Abstract
Purpose
The aim of the present study is to assess the molluscicidal, larvicidal and genotoxicological activities of papain and how it can affect the host-parasite interactions.
Methods
Toxicity of papain on snails by making series of concentrations to calculate LC50, and then study its larvicide effect on the free larval stages of S. mansoni and infection rate of snails.
Results
Papain has a molluscicidal activity on adult snails of Biomphalaria alexandrina with a lethal concentration LC50 equals to 43.1 mg/L. In addition, it has activity on miracidia with half Lethal time (LT50) of 16.11 min., and on cercariae with 12.1 min. compared to control ones. The sub lethal concentration LC10 and LC25 (6.9 or 24.1 mg/L, respectively) decreased the survival rate of snails at the first cercarial shedding, the rate of infection, the average total number of cercariae per snail, the shedding period and the life span of snails, while the prepatent period was significantly increased than the control ones. The morphological alterations in cercariae after exposure to papain were occurred where the cercariae lacked motility and some had a dark tail with complete detachment of head and tail. Compared to the control group, the levels of cytochrome oxidase subunit I (COI) and (ND1) genes significantly decreased in snails after exposure to papain.
Conclusions
Papain could be used as a potential molluscicide for elimination of schistosomiasis and decrease its transmission and deterioration of host-parasite interaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is a neglected helminthic disease that is caused by trematodes of the genus Schistosoma in poor and undeveloped countries [1,2,3]. Estimates showed that more than 236.60 million people worldwide needed preventive treatment for schistosomiasis by Schistosoma mansoni [4,5,6,7]. The World Health Organization has established road map guidelines for the elimination of schistosomiasis based on mass drug treatment by praziquantel [8,9,10] and snail control [11, 12]. Biomphalaria snails are freshwater gastropods that are responsible for the transmission of S. mansoni [13, 14]. In this host, the miracidia penetrate its tissues and develop to cercariae (the infective stage) that emerge from snails and search for the final host to complete their life cycle [15, 16]. To control schistosomiasis, the life cycle could be cut at the snail stage [4]. The effective molluscicide should be biodegradable and nontoxic to non-target organisms like fish [17, 18].
Niclosamide is the only drug approved by WHO to eradicate snails of schistosomiasis [19, 20], but it is toxic to the non-target aquatic species besides the high cost and the pollution that resulted from its use [2, 16, 21]. Many investigations were evolved to study the effects of different plant molluscicides on the biological aspects of the snails and the larval stages of S. mansoni [4, 22,23,24].
Plants and their secondary metabolites can be used as potential molluscicidal, herbicidal, antimicrobial and helminthicidal agents [18, 25,26,27,28]. The main reason for using plant molluscicides is they are cheap, biodegradable, easily handled and can provide a good income for poor farmers in undeveloped countries [23, 29,30,31,32].
Carica papaya L. (Caricaceae) is widely cultivated for food and has several industrial uses [33]. It is known to have antischistosomal and molluscicidal activities due to the presence of papain [34, 35]. Phytochemical screening of the ethanol extract of C. papaya seeds revealed the presence of polyphenols and glycosides, along with trace amounts of alkaloids, saponins, and flavonoids [36]. Papain is a purified protein extracted from the latex of the unripe papaya, is widely used in folk medicine [37]. It is a predominant enzyme that has the ability to cause a genotoxicity or mutagenicity in DNA [33]. Also, papain is a potent molluscicide against the harmful snail Lymnaea acuminata, the intermediate host for the liver fluke Fasciola gigantica [38]. Another study stated that the ethanolic extract of C. papaya seed (LC50 at 24 h: 53.38 mg/l) could be used as potent molluscicide against L. acuminata since this concentration is not toxic for Colisa fasciatus fish which is living in the same habitat with the snail [39]. The methanolic extract of C. papaya could be used as an eco-friendly alternate molluscicide for B. alexandrina snails with safety effects on D. magna with no mortality percentages of D. magna during the first 12 h of the exposure to 138.5 mg/l [31].
Therefore, the main objectives of this study were (i) to study papain larvicidal effects on the free larval stages of S. mansoni, (ii) to determine how papain can affect host-parasite interaction by specifying the prevalence of snail infection, the length of the prepatent and patent periods, the shedding of cercariae and, also, the survival rate and lifespan of exposed snails, and (iii) to elucidate its molluscicidal effect on DNA of exposed B. alexandrina snails by a real-time PCR.
Materials and Methods
Snails and Parasite
Snails
Adult B. alexandrina (9–11 mm) were obtained from a reared lab colony in Medical Malacology Laboratory, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Schistosoma mansoni Miracidia
Schistosoma mansoni eggs were obtained from the Schistosome Biological Supply Center (SBSC), Theodor Bilharz Research Institute, Giza, Egypt (SBSC/TBRI). This strain originated from Egypt and was obtained from Giza Governorate and has been maintained in albino mice Mus musculus CD1 strain. Eggs were left in a clean dechlorinated tap water (24 ± 1 °C) for hatching under a desk lamp light. Freshly hatched miracidia were pipetted in clean petri-dishes for further experiments [40].
Cercariae
Schistosoma mansoni cercariae were obtained from the SBSC/TBRI, Giza, Egypt. It was shed under illumination from the infected B. alexandrina snails and they were used for cercaricidal activity immediately after they were shed from these snails.
Snail Maintenance
Adult snails were reared in 15 × 24 × 10 cm plastic aquaria (4 L capacity) and provided with dechlorinated tap water which was changed each 3 days. Snails were fed on lettuce, blue green algae (Nostoc muscorum) and tetramine (fish food) (10 snails/ L). The temperature of aquaria was adjusted to 25 ± 2 °C, pH: 7 ± 0.2, with a photoperiodicity of 12 h light/12 h dark [41]. The egg masses were collected by fine forceps from small foam pieces that were placed on the water surface of the aquaria [4]. Egg masses were transferred to clean smaller plastic aquaria of 2 L capacity containing dechlorinated tap water and fed with blue green algae (Nostoc muscorum), tetramine and small pieces of CaCO3 [6]. After reaching a 4–5 mm shell diameter, they were subjected to the infection with miracidia.
Papain
Papain from Carica papaya latex (lyophilized powder, 23 kDa, Alfa Aesar) was obtained from ThermoFisher (Kandel) GmbH- Erienbachweg 2-76870 Kandel, Germany. To calculate LC50 and LC90, serial dilutions of papain (10, 15, 25, 50, 75, and 100 mg/l) were made with dechlorinated tap water.
Snail Infection
Three replicates, each of 20 lab-bred B. alexandrina snails (4–5 mm), were exposed to newly hatched S. mansoni miracidia, 10 miracidia/ snail for 2 h under illumination and were maintained till cercarial emergence.
Toxicity of Papain on Snails
Uninfected Adult Snails
Ten snails (diameter, 9–10 mm) were exposed to each concentration tested in a plastic aquarium [42] and three replicates were performed. Control snails of the same size were maintained only in dechlorinated tap water and their behavior was assessed against that of their papain-exposed congeners under the same experimental conditions [43]. The duration of exposure to papain lasted 24 h and was followed by a recovery period (in dechlorinated water) for 24 h. Mortality percentages were analyzed by a probability analysis [44] to determine sublethal concentrations of the substance [45].
Infected Snails
Three replicates, each with 20 laboratory-reared B. alexandrina snails (4–5 mm), were exposed to newly hatched S. mansoni miracidia (10 miracidia/ snail) for 2 h under illumination and the sublethal concentrations LC10 or LC25 (6.9 or 24.1 mg/L) of papain for 24 h and placed in dechlorinated tap water afterwards. Three other replicates, each comprising 20 snails, were exposed to miracidia under the same conditions and kept only in dechlorinated tap water until cercariae emerged to constitute a positive control group.
Examination of the Infected Snails for the Cercarial Shedding
This examination starts from day 21 post miracidial exposure. Snails were examined individually for cercarial shedding in multi-dishes under artificial light for 2 h and 2 ml of dechlorinated tape water/snail. As soon as the initial shedding was observed, positive snails were separated individually in plastic cups and examined once a week till snails’ death. The emerged cercariae/ snail were pipetted to a small Petri dish, fixed in Bouin’s solution and counted under a stereomicroscope to count the total cercariae/ snail.
-
a)
The snail’s infection rate was calculated at the end of experiment by dividing number of shedding and positive snails on the number of survived snails at first shedding X100 [46].
-
b)
The survival rate was calculated by dividing the number of snails at first shedding by the total number of exposed snails [47].
-
c)
Mean prepatent period, mean length of shedding, and mean life- span of snails in each group of positive infection [48].
Papain Toxicity on Schistosome- free Larvae
Miracidicidal, cercaricidal activity and their lethal time (LT50, 90, and 99):
One hundred newly hatched miracidia/ 5 ml dechlorinated water were placed in a sterilized petri dish with 5 ml of LC50 concentration of papain (43.1 mg/L). In addition, freshly shed 100 cercariae/ 5 ml water were placed with the same concentration of papain. As well as, the control groups were assessed side by side as 100 miracidia or cercariae were kept in 10 ml of dechlorinated tap water [49]. Three replicates were used to detect the number of dead miracidia and cercariae with each concentration and control groups [50, 51]. Both larval stages were examined under a stereomicroscope to detect their morphology and motility after intervals of 10, 20, 30, 40, and 50 min. The stationary cercariae or miracidia were photographed by light microscope and they were considered as dead [13, 52]. Cercariae were considered dead when they stopped movement, sank down and when their tails were detached [51]. It was photographed by Automatic camera using Olympus System Microscope.
Lethal Time (LT50, 90, and 99) is the time of the death of 50%, 90% and 98% of S. mansoni larvae after exposure to the half lethal concentration LC50 (43.1 mg/l) of papain. Determination of the value is done by probit analysis [53].
Molecular Changes in Papain-Exposed Adult Snails
Snails were exposed to the sublethal concentrations either LC10 6.9 mg/L or LC25 24.1 mg/L of papain for 24 h followed by 24 h recovery in dechlorinated water. Snails were dissected and the head foot part was used in Gene regulation assay by quantitative PCR as reported by [13]. They used two primers to measure changes in B. alexandrina cytochrome oxidase subunit I (COI) and B. alexandrina NADH dehydrogenase subunit 1 (ND1) genes. Both genes were quantified using the StepOne Real-Time PCR System (Applied Biosystems, California, USA) duplicated real time runs [54].
B. alexandrina cytochrome oxidase subunit I (COI) product length is 449 bp.
Forward primer (GGTACTACTCTTGTTTTGATAGATG) Tm = 52 and.
Reverse primer (GCTGTAACCAACACAGATCATAC) Tm = 54.
B. alexandrina NADH dehydrogenase subunit 1 (ND1).
Forward primer (GGGATTCTGCAACCATTTGC).
Reverse primer (TTTCTGCTAATGTTGTTGTAAATCACAC) Tm = 55 for both and Product length is 439. The slides were coded independently and scored blindly.
Statistical Analyses
The half-lethal concentration values were defined using the Probit facility [44]. Student’s t-test was used to compare the means of the exposed and control groups [55]. Infection rate of every snail group was compared with control using the chi-square (χ2) test [56]. Significant differences were considered at p ≤ 0.05. Data were expressed as mean ± standard error of the mean (SEM). Data normality has been checked using the Shapiro-Wilk test [57].
Results
The present results showed that papain has a molluscicidal activity against adult B. alexandrina snails after 24 h of exposure (Table 1, and Fig. 1) with a median lethal concentration (LC50) 43.1 mg/l.
Snail’s Infection Parameters
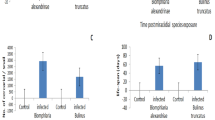
The present results showed that after exposure to the sub lethal concentration LC10 or LC25 (6.9 or 24.1 mg/l) of papain for 24 h and exposure to S. mansoni miracidia, both the survival rate at first cercarial shedding and the infection rates were significantly decreased (Fig. 2, A). Also, the present result confirmed that the mean total number of cercariae that were shed by each snail was significantly (p < 0.01) decreased than the control ones (Fig. 2, B).
In S. mansoni-infected snails, the prepatent period significantly increased (p < 0.01) after exposure to a LC10 (at 6.9 mg/l) or LC25 (at 24.1 mg/l) of papain for 24 h, whereas the shedding period was significantly lower (p < 0.01) than that observed in controls infected and not exposed to papain (Fig. 3, A). The lifespan of infected snails exposed to papain also significantly decreased (p < 0.01) compared to infected controls and was dependent on the concentration of the product used (Fig. 3, B).
Larvicidal Effect of LC50 (43.1 mg/l) of Papain
The present results showed that the sub lethal concentration LC50 (43.1 mg/l) of Papain has miracidial activity where all miracidia died after 40 min. Also, it has cercaricidal activity, where all cercariae died after 30 min of exposure compared to control ones (Fig. 4).
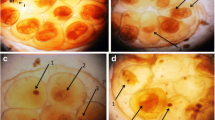
The present results showed some morphological alterations in cercariae were observed after exposure to the sub lethal concentration LC50 (43.1 mg/l) of papain, where they lack their motility and this is considered as the death of the larval stages. Also, cercariae showed darkness and swollen head, when exposed to papain for 30 min there was a complete detachment of the head and tail (Fig. 5).
Regarding the half Lethal Time (LT50) for miracidia and cercariae, the present results showed that the sub lethal concentration LC50 (43.1 mg/l) of papain has affected the S. mansoni larvae lethal time, where the half Lethal Time (LT50) for miracidiae was 16.11 min, while for cercariae was 12.1 min (Table 2).
The present results showed that the expression level of cytochrome oxidase subunit I (COI) and (ND1) genes were significantly decreased (p < 0.01) in exposed snails than the control group and this reduction is concentration dependent (Fig. 6).
Discussion
The present results showed that Papain has molluscicidal activity on adult B. alexandrina snails after 24 h with lethal concentration (LC50) was 43.1 mg/l. The molluscicidal activity of the lyophilized latex powder of Carica papaya (papain) was also confirmed on Lymnaea acuminata snail (LC50 at 96 h: 8.38 mg/l) and this toxicity is time and dose dependent [39]. Authors made another paper on the mechanism of the action of papain and how it affected the biological system, they found that papain (C. papaya latex) significantly inhibited the acetylcholinesterase (AChE), acid and alkaline phosphatase(ACP/ALP) activity in the nervous tissue of L. acuminata [58]. In 2018, another study showed that snail fed on pellets containing papain (40% of 24 h LC50) resulted in significant decreases in protein, amino acids, DNA, RNA and AChE levels in the gonadal/nervous tissue of Lymnaea acuminata and this is might be the cause for snails death [59]. Also, the C. papaya ethanolic leaf extracts has molluscicidal activity on B. globosus, the snail intermediate hosts of schistosomiasis [60].
The present results showed that after exposure to the sub lethal concentration LC10 or LC25 (6.9 or 24.1 mg/l) of papain for 24 h and exposure to S. mansoni miracidiae, both the survival rate at first cercarial shedding and the infection rates, mean total number of cercariae per snail, the shedding period and the life span of snails were significantly decreased, while the prepatent period was significantly increased than the control ones in concentration dependent way. The time-dependent toxic effects of papain might be correlated to the snails’ uptake of the active components which will increase in their body with the increase in the exposure duration [61].
The interaction between mollusks and their trematode parasites is dynamic and in which the trematode is either destroyed and eliminated by the host snail defensive system [62, 63]. These decreases might be due to the excessive production of the inhibitory compound by the infected snails and further histopathological damages which might lead to the reduction in these parameters [64] or might due to a disruption in the snail metabolism due to exposure to S. mansoni [65, 66]. Also, Papain can be used in vaccination of CD-1 mice and hamsters against S. mansoni and S. haematobium, respectively, where it decreased the worm burden, egg load, and the viability of the parasite ova in the small intestine and liver [67].
The present results showed that the Papain sub lethal concentration LC50 (43.1 mg/l) has miracidial and cercaricidal activities. This might be due to the toxic effect of papain on the enzymatic activities of the miracidiae which would lead to their death and hence decreased the infection rate of snails by these miracidiae [68]. Similarly, [69] reported 100% mortality of cercariae and miracidiae after 30 min and 60 min exposure to the sublethal concentration of Albizia anthelmintica saponin (LC50 17.6 ppm) and this resulted in a significant reduction of the survival and rate infection rate of the exposed snails. Authors reasoned these alterations to the high damages in the physiological and biological parameters of snails which made them unsuitable intermediate host for the parasite development [13]. These results were reflected on the reduction in all parameters of infection measured in the present study.
The present results showed some morphological alterations in cercariae after the exposure to papain, where they lack their motility and some has dark tail with complete detachment of head and tail. These results in good accordance with [51] who reported that the microscopic examination of cercariae exposed for 1 h to Solanum nigrum and Callistemon citrinius leaves extract where cercariae have a decrease in motility until motionless with shorten length. Some have abnormal shape, head separation from tail, and sometimes disintegration. Because of theses alterations, the measured infection parameters were decreased. Also, [70] reported miracicidicical and cercaricidal activities of Nerium oleander and Tecoma stans extract and correlated the mortality of these larvae to the presence of the bioactive secondary metabolites in their extracts [71]. Also, [72] confirmed the presence of the cercarial abnormalities after exposure of S. mansoni cercaria to different concentrations of Solanum nigrum, where, the cercariae showed swollen head, darkness of the tail and shortness, complete detachment of head and tail with loss of their contents [51].
The Lethal Time (LT) is a standard medium time measurement that can kill test animals. It was done to find out the time needed for LC50 concentration of papain [53]. The half Lethal Time (LT50) for miracidiae was 16.11 min, while for cercariae were 12.1 min compared to control ones. The causes of this mortality is due to the interference of papain with the protein biosynthesis process, RNA, DNA and AChE which would lead to the larval death [23, 58, 70].
Genotoxicological studies on DNA were considered as an effective tool for assessing the contamination of the aquatic ecosystems [6, 18, 73]. The main reason in this genotoxic effect is the increase in reactive oxygen species that responsible for these changes [74]. The present results showed that the expression of COI was significantly decreased compared to the expression of ND1in all groups. After exposure to the sub lethal concentration LC10 or LC25 of papain, the levels of cytochrome oxidase subunit I (COI) and (ND1) gene expression were significantly decreased than the control group in concentration- dependent manner. The toxicity of papain might be due to its nature as a secondary metabolite that resulted in DNA damages [75, 76]. Similar results were reported by [77] who found that C. papaya extract caused DNA damages in exposed B. alexandrina snails, including the changes in number, intensity and position of DNA bands and reasoned these genotoxic to the presence of the secondary metabolites. Also, these results were in a good accordance with [59] who reported change in the level of DNA and RNA in ovotestis of L. acuminata snails after exposure to papain, they reasoned these damages to that papain could reduce RNA and protein content as it affected the protein bio synthesis at the transcriptional level. Also, [13] reported significant decreases in the expression level of COI and ND1 genes in B. alexandrina snails exposed to LC10 or LC25 concentrations of saponin. [33] reported that during the short-term tests, papain had the ability to induce lesions in DNA, which may lead to genotoxicity, cytotoxicity, or mutagenicity.
Conclusion
This study elucidates the molluscicidal, larvicidal and genotoxicological effects of papain on B. alexandrina snail, free larval stages of S. mansoni. And hence, Papain could play an important role in the control of the intermediate snail population and ultimately elimination of schistosomiasis and decrease its transmission. The effects of field applications and the toxicity of papain to additional species are being investigated further.
Data Availability
Any enquiries for additional information are available upon request from the corresponding author.
References
Ibrahim A, Nasr S (2019) Cytochrome oxidase Subunit and Internal Transcribed Spacer Molecular Revelation of infection with Fasciola Sp. in Field- Collected Lymnaea natalensis snails. J Egypt Soc Parasitol 49:357–364. https://doi.org/10.21608/jesp.2019.68141
WHO, Schistosomiasis (2022) accessed March 18, World Heal. Organ. Fact Sheet;Schistosomiasis (2022) 8 January 2022. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
dos Santos AJ, Lima SVMA, de Sousa AFL, Vasconcelos dos Santos A, de Santos IG, Bezerra Santos M, Feitosa VLC, dos Santos AD, Primão JCM, de Andrade D, Silva JRS (2023) Knowledge, attitude and practices towards the Prevention of Schistosomiasis Mansoni in an endemic area of Alagoas, Northeast Brazil, Trop. Med Infect Dis 8:34. https://doi.org/10.3390/TROPICALMED8010034/S1
Sheir SK, Elmongy EI, Mohamad AH, Osman GY, Bendary SE, Ahmed AAS, Binsuwaidan R (2023) El-Sayed, Molluscicidal and Larvicidal Potency of N-Heterocylic analogs against Biomophalaria Alexandrina Snails and Schistosoma mansoni Larval stages. Pharmaceutics 15. https://doi.org/10.3390/PHARMACEUTICS15041200/S1
WHO, World Health Organization: Schistosomiasis (2023) https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed March 24, 2023)
Abdel-Tawab H, Ibrahim AM, Hussein T, Mohamed F (2022) Mechanism of action and toxicological evaluation of engineered layered double hydroxide nanomaterials in Biomphalaria alexandrina snails. Environ Sci Pollut Res Int 29. https://doi.org/10.1007/S11356-021-16332-W
Mansour SM, Ibrahim AM (2023) Differentiation between Bulinus Truncatus and Bulinus hexaploidus by morphological characters, chromosomal study and compatibility with Schistosoma haematobium. Exp Parasitol 108502. https://doi.org/10.1016/J.EXPPARA.2023.108502
Ibrahim AM, Morad MY, El-Khadragy MF, Hammam OA (2022) The antioxidant and anti-inflammatory effects of Eremina desertorum snail mucin on experimentally-induced intestinal inflammation and testicular damage. Biosci Rep 42:BSR20221020
Khalil RG, Ibrahim AM, Bakery HH, Juglone (2022) A novel immunomodulatory, antifibrotic, and schistosomicidal agent to ameliorate liver damage in murine schistosomiasis mansoni. Int Immunopharmacol 113:109415. https://doi.org/10.1016/j.intimp.2022.109415
Ibrahim AM, Abdel-Tawab H, Hussein T (2019) Pentoxifylline and/or praziquantel reduce murine schistosomiasis mansoni histopathology via amelioration of liver functions. Egypt J Aquat Biol Fish 23:121–133. https://doi.org/10.21608/ejabf.2019.67229
Assefa A, Erko B, Gundersen SG, Medhin G, Berhe N (2021) Current status of Schistosoma mansoni infection among previously treated rural communities in the Abbey and Didessa Valleys, Western Ethiopia: implications for sustainable control. PLoS ONE 16. https://doi.org/10.1371/JOURNAL.PONE.0247312
Ibrahim AM, Abdel-Tawab H (2020) Cystoseira barbata Marine algae have a molluscicidal activity against Biomphalaria alexandrina snails supported by scanning electron microscopy, hematological and histopathological alterations, and larvicidal activity against the infective stages of Schis, Biologia (Bratisl). 1–10
Ibrahim AM, El-karim RMG, Ali RE, Nasr SM (2023) Toxicological effects of saponin on the free larval stages of Schistosoma mansoni, infection rate, some biochemical and molecular parameters of Biomphalaria alexandrina snails. Pestic Biochem Physiol 191:105357. https://doi.org/10.1016/j.pestbp.2023.105357
Abdel-Ghaffar F, Ahmed AK, Bakry F, Rabei I, Ibrahim A (2016) The impact of three herbicides on Biological and histological aspects of Biomphalaria Alexandrina, intermediate host of Schistosoma mansoni. Malacologia 59:197–210. https://doi.org/10.4002/040.059.0201
Ibrahim AM, Bakry FA (2019) Assessment of the molluscicidal impact of extracted chlorophyllin on some biochemical parameters in the nervous tissue and histological changes in Biomphalaria Alexandrina and Lymnaea natalensis snails. Invertebr Neurosci 19. https://doi.org/10.1007/s10158-019-0230-1
Rocha-Filho CAA, Albuquerque LP, Silva LRS, Silva PCB, Coelho LCBB, Navarro DMAF, Albuquerque MCPA, Melo AMMA, Napoleão TH (2015) Pontual, others, Assessment of toxicity of Moringa oleifera flower extract to Biomphalaria glabrata, Schistosoma mansoni and Artemia salina. Chemosphere 132:188–192
El-Seedi HR, Khalifa SAM, Mohamed AH, Yosri N, Zhao C, El-Wakeil N, Attia NF, Xu B, AbdElhafez AER, Boskabady MH, Elseedy S, Efferth T, Verpoorte R (2022) Plant extracts and compounds for combating schistosomiasis. Phytochem Rev 2022:1–116. https://doi.org/10.1007/S11101-022-09836-X
Morad MY, El-Sayed H, El-Khadragy MF, Abdelsalam A, Ahmed EZ, Ibrahim AM (2023) Metabolomic Profiling, Antibacterial, and Molluscicidal Properties of the Medicinal Plants Calotropis procera and Atriplex halimus: In Silico Molecular Docking Study, Plants 2023, Vol. 12, Page 477 12 477. https://doi.org/10.3390/PLANTS12030477
Ibrahim AM, Ahmed AK, Bakry FA, Abdel-Ghaffar F (2018) Hematological, physiological and genotoxicological effects of Match 5% EC insecticide on Biomphalaria alexandrina snails. Ecotoxicol Environ Saf 147. https://doi.org/10.1016/j.ecoenv.2017.09.059
Augusto R, de Mello-Silva C (2018) Phytochemical molluscicides and schistosomiasis: what we know and what we still need to learn. Vet Sci 5:94. https://doi.org/10.3390/vetsci5040094
Ibrahim AM, Mohamed F, Al-Quraishy S, Abdel-Baki AAS, Abdel-Tawab H (2021) Green synthesis of Cerium oxide / Moringa oleifera seed extract nano-composite and its molluscicidsal activities against biomophalaria alexanderina. J King Saud Univ - Sci 33:101368. https://doi.org/10.1016/J.JKSUS.2021.101368
Muhsin MA, Wang X, Kabole FM, Zilabumba J, Yang K (2022) The indispensability of Snail Control for Accelerating Schistosomiasis Elimination: evidence from Zanzibar. Trop Med Infect Dis 7:347–347. https://doi.org/10.3390/TROPICALMED7110347
Ibrahim AM, Abdalla AM (2017) Impact of Moringa oleifera seed aqueous extract on some biological, biochemical, and histological aspects of Biomphalaria alexandrina snails. Environ Sci Pollut Res 24:28072–28078. https://doi.org/10.1007/s11356-017-0397-0
Ibrahim AM, Ghazy M, El-sayed H, El-hameed RMA, Khalil RG, Korany SM, Aloufi AS, Hammam OA, Morad MY (2023) and In Silico Molecular Docking Study of Fungal-Mediated Selenium Oxide Nanoparticles on Biomphalaria alexandrina (Ehrenberg, 1831) Snails
Zulfiqar S, Abidi ALI, Shakir HA, Ali SS (2017) Preliminary screening of some plants of Punjab. Pakistan Phytochemicals 63:179–191
Rashidian G, Shahin K, Elshopakey GE, Mahboub HH, Fahim A, Elabd H, Prokić MD, Faggio C (2022) The Dietary effects of Nutmeg (Myristica fragrans) extract on growth, Hematological Parameters, immunity, antioxidant status, and Disease Resistance of Common Carp (Cyprinus carpio) against Aeromonas hydrophila. J Mar Sci Eng 10. https://doi.org/10.3390/JMSE10030325
Elabd H, Faggio C, Mahboub HH, Emam MA, Kamel S, El Kammar R, Abdelnaeim NS, Shaheen A, Tresnakova N, Matter A (2022) Mucuna pruriens seeds extract boosts growth, immunity, testicular histology, and expression of immune-related genes of mono-sex Nile tilapia (Oreochromisniloticus), Fish Shellfish Immunol. 127:672–680. https://doi.org/10.1016/J.FSI.2022.06.055
Ibrahim AM, Al AA, Hebat F, Dokmak AA (2023) Ovicidal, immunotoxic and endocrine disrupting effects of saponin on Bulinus Truncatus snails with special emphasize on the oxidative stress parameters, genotoxicological, and histopathological alterations. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-27668-w
Al-Obaidi MJ, Abbas AH, Hanoon AY, Ibrahim K (2018) Using of Thymus Vulgaris extracts to control the snail vector in Schistosomiasis (Part Ii). Iraqi J Agric Sci 49
Ibrahim AM, Ghoname SI (2018) Molluscicidal impacts of Anagallis arvensis aqueous extract on biological, hormonal, histological and molecular aspects of Biomphalaria alexandrina snails. Exp Parasitol 192:36–41. https://doi.org/10.1016/j.exppara.2018.07.014
Ibrahim AM, Sayed SSM (2021) Assessment of the molluscicidal activity of the methanolic seed extracts of Ziziphus spina-christi and Carica papaya on immunological and molecular aspects of Biomphalaria alexandrina snails. Aquac Res 52:2014–2024. https://doi.org/10.1111/ARE.15050
Saidi R, Khanous L, Khadim Allah S, Hamdi B, Ayadi A, Damak M, Hammami H, Mezghani-Jarraya R (2017) Antifungal, molluscicidal and larvicidal assessment of anemonin and Clematis flammula L. extracts against mollusc Galba truncatula, intermediate host of Fasciola hepatica in Tunisia, Asian Pac. J Trop Med 10:967–973. https://doi.org/10.1016/j.apjtm.2017.09.008
Silva CRD, Oliveira MBN, Motta ES, Almeida GSD, Varanda LL, De Pádula M, Leito AC, Caldeira-De-Arajo A (2010) Genotoxic and cytotoxic safety evaluation of Papain (Carica papaya L.) using in Vitro assays. J Biomed Biotechnol 2010. https://doi.org/10.1155/2010/197898
Mose MJ, Kutima H, Waihenya R, Yole D (2013) Evaluating the antischistosomal activity of crude extracts of Carica Papaya against Schistosoma Mansoni: the interplay of Cellular and Humoral Immunity. J Biomed Pharm Res 2
Ibrahim AM, Ghareeb MA (2020) Preliminary phytochemical screening, total phenolic content, in Vitro antioxidant and molluscicidal activities of the Methanolic Extract of Five Medicinal Plants on Biomphalaria Alexandrina Snails. J Herbs Spices Med Plants 26. https://doi.org/10.1080/10496475.2019.1666769
Udoh F, biology PU-P (2005) undefined Hepatotoxicity of the Methanol Extract of Carica papaya. (Paw-Paw) Seeds in Wistar Rats, Taylor Fr. Udoh, PB UdohPharmaceutical Biol. 2005•Taylor Fr. 43 (2005) 349–352. https://doi.org/10.1080/13880200590951810
El-Zalaki E (2021) Preparation and properties of Papain precipitated from fresh latex of Papaya fruits (Carica papaya), Alexandria J. Food Sci Technol 18:27–32. https://doi.org/10.21608/ajfs.2021.187130
Hanif F, Singh DK (2013) Binary combination of Carica papaya, Areca catechu and Myristica fragrans with piperonyl butoxide / MGK-264 against freshwater snail lymnaea acuminata. Trop Life Sci Res 24(2):1–11
Jaiswal P, Singh DK (2008) Molluscicidal activity of Carica papaya and Areca catechu against the freshwater snail Lymnaea Acuminata. Vet Parasitol 152:264–270. https://doi.org/10.1016/J.VETPAR.2007.12.033
Chernin E (1970) Behavioral responses of miracidia of Schistosoma mansoni and other trematodes to substances emitted by snails. J Parasitol 56:287–296. https://doi.org/10.2307/3277659
Ibrahim AM, Bakry FA (2019) Assessment of the molluscicidal impact of extracted chlorophyllin on some biochemical parameters in the nervous tissue and histological changes in Biomphalaria Alexandrina and Lymnaea natalensis snails. Invertebr Neurosci 19:7. https://doi.org/10.1007/s10158-019-0230-1
WHO, Report of the Scientific working Group on Plant Molluscicide & Guidelines for evaluation of plant molluscicides, Geneva: WHO,(TDR/SCHSWE (4)/83.3 (1983)
Mossalem HS, Ibrahim AM (2019) The ameliorative potential of the ethanol extract of the plant ocimum basilicum on biomphalaria alexandrina snails exposed to the insecticide bestacid. Egypt J Aquat Biol Fish 23. https://doi.org/10.21608/ejabf.2019.26691
Finney DJ (1971) Probit analysis (3rdedn.) Combrige University Press
Ibrahim AM, Saleh HA, Zayed K, Mahassen G (2021) Colchicum Ritchii flower: a new molluscicidal plant for Biomphalaria alexandrina snails and the infective stages of Schistosoma mansoni alexandrina snails and the infective stages of Schistosoma mansoni. Molluscan Res 0:1–9. https://doi.org/10.1080/13235818.2021.2003982
Théron A, Pages JR, Rognon A (1997) Schistosoma mansoni: distribution patterns of miracidia among Biomphalaria glabrata snail as related to host susceptibility and sporocyst regulatory processes. Exp Parasitol 85:1–9. https://doi.org/10.1006/EXPR.1996.4106
Théron A, Coustau C (2005) Are Biomphalaria snails resistant to Schistosoma mansoni? J Helminthol 79:187–191. https://doi.org/10.1079/JOH2005299
Mostafa OMS, El-Dafrawy SM (2011) Susceptibility of Biomphalaria spp. to infection with Schistosoma mansoni in sympatric and allopatric combinations with observations on the genetic variability between snails. Vet Parasitol 180:226–231. https://doi.org/10.1016/j.vetpar.2011.03.019
Ritchie LS, Lopez VA, Cora JM (1974) Prolonged applications of an organotin against Biomphalaria glabrata and Schistosoma mansoni. Molluscicides Schistosomiasis Control 77:77–88
Ibrahim AM, Sayed DA (2019) Toxicological impact of oxyfluorfen 24% herbicide on the reproductive system, antioxidant enzymes, and endocrine disruption of Biomphalaria alexandrina (Ehrenberg, 1831) snails, Environ. Sci Pollut Res 26:7960–7968. https://doi.org/10.1007/s11356-019-04251-w
Sadek G, Harba N, Faheem M (2019) In vitro assessment of Schistosoma mansoni cercaricidal activities of Solanum nigrum and Callistemon Citrinius leaves extracts and cercarial genetic changes by RAPD-PCR, Parasitol. United J 12:18–25. https://doi.org/10.21608/puj.2019.7468.1029
Morad MY, El-Sayed H, Elhenawy AA, Korany SM, Alofi AS, Ibrahim AM (2022) Myco-Synthesized Molluscicidal and Larvicidal Selenium Nanoparticles: A New Strategy to Control Biomphalaria alexandrina Snails and Larvae of Schistosoma mansoni with an In Silico Study on Induced Oxidative Stress, J. Fungi Vol. 8, Page 262 8 (2022) 262. https://doi.org/10.3390/JOF8030262
Fatimah G, Rahayu R (2020) Hasmiwati, Lethal concentration (LC 50, 90, and 98) and lethal time (LT 50, 90, and 98) at various temephos concentrations of Aedes aegypti L. larvae. Int J Mosq Res 7:01–03. http://www.dipterajournal.com
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3. https://doi.org/10.1186/GB-2002-3-7-RESEARCH0034
Sokal RR, Rohlf FJ, Introduction to, Biostatistics WHH, Free (1973) Co. 253–280. https://scholar.google.com/scholar?hl=en%26as_sdt=0%252C5%26q=Sokal%2BRR%252C%2Band%2BRohlf%2BFJ%252C%2B%25281995%2529%2BIntroduction%2Bto%2B%2BBiostatistics.%2BW.H.%2BFreeman%2Band%2BCo.%252C%2BSan%2BFrancisco.%252C%2Bpp%253A%2B271-%2B273%26btnG= (accessed June 18, 2023)
Southwood KE (1978) Substantive Theory and Statistical Interaction: five models. 83:1154–1203. https://doi.org/10.1086/226678
Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A (2019) Ann Card Anaesth 22:67. https://doi.org/10.4103/ACA.ACA_157_18. Descriptive Statistics and Normality Tests for Statistical Data
Jaiswal P, Singh VK, Singh DK (2008) Enzyme inhibition by molluscicidal component of Areca catechu and Carica papaya in the nervous tissue of vector snail Lymnaea Acuminata. Pestic Biochem Physiol 92:164–168. https://doi.org/10.1016/j.pestbp.2008.07.011
Srivastava AK (2018) Role of abiotic factors and Plant Molluscicides against Reproduction of snail. Open Access J Biomed Eng Biosci 2:182–184. https://doi.org/10.32474/oajbeb.2018.02.000140
Adetunji VO, Salawu OT (2010) Efficacy of ethanolic leaf extracts of Carica papaya and Terminalia catappa as molluscicides against the snail intermediate hosts of schistosomiasis. J Med Plants Res 4:2348–2352. https://doi.org/10.5897/JMPR10.468
Upadhyay A, Singh VK, Singh DK (2013) Caracterização do componente moluscicida das folhas da Moringa oleifera E das frutas da Momordica charantia e seus modos de ação sobre o caramujo Lymnaea Acuminata. Rev Inst Med Trop Sao Paulo 55:251–259. https://doi.org/10.1590/S0036-46652013000400006
Loker ES, Bayne CJ (1986) Immunity to tremotode larvae in the snail Biomphalaria., Symp. Zool. Soc. Lond. 199–220. https://scholar.google.com.eg/scholar?hl=en%26as_sdt=0%25252C5%26q=Immunity%2Bto%2Btrematode%2Blarvae%2Bin%2Bthe%2Bsnail%2BBiomphalaria%26btnG= (accessed July 2, 2018)
van der Knaap WPW, Loker ES (1990) Immune mechanisms in trematode-snail interactions. Parasitol Today 6:175–182. https://doi.org/10.1016/0169-4758(90)90349-9
El-Sayed K, El-Dafrawy S, El-Din S A (1999) Influence of Schistosoma mansoni infection on Biomphalaria alexandrina snails under laboratory conditions. J Zool Egypt 33:343–354. https://scholar.google.com/scholar?hl=en%26as_sdt=0%252C5%26q=El-Sayed%2BK%252C%2BEl-Dafrawy%2BS%252C%2BSharaf%2BEl-Din%2BA%252C%2B%25281999%2529.%2BInfluence%2Bof%2BSchistosoma%2Bmansoni%2Binfection%2Bon%2BBiomphalaria%2Balexandrina%2Bsnails%2Bunder%2Blaboratory%2Bconditions.%2BJ.%2BZool.%2BEgypt.%252C%2B33%253A%02B343-%2B3 (accessed June 18, 2023)
Ibrahim AM, Hammam OA, EL-dafrawy SM (2018) Infected freshwater snails, biomphalaria alexandrina, the intermediate host of schistosoma mansoni. J Egypt Soc Parasitol 48:503–507. https://doi.org/10.21608/JESP.2018.76544
El-Dafrawy SM, El Din AT, Bakry FA, Dosouky AM (2001) Effect of double infection with Schistosoma mansoni and Echinostoma liei on some physiological parameters of Biomphalaria Alexandrina. J Egypt Soc Parasitol 31:433–447. https://pubmed.ncbi.nlm.nih.gov/11478444/ (accessed June 18, 2023)
Tallima H, El Dahab MA, El R, Ridi (2019) Role of T lymphocytes and papain enzymatic activity in the protection induced by the cysteine protease against Schistosoma mansoni in mice. J Adv Res 17:73–84. https://doi.org/10.1016/J.JARE.2018.12.008
Morley NJ, Crane M, Lewis JW (2003) Cadmium toxicity and snail–digenean interactions in a population of Lymnaea spp. J Helminthol 77:49–55. https://doi.org/10.1079/joh2002148
Bahgat DM, Mossalem HS, Al-Sayed E, Eldahshan OA, Singab ANB (2018) Abu El Einin, influence of saponin fraction from albizia anthelmintica on biomphalaria alexandrina snail; the intermediate host of schistosoma mansoni in Egypt. Egypt J Aquat Biol Fish 22:231–240. https://doi.org/10.21608/ejabf.2018.20446
Ibrahim AM, Ahmed AK, Hammam OA, Abdel-Ghaffar F (2022) Immunotoxical, neurotoxical, histopathological and immunohistopathological alterations of Nerium oleander and Tecoma stans methanolic extract on Biomphalaria alexandrina snails. Acta Trop 230:106405. https://doi.org/10.1016/j.actatropica.2022.106405
Wang H, Cai WM, Wang WX, Yang JM (2006) Molluscicidal activity of Nerium Indicum Mill, Pterocarya stenoptera DC, and Rumex Japonicum Houtt on Oncomelania Hupensis. Biomed Environ Sci 19:245–248. https://www.researchgate.net/publication/6750269_Molluscicidal_Activity_of_Nerium_indicum_Mill_Pterocarya_stenoptera_DC_and_Rumex_japonicum_Houtt_on_Oncomelania_hupensis accessed March 10, 2022
Pereira ASA, Cavalcanti NL, Nascimento GAF, Nascimento-Silva JLG, Padilha RJR, Viegas LFW, Alves LC, Lima-Filho JL, Chaves MEC (2013) Morphological and morphometric study of cercariae and adult worms of Schistosoma mansoni (SLM strain) isolated from infected mice. Parasitol Res 112:1087–1096. https://doi.org/10.1007/s00436-012-3235-9
Jiang N, Naz S, Ma Y, Ullah Q, Khan MZ, Wang J, Lu X, Luosang D-Z, Tabassum S, Chatha AMM, Basang W-D (2023) An overview of Comet Assay Application for detecting DNA damage in aquatic animals. Agriculture 13:623. https://doi.org/10.3390/agriculture13030623
Xu D, Li C, Wen Y, Liu W (2013) Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ Pollut 174:121–127. https://doi.org/10.1016/j.envpol.2012.10.030
Silva YRR, Silva LD, Rocha TL, Dos Santos DB, Bezerra JCB, Machado KB, DE PAULA JAM, Amaral VCS (2020) Molluscicidal activity of Persea americana Mill. (Lauraceae) stem bark ethanolic extract against the snail Biomphalaria glabrata (say, 1818): a novel plant-derived molluscicide? Acad Bras Cienc 92:1–16. https://doi.org/10.1590/0001-3765202020200715
Ross IA (2003) Persea americana, Med. Plants World 383–392. https://doi.org/10.1007/978-1-59259-365-1_21
Ibrahim AM, Sayed SSM (2020) Assessment of the molluscicidal activity of the methanolic seed extracts of Ziziphus spina-christi and Carica papaya on immunological and molecular aspects of Biomphalaria alexandrina snails. Aquac Res 52:2014–2024. https://doi.org/10.1111/are.15050
Funding
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
The Research Ethics Committee (REC) at Theodor Bilharz Research Institute (TBRI) has reviewed the study protocol of the research work and found that the research work is exempted from review as the research work does not involve human or experimental animals. TBRI-REC operates in a manner consistent with National Institutes of Health (NIH) guide for care and use of laboratory animals (eighth edition).
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A.M., Nasr, S.M. Evaluation of the Effects of Papain on Schistosoma mansoni: Miracidial Infection Capacity, Infection Prevalence, Cercarial Shedding and Molecular Changes in Biomphalaria alexandrina. Acta Parasit. (2024). https://doi.org/10.1007/s11686-024-00898-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11686-024-00898-9