therapeutic targets in Alzheimer’s disease. J Am Geriat Soc 51:1143–1148
Guermonprez M, Pinkas M, Tork M (1997) Chionanthus virginiana. In: Matiere Medicale Homeopathique, 2nd edn, pp 144–145
Gülçin İ (2006a) Antioxidant and antiradical activities of l-Carnitine. Life Sci 78:803–811
Gülçin İ (2006b) Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicology 217:213–220
Gülçin İ (2007) Comparison of in vitro antioxidant and antiradical activities of l-tyrosine and l-dopa. Amino Acids 32:431–438
Gülçin İ, Daştan A (2007) Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enzym Inhib Med Chem 22:685–695
Gülçin İ, Büyükokuroğlu ME, Oktay M, Küfrevioğlu Öİ (2002a) On the in vitro antioxidant properties of melatonin. J Pineal Res 33:167–171
Gülçin İ, Oktay M, Küfrevioğlu Öİ, Aslan A (2002b) Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 79:325–329
Gülçin İ, Büyükokuroğlu ME, Oktay M, Küfrevioğlu Öİ (2003a) Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. Subsp. pallsiana (Lamb.) Holmboe. J Ethnopharmacol 86:51–58
Gülçin İ, Oktay M, Kireçci E, Küfrevioğlu Öİ (2003b) Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem 83:371–382
Gülçin İ, Beydemir Ş, Alici HA, Elmastaş M, Büyükokuroğlu ME (2004a) In vitro antioxidant properties of morphine. Pharmacol Res 49:59–66
Gülçin İ, Küfrevioğlu Öİ, Oktay M, Büyükokuroğlu ME (2004b) Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 90:205–215
Gülçin İ, Şat İG, Beydemir Ş, Elmastaş M, Küfrevioğlu Öİ (2004c) Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem 87:393–400
Gülçin İ, Şat İG, Beydemir Ş, Küfrevioğlu Öİ (2004d) Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.). Ital J Food Sci 16:17–30
Gülçin İ, Berashvili D, Gepdiremen A (2005) Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J Ethnopharmacol 101:287–293
Gülçin İ, Elias R, Gepdiremen A, Boyer L (2006a) Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 223:759–767
Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R (2006b) Screening of antioxidant and antiradical activity of monodesmosides and crude extract from Leontice smirnowii Tuber. Phytomedicine 20:130–134
Gülçin İ, Elmastas M, Aboul-Enein HY (2007) Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum) assayed by different methodologies. Phytother Res 21:354–361
Haber F, Weiss J (1934) The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond Ser A 147:332–351
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry and role in human disease. Am J Med 91:14–22
Halliwell B, Gutteridge JMC (1984) Oxygen toxicology, oxygen radicals, transition metals and disease. Biochem J 219:1–4
Halliwell B, Gutteridge JM (1989) Free radicals in biology and medicine. Clarendon Press, Oxford, pp 23–30
Hamdi HK, Castellon R (2005) Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem Biophys Res Commun 334:769–778
Hamdi HK, Tavis JH, Castellon R (2003) In: USPTO, Antigen Biologicals Corporation, USA
Harborne JB, Green PS (1980) A chemotaxonomic survey of flavonoids in leaves of the Oleaceae. Bot J Linn Soc 81:155–167
He ZD, Dong H, Xu HX, Ye WC, Sun HD, But PPH (2001) Secoiridoid constituents from the fruits of Ligustrum lucidum. Phytochemistry 56:327–330
Kehrer JP (2000) The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149:43–50
Kinsella JE, Frankel E, German B, Kanner J (1993) Possible mechanism for the protective role of the antioxidant in wine and plant foods. Food Technol 47:85–89
Kumarasamy Y, Nahar L, Cox PJ, Jaspars M, Sarker SD (2003) Bioactivity of secoiridoid glycosides from Centaurium erythraea. Phytomedicine 10:344–347
Lai LS, Chou ST, Chao WW (2001) Studies on the antioxidative activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem 49:963–968
Liu Q, Zhu G, Huang P (1991) Anti-inflammatory, analgesic and sedative effects of Leontice kiangnanensis. Zhongguo Zhong Yao Za Zhi 161:50–65
Liyana-Pathirana CM, Shahidi F (2006) Antioxidant properties of commercial soft and hard winter wheats (Triticum aestivum L.) and their milling fractions. J Sci Food Agric 86:477–485
Manna C, D’Angelo S, Migliardi V, Loffredi E, Mazzoni O, Morrica P, Galletti P, Zappia V (2002) Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J Agric Food Chem 50:6521–6526
Miller DD (1996) Mineral. In: Fennema OR (ed) Food chemistry. Marcel Deckker, New York, pp 618–649
Miller NJ, Castelluccio C, Tijburg L, Rice-Evans CA (1996) The antioxidant properties of thioflavines and their gallate esters—radical scavengers or metal chelator? FEBS Lett 392:40–44
Min DB (1998) Lipid oxidation of edible oil. In: Akoh CC, Min DB (eds) Food lipids chemistry, nutrition and biotechnology. Marcel Dekker, New York, pp 283–296
Mitsuda H, Yuasumoto K, Iwami K (1996) Antioxidation action of indole compounds during the autoxidation of linoleic acid. Eiyo to Shokuryo 19:210–214
Oktay M, Gülçin İ, Küfrevioğlu Öİ (2003) Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wiss Technol 36:263–271
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315
Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codına C (2002) Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agric Food Chem 50:6882–6890
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Pourra H, Le Men J, Boustany N (1954) Ursolic acid; disturbtion of ursolic acid in Oleaceae. Ann Pharm Fr 12:59–62
Pryor WA (1991) The antioxidant nutrient and disease prevention—what do we know and what do we need to find out? Am J Clin Nutr 53:391–393
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10:1003–1008
Sherwin ER (1990) Antioxidants. In: Branen AL, Davidson PM, Salminen S (eds) Food additives. Marvel Dekker inc, New York, pp 139–193
Singh RP, Murthy KNC, Jayaprakasha GK (2002) Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 50:81–86
Steinegger E, Jacober H (1959) Presence of phillyrin in the Oleaceae. Structure of chionanthins. Pharm Acta Helv 34:585–592
Strlic M, Radovic T, Kolar J, Pihlar B (2002) Anti- and prooxidative properties of gallic acid in Fenton-type systems. J Agric Food Chem 50:6313–6317
Sun B, Fukuhara M (1997) Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug metabolizing enzymes in mice. Toxicology 122:61–72
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 46:4113–4117
Visioli F, Bellosta S, Galli C (1998) Oleuropein, the bitter principle of olives, enhances nitric oxide production by mouse macrophages. Life Sci 62:541–546
Wichi HP (1988) Enhanced tumor development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium. Food Chem Toxicol 26:717–723
Wickens AP (2001) Aging and the free radical theory. Reportor Physiol 128:379–391
Wong PYY, Kitts DD (2001) An iron binding assay to measure activity of known food sequestering agents: studies with buttermilk solids. Food Chem 72:245–254
Yamaguchi F, Ariga T, Yoshimira Y, Nakazawa H (2000) Antioxidant and antiglycation of carcinol from Garcinia indica fruit rind. J Agric Food Chem 48:180–185
Yen GC, Duh PD (1994) Scavenging effect of methanolic extract of peanut hulls on free radical and active oxygen species. J Agric Food Chem 42:629–632
Zhu QY, Hackman RM, Ensunsa JL, Holt R, Keen CL (2002) Antioxidative activities of oolong tea. J Agric Food Chem 50:6929–6934
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gülçin, İ., Elias, R., Gepdiremen, A. et al. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.). Wood Sci Technol 43, 195–212 (2009). https://doi.org/10.1007/s00226-008-0234-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-008-0234-1