Abstract
Schistosomiasis seriously affects human health in tropical regions. Its prevention is more important than treatment, raising the need for effective control methods. Recently, the role of nanomaterials in medical science has been growing. The present study aimed to evaluate the potential effects of silver (Ag) and gold (Au) nanoparticles (NPs) on Biomphalaria alexandrina snails and Schistosoma mansoni cercariae in vitro and to assess their effects on the infectivity of cercariae in vivo. The in vitro study proved that Ag and Au NPs were effective in killing B. alexandrina snails, with 30 μg/ml Ag and 160 μg/ml Au causing 100% mortality. The LC50 of 9.68 μg/ml for Ag NPs and 133.7 μg/ml for Au NPs prevented snail infection with S. mansoni miracidia. Furthermore, Ag NPs at 50 μg/ml and Au NPs at 100 μg/ml increased the mortality of S. mansoni cercariae in a dose- and time-dependent manner, reaching 100% mortality after 1 h. The in vivo study found that Ag NPs prevented the occurrence of infection when cercariae were treated before the infection by either the tail immersion (TI) or subcutaneous (SC) route, as proven by parasitological parameters and by the absence of granuloma formation in hepatic tissue. Meanwhile, infection of mice by untreated cercariae followed by treatment with NPs 1 h post-infection (PI) caused a decrease in egg count/g intestine and egg count/g liver in the TI-infected group only. The oogram patterns and granuloma formation results were similar between infection control and the SC-infected group. On the other hand, Au NPs led to a decrease in total worm burden (TWB) in all tested groups, with a decrease in egg count/g intestine and egg count/g liver in TI-infected groups with either pre-treated or post-treated cercariae, in contrast to SC-infected groups. However, the oogram patterns and granuloma formation showed similar results to infection control. Ag and Au NPs have potential as molluscicides and cercaricides in vitro and can prevent or modulate the infectivity of cercariae in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is the second most prevalent parasitic disease in the world after malaria. It affects more than 260 million people and leads to 200,000 deaths per year (WHO 2015). There are approximately 12.7 million infected individuals in the Middle East and North Africa region, with approximately 7.2 million in Egypt (Hotez et al. 2012). The transmission has been greatly reduced in Egypt, where a non-integrated intervention strategy is still used and transmission of infection in high-risk areas in the Nile Delta remains uninterrupted. Therefore, improved, more comprehensive control strategies are still in need (Barakat et al. 2014).
Recently, nanoparticles (NPs) have been widely used in the biomedical sciences for facilitating antimicrobial drugs’ administration and minimizing their side effects. The NPs’ ultra-small size, large surface-area-to-mass ratio, and high reactivity add to their benefits (Zhang et al. 2010). Silver (Ag) and gold (Au) NPs are highly effective against bacteria, viruses, and eukaryotic microorganisms, as well as having the ability to destroy cancer cells (Gong et al. 2007; Kuo 2009).
Plant-synthesized Ag NPs are effective ovicides, larvicides, pupicides, adulticides, and oviposition deterrents against mosquito species of medical and veterinary importance (Jayaseelan et al. 2011; Haldar et al. 2013; Govindarajan et al. 2015; Lallawmawma et al. 2015; Dinesh et al. 2015; Chitra et al. 2015; Benelli 2016). Au NPs have also shown potential as insecticides (Teimouri et al. 2018). The effects of Au NPs on arthropod vectors, such as ticks, tsetse flies, tabanids, sandflies, and blackflies, and additive-related ecotoxicological assays are recorded (Benelli 2018).
NPs have potent antiparasitic effects by acting as a drug vehicle for conventionally used drugs, such as praziquantel in the treatment of schistosomiasis (Kolenyak-Santos et al. 2014) and doxycycline and ivermectin in the treatment of filariasis (Binnebose et al. 2015). Furthermore, NPs themselves can have antiparasitic action directly, as Ag, chitosan, and curcumin NPs have anti-giardia effects (Said et al. 2012), and Au NPs have an anti-neuroschistosomal effect in mice infected with Schistosoma mansoni (Dkhil et al. 2015a).
Only a few studies have investigated NPs as cercaricides or molluscicides. Ag NPs show a toxic effect on S. mansoni cercariae, inhibiting their penetration into linolenic acid-impregnated agar (King and Highashi 1992). They induce Schistosoma japonicum cercarial tail-shedding and agitated behavior and block cercarial infectivity (Cheng et al. 2013). Au NPs lead to a reduction in reproductive capacity and death of Biomphalaria alexandrina (El-Sayed and El-Sherbini 2006, El-Hommossany and El-Sherbibni 2011) and Bulinus truncatus snails (Ragheb 2009).
In light of the above, Ag and Au NPs can have a role in preventing schistosomiasis, so this study was carried out to evaluate the potential effects of Ag and Au NPs on B. alexandrina snails and S. mansoni cercariae in vitro and to assess their effects on the infectivity of cercariae in vivo.
Materials and methods
The study was done in Medical Malacology Laboratory and Animal House at Theodor Bilharz Research Institute (TBRI) Giza, Egypt. All reagents were purchased from Sigma Aldrich (USA) except Ag and Au NPs from Nanotech Company (Giza, Egypt).
In vitro study
Snail and parasite collection
Healthy B. alexandrina snails were collected from different water streams at Giza Governorate, Egypt, during the spring of 2016. Snails were cultivated according to Mossalem and Elenain (2014). S. mansoni miracidia were obtained from cleaned eggs extracted from the intestines of infected mice by hatching them in dechlorinated tap water (25 ± 1 °C) (Eissa et al. 2011). S. mansoni cercariae were obtained from experimentally infected B. alexandrina snails which were individually placed in 24-well culture plates containing 1 ml of distilled water (dH2O) followed by exposure to artificial light for 1 h. Emerged cercariae were observed under dissecting microscope and counted (Kiros et al. 2014).
Detection of molluscicidal rate
Serial dilutions from each drug were prepared in dH2O (Oteifa et al. 1975). For each concentration, 10 snails were used (each snail 6–8 mm in diameter) and this was repeated in triplicates. Each snail was exposed to each concentration for 24 h then allowed to recover for the next 24 h. The result was documented on the 3rd day; all were maintained at 25° ± 1 °C and pH 7.4. Control snails were maintained under the same experimental conditions in dechlorinated water (Litchfield and Wilcoxon 1949). The dose 100 μg/ml was chosen empirically as a starting dose; it showed a 100% lethal effect with Ag NPs while for Au NPs it was increased to 200 μg/ml to reach a 100% lethal effect. Snails were considered dead if they did not move and showed a discolored body retracted into the shell (Vijay 2010). Percentage of snail mortality was detected and the LC50 and LC90 values of each drug were determined.
Effect of Ag and Au NPs on snail infection with S. mansoni miracidia
B. alexandrina snails were exposed to the LC50 (9.68 μg/ml Ag and 133.7 μg/ml Au) followed by testing its susceptibility to infection with S. mansoni miracidia. Infection of the snails was done by exposing 50 snails to 500 miracidia maintained at room temperature for 3 weeks in a plastic container. Infected snails were used as control group (Massoud et al. 2004). Evaluation of the infection rate of snails was checked to start from the 3rd week post-infection (PI) to assess their infection. The snails were examined for cercarial shedding by the distribution of the snails in 24-well culture plate left in light for 1 h. Then the plate was examined under dissecting microscope to determine the infection rate of snails; by counting the number of snails which acquired infection and shed cercariae (El-Assal et al. 1997).
Testing Ag and Au NPs cercaricidal potential
Previously infected B. alexandrina snails with S. mansoni miracidia were exposed to light for 1 h to shed cercariae, which were distributed in petri dishes (100 cercariae in each dish) then exposed to a range of dilutions for each drug. The same number of cercariae was placed in new petri dishes containing dechlorinated water as a control group (Kiros et al. 2014). Serial dilutions of Ag NPs (50, 25, 10, 5, and 3 μg/ml) and of Au NPs (100, 75, 50, 25, 10, and 5 μg/ml) were tested in duplicates. The cercariae were observed under a dissecting microscope at successive intervals of 15, 30, 45, and 60 min. Cercariae were considered dead when they stopped movement sank down or showed a detached tail (Eissa et al. 2011). Percentage of cercarial mortality was detected and the LC50 and LC90 values of each drug were determined (Rug and Ruppel 2000). For examining the cercariae by transmission electron microscopy (TEM), a number of the treated cercariae (exposed to each of Ag and Au NPs) and control cercariae was put in eppendorfs and concentrated by centrifugation at 1400 rpm at 15 °C for 5 min, then sediments were fixed in a 10% glutaraldehyde. Ultrathin sections were examined by TEM after staining with uranyl acetate and lead citrate (Chisty et al. 2004).
In vivo study
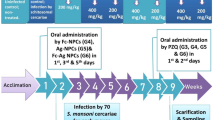
The study included 60 male Swiss Albino mice, 6–8 weeks old, weighing 20–25 g, purchased and housed at the Animal House, TBRI, after fulfilling all animal ethical considerations.
Routes of experimental infection
The LC90 for each drug (Ag-Au NPs), as estimated from the present cercaricidal study, was used. S. mansoni cercariae were obtained from laboratory-bred infected B. alexandrina snails. Two routes of experimental infection were applied where each mouse was infected with 60 ± 10 S. mansoni cercariae suspended in 0.2–0.3 ml diluents. Infection by tail immersion (TI) was done according to Tendler and Pinto (1981) and by subcutaneous injection (SC) according to Peters and Warren (1969). Control groups of mice were infected with cercariae suspended in dH2O. Experimental groups included two groups of mice infected with cercariae suspended in Ag and Au NPs (pre-treated cercariae) and another two groups infected with non-treated cercariae suspended in dH2O, then the infected mice were treated by injection of Ag and Au NPs 1 h PI targeting early stages of infection.
Infection of mice and experimental design
Control groups (C)
Group I (infection control): mice infected with non-treated cercariae, divided into Ia: six mice infected by SC injection, and Ib: six mice infected by TI technique.
Group II (negative control): six non-infected healthy mice.
Group III (drug control): six non-infected mice treated by nano drugs divided into IIIa: three mice treated with Ag NPs, and IIIb: three mice treated with Au NPs.
Experimental groups
Group IV: 12 mice infected with treated cercariae by TI, divided into IVa: 6 mice infected with Ag NPs, and IVb: 6 mice infected with Au NPs.
Group V: 12 mice infected with treated cercariae by SC route divided into Va: 6 mice infected with Ag NPs, and Vb: 6 mice infected with Au NPs.
Group VI: six mice infected with non-treated cercariae by SC route then after 1 h PI mice were injected with the NPs, they were divided into VIa: three mice injected with Ag NPs, and VIb: three mice injected with Au NPs.
Group VII: six mice infected with non-treated cercariae by TI then after 1 h PI mice were injected with the NPs, they were divided into VIIa: three mice treated with Ag NPs, and VIIb: three mice treated with Au NPs.
Evaluation of the antischistosomal activity of Ag and Au NPs
After 6 weeks, PI mice were decapitated and each mouse was subjected to two types of assessments. Parasitological assessment by total worm burden (TWB) (Smithers and Terry 1965), tissue egg count/g intestine and liver (Cheever 1968), and lastly oogram pattern (Pellegrino and Faria 1965) were done. Histopathological assessment by examination of liver tissue (Druray and Wallington 1980) was also performed.
Statistical analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version 20.0 to calculate the LC50 and LC90 by probit analysis. Analytical statistics included the following: a one-way analysis of variance (F) (ANOVA), Chi-square test of significance, and Post Hoc test. The confidence interval was set to 95% and the margin of error accepted was set to 5%. Probability (P value) was calculated (P value < 0.05 was considered significant, P value < 0.001 was considered as highly significant, and P value > 0.05 was considered insignificant) (P1: comparative performance between Ag and Au, P2: comparative performance between Ag and control, and P3: comparative performance between Au and control).
Results
In the in vitro study, the molluscicide assay revealed that Ag NPs at concentrations of 100, 50, 40, and 30 μg/ml led to death of 100% of B. alexandrina snails, while at concentrations of 25, 10, 5, and 3 μg/ml, they led to 80, 60, 40, and 20% snail death, respectively. Au NPs at concentrations of 200, 180, and 160 μg/ml led to the death of 100% of snails, while at concentrations of 140, 120, and 100 μg/ml, they led to 60, 20, and 0% snail death, respectively. The LC50 of Ag NPs was 9.68 μg/ml, and the LC90 was 24.75 μg/ml, while for Au NPs, the LC50 was 133.7 μg/ml and LC90 was 150.7 μg/ml. Ag NPs showed a highly effective molluscicidal potential, even at lower concentrations than Au NPs, albeit with no statistically significant difference (P > 0.05) between the effects of Ag and Au NPs (P = 0.192). Moreover, there was a highly statistically significant difference (P < 0.001) when comparing Ag or Au NPs with the control group, demonstrating their significant molluscicidal potential (Table 1, Fig. 1).
The effect of the LC50 of each of Ag and Au NPs on B. alexandrina snails revealed an absence of cercarial shedding in comparison to the control ones.
The cercariae showed changes in behavioral pattern, such as swimming and contraction, and morphological alterations, such as tail shedding (Fig. 2). Ag NPs showed a cercaricidal potency of 100% lethality at all time intervals from 30 min up to 60 min with concentrations of 50, 25, and 10 μg/ml. Au NPs showed a cercaricidal potency of 100% lethality at 60 min with concentrations 100, 75, and 50 μg/ml and at 45 min with concentrations 100 and 75 μg/ml. The mortality rate increased with the increase in time of exposure. The dilution titer continued to 3 μg/ml for Ag and 5 μg/ml for Au NPs, where they reached 0% lethality (Fig. 3). The starting values of tabulation for statistical input were the concentrations showing different lethal effects. At all time intervals, Ag NPs at concentrations above 50 μg/ml were 100% lethal, whereas only Au NP concentrations above 100 μg/ml were 100% lethal at all time points. For evaluating the cercaricidal potency, the results of different concentrations at 15 min were chosen to calculate the LC50 and LC90 of each drug, as the best outcome is obtained from the shortest time with the minimal drug concentration. Ag NPs’ LC50 was 19.6 μg/ml, and their LC90 was 39.7 μg/ml, while Au NPs’ LC50 was 127 μg/ml and LC90 was 244 μg/ml. Ag NPs showed a highly effective cercaricidal potency with a lower range of concentrations than Au NPs (Fig. 3).
By TEM, cercariae exposed to Ag 10 μg/ml and Au 100 μg/ml showed progressive morphological (ultrastructural) changes at 30 and 45 min post-exposure, respectively, compared to negative-control cercariae. These concentrations were chosen at this time of exposure because they were 100% lethal (Fig. 4).
TEM showing changes in the cercariae of S. mansoni on exposing to Ag NPs and Au NPs. a, b Control cercariae showing intact corrugated tegument (T) with spines (arrow) and compact muscle layer (M) without cellular swelling or edema. c, d Cercariae treated with Ag NPs (10 μg/ml) 30 min after exposure showing; thinning of tegument (T) with focal loss of spines and edematous swelling of the muscle layer and deeper parenchyma (arrow). e–h Cercariae treated with Au NPs (100 μg/ml) 45 min after exposure showing; e loss of external glycocalyx leading to thinning of the tegument (T) with focal loss of spines and edematous swelling of the muscle layer (M) and deeper parenchyma; f migration of cytoplasmic granules (cg) towards the tegument (arrow); g, h tegumental thinning (T) leading to external protrusion with focal breach of continuity of the tegument and a severe degeneration leading to focal lysis of the tegument causing expulsion of sub-tegumental materials (arrow)
Assessing the effects of NPs in vivo using the parasitological parameters TWB, tissue egg count/g intestine, and tissue egg count/g liver with either route of infection, TI or SC, showed an overall stronger effect of Ag than Au NPs. The effect of Ag NPs was better when applied pre- than post-infection. There were significant outcomes among all treated groups and the control in all the experiments (Tables 2, 3, 4, 5, 6, and 7, Figs. 5, 6, and 7).
The oogram pattern in SC infection with treated cercariae showed a highly significantly different outcome when comparing Ag with Au NPs and Ag NPs with control groups (P < 0.001), though there were similar outcomes between Au NPs and control groups (P > 0.05). At 1 h PI, there was no significantly different outcome (P > 0.05) between the treated groups and the control (Table 8, Fig. 8). The oogram pattern in TI infection with treated cercariae showed a highly significant difference (P ≤ 0.001) between all groups in the mean numbers of immature, mature, and dead eggs (Fig. 9), except for a non-significantly different outcome (P > 0.05) between Au NPs and control groups in the mean number of dead eggs. At 1 h PI, there was no significantly different outcome (P > 0.05) between both drugs, whereas there was a significantly different outcome (P < 0.001) between each drug and the control, in the mean numbers of immature and mature eggs (Table 9, Fig. 10).
In histopathological examination, only in the Ag NP-treated cercariae group were normal hepatic architecture and histological patterns seen; otherwise, all other groups showed abnormal changes (Fig. 11).
Histopathological changes in hepatic tissue in different groups 6 weeks PI (Hx and E stain ×400). a Positive control group showing severe inflammatory response in the liver indicated by inflammatory cellular infiltration, cytoplasmic vacuolation, degeneration of hepatocytes, dilated hepatic sinusoids and more Kupffer cells with intact ova in the center; b Ag NPs-treated cercariae showing normal hepatic architecture; c Au NPs-treated cercariae; d Ag NPs treated 1 h PI; e Au NPs treated 1 h PI. c–e showed the same picture as control group
Discussion
The in vitro study revealed that Ag and Au NPs have molluscicidal activity against B. alexandrina in a concentration-dependent manner. These results were in parallel to Abdel-Hamid and Mekawey (2014), who reported a dose-dependent molluscicidal effect of Ag NPs on B. alexandrina. Ali et al. (2014) demonstrated that Ag NPs were lethal to freshwater snail Lymnaea luteola which was in a dose- and time-dependent manner. Moreover, Bernot and Brandenburg (2013) and Goncalves et al. (2017), indicated that even very low concentrations of Ag NPs can affect all the vital rates of Physa acuta snails. In the current study, snails were examined after the 3rd week of infection to assess whether or not they had acquired infection after applying each drug individually. The study recorded the absence of cercarial shedding. This could be due to either changes induced by the drugs on snails, so that they could not acquire the infection, or that the drugs led to the death of the miracidia.
Regarding the cercaricidal potency, Ag and Au NPs were effective in a dose- and time-dependent manner. Both drugs induced agitated behavior followed by death, with the appearance of swollen cercariae. The study revealed that the effect of Ag NPs was far beyond Au NPs. By the end of the 1st hour, all tested concentrations of Ag NPs were lethal to all cercariae, while Au NPs concentrations below 50 μg/ml were not lethal. To our knowledge, this is the first study to assess Au NPs’ cercaricidal activity against S. mansoni.
King and Highashi (1992) reported that Ag ions at concentrations above 0.09 mM were toxic to S. mansoni cercariae, and concentrations below that were non-toxic but inhibited their penetration into the linolenic acid-impregnated agar. Cheng et al. (2013) reported similar results to our study when testing Ag NPs on S. japonicum cercariae and found that it rapidly induced cercarial tail-shedding, agitated behavior and a decrease in cercarial secretion. They used higher concentrations of Ag NPs which completely blocked cercarial infectivity after 30 min of exposure. Although their concentrations were higher than that employed in the present study, they showed nearly the same impact on cercariae, and this could be attributed to the different biochemical manufacturing processes of the NPs. The potent effect of Ag NPs on cercariae was attributed to Ag ions, which are continuously released in water suspension and can bind to the papillar sites on the cercarial surface thereby, inhibiting fatty acid-induced cercarial acetabular gland secretion (Liu and Hurt 2010).
The ultrastructural changes recorded in the present study were in the form of thinning of tegument with focal loss of spines, oedematous swelling of the muscle layer and deeper parenchyma with an external protrusion and focal breach of continuity of the tegument. These findings were similar to findings by Chisty et al. (2004), who used hinokitiol, a plant that potently protects the skin, against cercarial penetration, and Eissa et al. (2011), who used miltefosine, a promising schistosomicidal compound.
The in vivo study applied different routes of infection to exclude the impact of the route on the drug administration on its cercaricidal potency. In the present work, the timing of 1 h PI was chosen to assess drug potency at preventing early infection. A higher infection rate was reported by the TI route than SC injection through the whole study. In subcutaneously infected mice with S. mansoni cercariae, approximately 70% of the larval forms are destroyed before reaching maturity (Holanda et al. 1974 and Tendler et al. 1985), while by TI the loss is not more than 20% of the infecting cercariae during the skin phase (Mangold and Dean 1983). In this context, it seems that experimental infection by the TI route leads to greater infection than SC (Vilar and Pinto 2005).
By either route, the in vivo study showed that Ag NPs led to the absence of adult worms in mice infected by treated cercariae, while in mice treated 1 h PI they led only to a decrease in TWB. This is in accordance with Cheng et al. (2013), who used Ag NP-treated cercariae by SC infection and proved that at high concentrations, they caused an absence of adult worms and at lower concentrations caused a decrease in TWB. Those effects were attributed to Ag ions, which at high concentrations were lethal to cercariae but at low concentration led only to tail loss. Moreover, they mentioned that cercariae without tails can still penetrate into mice and develop into adult worms, in spite of the decreased infection rate.
Within all tested groups, Au NPs showed an overall decrease in the TWB, which can be interpreted by the finding of Dkhil et al. (2015b) that Au NPs has a strong ability to scavenge free radicals.
The effect of Ag and Au NPs on tissue egg load/g intestine and tissue egg load/g liver was assessed, as an important parameter for both the magnitude of infection and efficacy of antischistosomal drugs. A higher egg load/g intestine than that in liver was recorded in infection control mice at 6 weeks PI. Friedman (2004) stated that the highest number of eggs is deposited in the liver at the beginning of infection, and then the number of ova decreases with infection time, associated with a corresponding increase in the number of ova in the intestine. Ag NP-treated cercariae injected by either route showed an absence of eggs in the intestine and liver, which highlights the potency of the drug. This finding is in line with Cheng et al. (2013).
By TI, Au NPs showed a significant decrease in the count of eggs/g intestine and eggs/g liver compared to infection control in all groups of infected mice. The effect of NPs of decreasing the number of ova in hepatic tissue of infected mice can be attributed to being an antioxidant (Abdallahi et al. 2001). Selenium NPs increase the level of glutathione and reduce the levels of nitrite/nitrate and malondialdehyde (Dkhil et al. 2016).
In the present study by either route, no eggs were detected in mice infected with Ag NP-treated cercariae. This was due to the absence of adult worms in these mice, with a subsequent absence of eggs. However, post-treated Ag NP groups and all groups of Au NPs (pre-treated and post-treated) showed similar results to infection control. The different outcomes from previous studies may be attributed to the longer study durations or repeated dosing schedules of the tested drugs (Cancado et al. 1965; Farid et al. 2013; Abaza et al. 2013).
Histopathological examination of the liver of mice subcutaneously infected with Ag NP-treated cercariae showed no granuloma formation, confirming the earlier results in the current work and verifying the drug’s potency. In contrast, the rest of the groups (Ag treated 1 h PI and all groups of Au) showed a similar histopathological picture to that of infection control as a sequel to the presence of eggs. This was contrary to Dkhil et al. (2015a, b), who used Ag NPs twice, on days 46 and 49 PI, and reported a decrease in granuloma diameter and to earlier studies that showed that Au NPs improved the histopathological changes in the spleen and brain from mice infected with S. mansoni (Dkhil et al. 2017). Again, this different outcome may be attributed to the different intervals in each study.
Conclusions
The present study clarified the in vitro role of Ag and Au NPs on S. mansoni as molluscicides and cercaricides and how they can prevent or modulate the infectivity of cercariae in vivo, eliciting their preventive role against schistosomiasis. The greater efficacy of Ag NPs than Au was highlighted. The applicability of these NPs in the field as preventive ointments or lotions is needed over longer intervals to see the potential of these NPs as chemotherapeutics or preventive drugs. More studies are required to track the different mechanisms of action of each NP, as well as using different ranges of concentrations. Further studies applying different sizes and shapes of NPs should be comparatively evaluated to assess whether different manufacturing criteria will alter the response.
References
Abaza SA, El-Moamly AA, Ismail OA, Alabbassy MM (2013) Cysteine protease inhibitors (phenyl vinyl sulfone and valproic acid) in the treatment of schistosomiasis mansoni-infected mice: an experimental study to evaluate their role in comparison to praziquantel. PUJ 6(1):99–108
Abdallahi OM, Bensalem H, Diagana M, De Reggi M, Gharib B (2001) Inhibition of nitric oxide synthase activity reduces liver injury in murine schistosomiasis. Parasitology 122 (3):309–315
Abdel-Hamid H, Mekawey AA (2014) Biological and hematological responses of Biomphalaria alexandrina to mycobiosynthsis silver nanoparticles. J Egypt Soc Parasitol 44(3):627–637
Ali D, Yadav PG, Kumar S, Ali H, Alarifi S, Harrath AH (2014) Sensitivity of freshwater pulmonate snail Lymnaea luteola L., to silver nanoparticles. Chemosphere 104:134–140
Barakat R, El Morshedy H, Farghaly A (2014) Human schistosomiasis in the Middle East and North Africa region. In MA McDowell and S Rafati (eds.), Neglected tropical diseases—Middle East and North Africa. Springer, Vienna, pp 23–57
Benelli G (2016) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res 115:23–34
Benelli G (2018) Gold nanoparticles—against parasites and insect vectors. Acta Trop 178:73–80. https://doi.org/10.1016/j..2017.10.021
Bernot RJ, Brandenburg M (2013) Freshwater snail vital rates affected by non-lethal concentrations of silver nanoparticles. Hydrobiologia 714:25–34
Binnebose AM, Haughney SL, Martin R, Imerman PM, Narasimhan B, Bellaire BH (2015) Polyanhydride nanoparticle delivery platform dramatically enhances killing of filarial worms. PLoS Negl Trop Dis 9(10):e0004173. https://doi.org/10.1371/journal.pntd.0004173.e-Collection2015
Cancado JR, Da Cunha AS, Carvalho DG, Cambraia JNS (1965) Evaluation of the treatment of human Schistosoma mansoni infection by the quantitative oogram technique. Bull Org Mond Sant Bull Wld Hlth Org 33: 557–566
Cheever AW (1968) Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull WHO 39:328–331
Cheng Y, Chen X, Song W, Kong Z, Li P, Liu Y (2013) Contribution of silver ions to the inhibition of infectivity of Schistosoma japonicum cercariae caused by silver nanoparticles. Parasitology 140:617–625
Chisty MM, Nargis M, Inaba T, Ishita K, Osanai A, Kamiya H (2004) Transmission Electron microscopy of Schistosoma mansoni cercariae treated with Hinokitiol (β-thujaplicin), a compound for potential skin application against cercarial penetration. Tohoku J Exp Med 202(1):63–67
Chitra G, Balasubramani G, Ramkumar R, Sowmiya R, Perumal P (2015) Mukia maderaspatana (Cucurbitaceae) extract-mediated synthesis of silver nanoparticles to control Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Parasitol Res 114:1407–1415
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res 114:1519–1529
Dkhil MA, Bauomy AA, Diab MS, Wahab R, Delic D, Al-Quraishy S (2015a) Impact of gold nanoparticles on brain of mice infected with Schistosoma mansoni. Parasitol Res 114(10):3711–3719
Dkhil MA, Diab MS, Bauomy AA, Al-Quraishy S (2015b) Antioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasis. Int J Nanomedicine 10:7467–7475
Dkhil MA, Bauomy AA, Diab MSM, Al-Quraishy S (2016) Protective role of selenium nanoparticles against Schistosoma mansoni induced hepatic injury in mice. Biomed Res 27(1):214–219
Dkhil MA, Khalil MF, Diab MS, Bauomy AA, Al-Quraishy S (2017) Effect of gold nanoparticles on mice splenomegaly induced by schistosomiasis mansoni. Saudi J Biol Sci 24(6):1418–1423
Druray RA, Wallington EA (1980) Carletonʼs histological technique. 5th edition Oxford University Press, Oxford New York pp: 195–210
Eissa M, Bardicy S, Tadros T (2011) Bioactivity of miltefosine against aquatic stages of Schistosoma mansoni Schistosoma haematobium and their snail hosts supported by scanning electron microscopy. Parasit Vectors 73:4–11
El-Assal FM, Shoukry NM, Soliman GN, Mansour NS (1997) Infection of laboratory-bred Biomphalaria alexandrina from Giza and Alexandria governorates with Schistosoma mansoni from Giza in relation to snail size and number of penetrated miracidia. J Egypt Soc Parasitol 27(3):739–754
El-Hommossany K, El-Sherbibni SA (2011) Impact of the photosensitizers hematoporphyrin coated gold nanoparticles on Biomphalaria alexandrina snails. Open J Animal Sci 1(2):54–60
El-Sayed KA, El-Sherbini SA (2006) Impact of 3ematoporphyrin and different laser sources on Biomphalaria alexandrina snails and their infection with Schistosoma mansoni. J Biol Chem 1:319–340
Farid A, Ismail A, Rabee I, Zalat R, El Amir A (2013) Study of the efficacy of cysteine protease inhibitors alone or combined with praziquantel as chemotherapy for mice schistosomiasis mansoni. Int J Med Hl Biomed Bioeng Pharma Eng 7(12):849–856
Friedman SL (2004) Mechanism of disease: mechanism of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1:98–105
Goncalves SF, Pavlaki MD, Lopes R, Hammes J, Gallego-urrea JA, Hassellöv M, Jurkschat K, Crossley A, Loureiro S (2017) Effects of silver nanoparticles on the freshwater snail Physa acuta: the role of test media and snails’ life cycle stage. Environ Toxicol Chem 36(1):243–253
Gong P, Li H, He X, Wang K, Hu J, Tan W (2007) Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology 18:604–611
Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Mehlhorn H, Barnard DR, Benelli G (2015) Novel synthesis of silver nanoparticles using Bauhinia variegata: a recent eco-friendly approach for mosquito control. Parasitol Res 115:723–733. https://doi.org/10.1007/s00436-015-4794-3
Haldar KM, Haldar B, Chandra G (2013) Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of Putranjiva, Drypetes roxburghii (wall.). Parasitol Res 112:1451–1459
Holanda JC, Pellegrino J, Gazzinelli G (1974) Infection of mice with cercariae and schistosomula of Schistosoma mansoni by intravenous and subcutaneous route. Rev Inst Med trop São Paulo 16:132–134
Hotez PJ, Savioli L, Fenwick A (2012) Neglected tropical diseases of the Middle East and North Africa: review of their prevalence distribution and opportunities of control. PLoS Negl Trop Dis 6(2):e1475
Jayaseelan C, Rahuman AA, Rajakumar G, Vishnu Kirthi A, Santhoshkumar T, Marimuthu S, Bagavan A, Kamaraj C, Zahir AA, Elango G (2011) Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifoliamiers. Parasitol Res 109:185–194
King CL, Highashi GI (1992) Schistosoma mansoni: silver ion (Ag+) stimulates and reversibly inhibits lipid-induced cercarial penetration. Exp Parasitol 75:31–39
Kiros G, Erko B, Giday M, Mekonnen Y (2014) Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes 7:220
Kolenyak-Santos F, Garnero C, Oliveira RN, Souza ALR, Chorilli M, Allegretti MRL, Longhi MV, Chaud MV, Gremi MPD (2014) Nanostructured lipid carriers as a strategy to improve the in vitro schistosomiasis activity of praziquantel. J Nanosci Nanotechnol 14:1–12
Kuo WS (2009) Antimicrobial gold nanorods with dual-modality photodynamic inactivation and hyperthermia. Chem Commun (32):4853–4855
Lallawmawma H, Sathishkumar G, Sarathbabu S, Ghatak S, Sivaramakrishnan S, Gurusubramanian G, Kumar NS (2015) Synthesis of silver and gold nanoparticles using Jasminum nervosum leaf extract and its larvicidal activity against filarial and arboviral vector Culex quinquefasciatus Say (Diptera: Culicidae). Environ Sci Pollut Res Int 22:17753–17768. https://doi.org/10.1007/s11356-015-5001-x
Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating concentration-effect experiments. J Pharmacol Exp Ther 96:99–113
Liu J, Hurt RH (2010) Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci and Technol 44:2169–2175
Mangold BL, Dean DA (1983) Autoradiographic analysis of Schistosoma mansoni migration from skin to lungs in naïve mice. Evidence that most attrition occurs after the skin phase. Am J Trop Med Hyg 32:785–789
Massoud A, Metwally DM, Khalifa KE, Habib FS (2004) Compatibility of Biomphalaria alexandrina snails to infection with Schistosoma mansoni after exposure to sublethal concentrations of Myrrh. J Egypt Soc Parasitol 34(3):995–1008
Mossalem HS, Elenain GL (2014) Evaluation of the pesticide emamectin and methanol extract of wheat bran against Biomphalaria alexandrina snails, their hemocytes and their infection with Schistosoma mansoni. AFSci Asian Online J 1(1):5–10
Oteifa BA, Mousa AH, Abou El-Hassan AA, Mohamed AM, El-Eman MA (1975) Effect of certain insecticides in the control of the freshwater snails, Biomphalaria alexandrina and Bulinus truncatus. Egypt J Bilh 2(2):221–243
Pellegrino J, Faria J (1965) The oogram method for the screening of drugs in schistosomiasis mansoni. Am J Trop Med Hyg 14:363–369
Peters PA, Warren KS (1969) A rapid method of infecting mice and other laboratory animals with Schistosoma mansoni: subcutaneous injection. J Parasitol 55(3):558
Ragheb M (2009) Histological studies on the effect of gold nanoparticles on Schistosoma haematobium intermediate host (Bulinus truncatus snails). MSc. Thesis, Zoology Department, Faculty of Science. Cairo University, Cairo
Rug M, Ruppel A (2000) Toxic activities of the plant Jatropha curcas against intermediate snail hosts and larvae of schistosomes. Trop Med Int Hlth 5:423–430
Said DE, El Samad LM, Gohar YM (2012) Validity of silver, chitosan, and curcumin nanoparticles as anti-Giardia agents. Parasitol Res 111:545–554
Smithers SR, Terry RJ (1965) Infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of adult worms. Parasitol 55:695–700
Teimouri M, Khosravi-Nejad F, Attar F, Saboury AA, Kostova I, Benelli G, Falahati M (2018) Gold nanoparticles fabrication by plant extracts: synthesis, characterization, degradation of 4-nitrophenol from industrial wastewater, and insecticidal activity—a review. J Clean Prod 184:740–753
Tendler M, Pinto RM (1981) A simple device to immobilize mice for infection with Schistosoma mansoni cercariae. J Parasitol 67:583–584
Tendler M, Pinto RM, Côrtes M, Gebara G (1985) Schistosoma mansoni: comparative evaluation of different routes of experimental infection. Rev Inst Med Trop São Paulo 27:111–114
Vijay P (2010) Evaluation of molluscicidal activity of some Indian medicinal plants against Lymnaea acuminate. Int J Appl Biol Pharm Technol 1:308–311
Vilar MM, Pinto RM (2005) Reappraisal of experimental infections with cercariae and schistosomula of a brazilian strain of Schistosoma mansoni in mice. Braz J Biol 65(4):729–733
WHO (2015) Weekly epidemiological record, Geneva, Switzerland: WHO 90 (5): 25–32
Zhang L, Pornpattananangkul D, HU CMJ, Huang CM (2010) Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem 17:585–594
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was started after being approved by the Ethical Committee of Scientific Research at TBRI after fulfilling all animal ethical considerations.
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Section Editor: Christoph G. Grevelding
Rights and permissions
About this article
Cite this article
Moustafa, M.A., Mossalem, H.S., Sarhan, R.M. et al. The potential effects of silver and gold nanoparticles as molluscicides and cercaricides on Schistosoma mansoni. Parasitol Res 117, 3867–3880 (2018). https://doi.org/10.1007/s00436-018-6093-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-6093-2