Abstract
Biomphalaria alexandrina and Lymnaea natalensis snails are the intermediate hosts of schistosomiasis and fasciolosis. The aim of the present study is to evaluate the molluscicidal activity of chlorophyll extract as a photodynamic substance against these snails and how it affected its tissues and the biological system. Chlorophyllin was extracted from deep-frozen Moringa oleifera leaves, and then it was transformed into water-soluble chlorophyllin. The present results showed that it had a molluscicidal activity on B. alexandrina snails (LC50 17.6 mg/l; LC90 20.9 mg/l) and L. natalensis snails (LC50 4.3 mg/l; LC90 6.8 mg/l). Exposing B. alexandrina snails to the sublethal concentrations (LC0, LC10, and LC25) resulted in a significant reduction in their survival rates. Regarding its effect on biochemical parameters, chlorophyllin significantly reduced the acetylcholinesterase activity, protein content, and alkaline and acid phosphatase activity in B. alexandrina nervous tissue compared to the control group. Histopathological changes occurred in the digestive gland of treated B. alexandrina snails where cells lost their nuclei, vacuolated, degenerated, and ruptured, and the lumen increased. Photosynthesizing materials like chlorophyllin are new approaches to control schistosomiasis and fasciolosis in developing countries by affecting their intermediate host. These materials were cheap and environmentally safe to replace the synthetic molluscicides for snail control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis and fasciolosis are widespread neglected tropical parasitic diseases that caused veterinary and human diseases and transmitted by snails (Chaturvedi et al. 2017; WHO 2017). About 200 million people worldwide are infected with schistosomiasis (Mahmoud et al. 2013), and about 250 million sheep and 350 million cattle are at risk of fasciolosis worldwide (Beesley et al. 2017). Human fascioliasis is considered now as a zoonosis of major global and regional importance (Soliman 2008) as it is affecting nearly 50 million people worldwide (Rahman et al. 2017).
Freshwater snails act as the intermediate hosts for a huge number of trematode parasites in humans and animals (Lee et al. 2017; Chontananarth et al. 2017). Freshwater snails of Biomphalaria sp. are the intermediate hosts of Schistosoma mansoni in Egypt (Ibrahim and Abdalla 2017), and Lymnaea sp. snails were the main snail host for the liver flukes, Fasciola hepatica or gigantica, which are widely distributed in Africa (Moema et al. 2008). Several strategies were used to control snail populations (Abd El-Ghany and Abd El-Ghany 2017). One of these preventive ways was by the use of the chemical molluscicides (Abdel-Ghaffar et al. 2016), but because they were poisonous to nontarget organisms and had high cost (WHO 2014), it stimulated the interest to find suitable natural molluscicides (Elsareh et al. 2016).
Plant-derived molluscicides are promising choices for controlling these snails (Kiros et al. 2014). Phytotherapy of snails by photodynamic material like chlorophyllin is a new approach to control schistosomiasis and fasciolosis in developing countries (Chaturvedi et al. 2017; Ragheb et al. 2018). The photodynamic substances are not toxic in darkness, but are activated by light. Upon reaction with oxygen, reactive singlet oxygen (ROS) is produced, which has highly cytotoxic effects (DeRosa 2002). ROS caused excessive oxidative stress in the cells, resulted in damage to cell membranes, proteins, DNA, and other cell structures. By simple chemical modifications, the hydrophobic chlorophyll can be made water-soluble in the form of chlorophyllin (Erzinger et al. 2011).
Water-soluble chlorophyllin exerts a pronounced photodynamic activity (Richter et al. 2014) that destroys parasites found in the aquatic ecosystems and acts as potent larvicides, cercaricides, and molluscicides against L. acuminate (Kumar and Singh 2016; Chaturvedi et al. 2017). Wohllebe et al. (2009) reported that water-soluble chlorophyllin when used at low concentrations was able to kill mosquito larvae and other small animals in the water body within a few hours under exposure of solar radiation. Mahmoud et al. (2013) reported that chlorophyllin even at low concentrations was able to kill L. stagnalis, Biomphalaria spp., and Physa marmorata snails within a few hours under exposure of solar radiation. Besides, it had a killing effect by about 70% and 100% on the snails’ eggs and the newly hatched snails, respectively, after 3-h exposure to solar radiation.
The objectives of the present research are to assess the molluscicidal activity of chlorophyllin as a photodynamic substance against B. alexandrina and L. natalensis snails and then to study the effect of its sublethal concentrations on some biological and biochemical parameters in the nervous tissue and the histological changes in B. alexandrina snails.
Materials and methods
Experimental animal
Adult B. alexandrina snails (Ehrenberg, 1831) (9.45 ± 1.9 mm) and L. natalensis (3.35 ± 0.30 mm) were maintained in medical malacology laboratory at Theodor Bilharz Research Institute (TBRI) and maintained in plastic aquaria (16 × 23 × 9 cm). The aquaria were provided with dechlorinated aerated tap water (10 snails/l), at pH 7 and at room temperature (22–25 °C) and covered with glass plates. Oven-dried lettuce leaves, blue green algae (Nostoc muscorum), and TetraMin (Fish food) were used for feeding. Water in aquaria was changed weekly with a photoperiodicity of 12-h light/12-h dark. Pieces of polyethylene sheets were used for collecting egg masses. Dead snails were removed immediately to prevent the water from being contaminated by decaying tissue.
Preparation of extracted chlorophyllin
The extraction was done according to Wohllebe et al. (2012), where chlorophyll was isolated from Moringa oleifera leaves using 100% ethanol at 55 °C for about 2 h. Then, 1 mg CaCO3/gm plant material was added to prevent the transformation of chlorophyll into pheophytin (Rahmani and Csallany 1991). The extract was filtered using Whatman filter papers, and 50 ml petroleum benzene was added with shaking, so the chlorophyll moved into the lipophilic benzene phase and the two phases were separated in a separatory funnel. This crude chlorophyll could be further purified by two to three reprecipitations from ether–petroleum ether, and then about 1.0 ml methanolic KOH was added (to break the ester bond between the chlorophyllin and the phytol tail by saponification). After separation of the methanolic KOH phase and the benzene phase, most of the chlorophyllin was found in the methanolic KOH phase. The methanol was evaporated in darkness, and the chlorophyllin concentration was determined using a spectrophotometer (Erzinger et al. 2015). The extract was stored in a dark flask at room temperature.
Toxicity experiment
A fresh stock solution of 1000 ppm (1000 mg/l) was prepared from extracted chlorophyllin on the basis of V/V using dechlorinated tap water. B. alexandrina snails (5–7 mm) were subjected to a series of concentrations of extracted chlorophyllin (22, 20, 18, 15, and 13 mg/l) to calculate LC50 and LC90 at room temperature (22–25 °C) with a photoperiodicity of 12-h light/12-h dark. Another snail group of the same size (5–7 mm) was dipped in dechlorinated water only as the control. Three replicates were used, each of 10 snails for each concentration and the control group. The exposure period was 24 h; after that, the snails were removed from the experimental test solution and washed thoroughly with dechlorinated tap water and transferred to containers with fresh dechlorinated tap water for another 24 h of recovery, and then, the percentages of observed mortalities were recorded. No-observed-effect level (NOEL) is the greatest concentration or amount of a substance, found by experiment or observation, that causes no alteration of morphology, functional capacity, growth, development, or life span of the target organism distinguishable from those observed in normal (control) organisms of the same species and strain under the same defined conditions of exposure. Mortality of snails was recorded at 24 h (WHO 1983) and analyzed to obtain the lethal concentration values and slope value by probit analysis (Bull WHO 1965).
Effect on survival rate of snails
Biomphalaria alexandrina snails (8–10 mm) were divided into four groups (30 snails each); three groups were exposed to the extracted chlorophyllin at NOEL, LC10, and LC25 for 24 h (exposure); then, the snails were removed, washed thoroughly with dechlorinated tap water, and transferred to containers with fresh dechlorinated tap water for 6 days of recovery, and this was done for 2 weeks and then followed by 2 weeks of recovery. The fourth group was left in dechlorinated water as the control, and all the experiments were repeated three times.
Biochemical alterations in the nervous tissue
Acetylcholinesterase (AChE)
Acetylcholinesterase activity was measured according to the method of Ellman et al. (1961) as modified by Singh and Agarwal (1991). The nervous tissue of B. alexandrina was taken around the buccal mass and homogenized in 1.0 ml of 0.1 M phosphate buffer pH 8.0 for 5 min in an ice bath and then centrifuged at 1000g for 30 min at 4 °C. The supernatant was used as an enzyme source. The enzyme activity was expressed in mg tissue unit (μmol/mg).
Acid and alkaline phosphatase (ACP/ALP)
Phosphatases were measured by the method of Bergmeyer (1985) modified by Singh and Agarwal (1991). Tissue homogenate (2%, w/v) was prepared in ice cold 0.9% NaCl and centrifuged at 5000g for 20 min at 4 °C. The activity of both phosphatases was expressed in mg tissue unit (μmol/mg).
Protein
Estimation of protein was made according to the method of Lowry et al. (1994). The results have been expressed as μg/mg tissue.
Histological study
Adult snails (8–10 mm) were exposed to the extracted chlorophyllin at (NOEL, LC10, or LC25) for 24 h (exposure); then, the snails were removed from the experimental test solution, washed thoroughly with dechlorinated tap water, and transferred to containers with fresh dechlorinated tap water for another 24 h of recovery, and this was done for 2 weeks. To study the changes in the histology of the digestive gland of treated snails compared with control snails, the digestive gland was dissected, fixed in Bouin’s solution, embedded in paraffin wax, sectioned, and stained with hematoxylin and eosin (Mohamed and Saad 1990). The sections were examined under the light microscope (Olympus System Microscope BX2 Series) and photographed by a Zeiss Video camera, Germany.
Statistical analysis
Lethal concentration values were defined by probit analysis (Finney 1971), and the data were analyzed using the Graph Pad Prism 6.04 software for Windows (Graph Pad Software, San Diego, California, USA; 1992–2014).
Results
The molluscicidal activity of the extracted chlorophyllin for adult B. alexandrina and L. natalensis snails after 24 h of exposure followed by another 24 h for recovery is represented in Table 1 and Fig. 1. These results showed that it was toxic to B. alexandrina snails at LC50 17.6 mg/l and to L. natalensis snails at LC50 4.3 mg/l.
By studying the effect of the sublethal concentrations (NOEL, LC10, and LC25) on B. alexandrina snails, the survival rates of these snails were highly significantly reduced (p < 0.001) compared to the control group and this reduction was concentration dependent (Fig. 2).
Regarding the biochemical alterations in the nervous tissue of B. alexandrina snails exposed to the sublethal concentrations (LC25) of chlorophyllin for 2 weeks of the exposure, there was a significant (p < 0.05) decrease in acetylcholine esterase activities, acid and alkaline phosphatase levels, and protein content (Table 2).
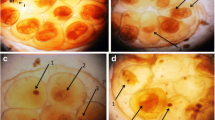
The normal digestive gland of B. alexandrina snails consists of two main cell types: the digestive cells which are columnar with round apices and the secretory cells which are pyramidal in shape (Fig. 3a). Histopathological examinations showed that there were deleterious effects in the snails that were exposed to LC25, which are represented by a deformation in the secretory cells, and a rupture of connective tissue between tubules and disintegration in the digestive cells (Fig. 3d), while less and moderate effects were observed in the digestive gland of snails groups that were exposed to NOEL and LC10, respectively, when compared to the control group (Fig. 3b, c).
Sections of the digestive gland (hematoxylin and eosin stain) of normal (control) B. alexandrina snails a columnar digestive cells (DC) and pyramidal secretory cells (SC) surround the lumen (L) and presence of connective tissues (CT). Snails exposed to NOEL, b dense enlarged secretory cells (SC) and ruptured digestive cells (RDC). Snails exposed to LC10, c cell membrane of some digestive cells disappeared which led to vacuole (V) formation and degenerated digestive cells (DDC). Snails exposed to LC25, d deformation in secretory cells (thin arrows) and disintegration in digestive cells (DDC)
Discussion
Water-soluble chlorophyllin can act as a molluscicidal agent (Chaturvedi et al. 2017). It is a photodynamic active substance that is excited by light (Singh et al. 2010). The present study showed that the calculated molluscicidal activity of the extracted chlorophyllin on B. alexandrina snails was LC50 17.6 mg/l and that on L. natalensis snails was LC50 4.3 mg/l. These results agreed with that of Elhadad et al. (2018) who stated that the exposure of B. alexandrina snails to LC50 of chlorophyllin (50 mg/l) greatly increased the mortality of these snails after 4 h of sunlight exposure to exert its photodynamic action. Erzinger et al. (2015) indicated that under short-term exposure for 24 h (one light/dark cycle), chlorophyllin can be used to control the vectors of parasitic diseases and that Astyanax bimaculatus fish showed a greater resistance (EC50 values of 29.96 mg/l).
The present investigation indicated that the survival rate of B. alexandrina snails exposed to these sublethal concentrations was highly significantly reduced (p < 0.05) compared to the control group and this reduction was concentration dependent. This is in agreement with Ragheb et al. (2018) who showed a marked reduction in the survivorship of B. alexandrina exposed to LC10 (5 × 10−6 mol/l) copper and magnesium chlorophyllin in the light. Also, Wohllebe et al. (2009) stated that water-soluble chlorophyllin under exposure of solar radiation was able to kill mosquito larvae and other small animals within a few hours. (LD50 value in Culex sp. larvae was 6.88 mg/l, and that in Chaoborus sp. larvae was about 24.18 mg/l.) Chaturvedi et al. (2017) confirmed that chlorophyllin enters the snail’s body through its surface which causes effective killing of L. acuminata snails.
Acetylcholine is a neurotransmitter that regulates the animal’s behavior. The acetylcholinesterase enzyme is used as a biomarker in various toxicological studies (Matozzo et al. 2005). This enzyme is involved in cholinergic neurotransmission of impulses by breaking acetylcholine into acetic acid and choline (Brien 1976). So, the inhibition in this enzyme will lead to the accumulation of acetylcholine at the nerve synapses, producing paralysis and general lack of coordination in neuromuscular system and eventual death (Jaiswal et al. 2010). Alkaline phosphatase is useful in protein synthesis and other secretory activities (Ibrahim et al. 1977) in gastropod; its inhibition may result in reduction in protein level. Acid phosphatase (ACP) is a lysosomal enzyme (Aruna et al. 1979) which has an important role in autolysis, pathological necrosis, and overall catabolism (Abou-Donia 1978).
The present results showed that there were significant (p < 0.05) decreases in acetylcholinesterase, acid and alkaline phosphatase, and protein levels in the nervous tissue of B. alexandrina snails exposed to LC25 of chlorophyllin. These results are in accordance with Kumar and Singh (2016) who showed that chlorophyllin bait caused strong inhibition in the activities of acetylcholinesterase and cytochrome oxidase with a dose-dependent manner in the nervous tissue of Lymnaea acuminata with exposure of sunlight and red light. Also, Chaturvedi et al. (2017) stated that treatment of L. acuminata snails with 80% of 4-h LC50 (264.80 mg/l) of chlorophyllin caused maximum reduction in protein, amino acid, DNA, RNA and enzymes acetylcholinesterase, alkaline phosphatase and acid phosphatase activity in their nervous tissue. The reduction in protein levels may be due to the direct interference of the chlorophyllin with the protein biosynthesis (Kumar and Singh 2016; Chaturvedi et al. 2017) and the decrease in alkaline phosphatase.
Histopathological examinations showed that there were deleterious effects in the digestive gland of B. alexandrina snails that were exposed to LC25, which are represented by a deformation in the secretory cells, and a rupture of connective tissue between tubules and disintegration in the digestive cells, while less and moderate effects were observed in the digestive gland of snails groups that were exposed to NOEL and LC10 when compared to the control group, and these effects were mirrored on their decreased survival rates. Elhadad et al. (2018) stated that the digestive gland of B. alexandrina snails treated with LC50 of chlorophyllin (50 mg/l) showed degenerations in the digestive cells with shrinkage of the supporting connective tissue, while the tubular epithelial cells lost their regular shape and have ruptured cell tips.
Conclusion
Chlorophyllin is a cheap substance that is found in every green plant and causes deleterious effects on snails that transmit diseases like B. alexandrina and L. natalensis. So, it can be used as a molluscicidal agent as it is a natural and biodegradable material.
References
Abd El-Ghany AM, Abd El-Ghany NM (2017) Molluscicidal activity of Bacillus thuringiensis strains against Biomphalaria alexandrina snails. Beni Suef Univ J Basic Appl Sci 6:391–393. https://doi.org/10.1016/j.bjbas.2017.05.003
Abdel-Ghaffar F, Ahmed AK, Bakry F, Rabei I, Ibrahim AM (2016) The impact of three herbicides on biological and histological aspects of Biomphalaria alexandrina, intermediate host of Schistosoma mansoni. Malacologia 59(2):197–210
Abou-Donia M (1978) Increased acid phosphatase activity in hens following an oral dose of leptophos. Toxicol Lett 2:199–203
Aruna P, Sreeramulu Chetty C, Chandramohan Naidu R, Swami KS (1979) Acid phosphatase activity in the Indian apple snail, Pila globosa (Swainson), during aestivation and starvation stress. Proc Anim Sci 88:363–365. https://doi.org/10.1007/BF03179115
Beesley NJ, Williams DJL, Paterson S, Hodgkinson J (2017) Fasciola hepatica demonstrates high levels of genetic diversity, a lack of population structure and high gene flow: possible implications for drug resistance. Int J Parasitol 47:11–20. https://doi.org/10.1016/j.ijpara.2016.09.007
Bergmeyer H (1985) Methods of enzymatic analysis, methods of enzymatic analysis. Metabolites 3: lipids, amino acids and related compounds, vol 8. VCH Verlagsgesellschaft, Weinheim
Brien RDO (1976) Acetylcholinesterase and its inhibition. In: Wilkinson CF (ed) Insecticide biochemistry and physiology. Plenum Press, New York, pp 271–273
Chaturvedi D, Vinay D, Singh K (2017) Assessment the effect of photodynamic chlorophyllin on biochemical changes in the cerebral ganglion of snail lymnaea acuminata. Int J Pharma Sci Res 8:68–75
Chontananarth T, Tejangkura T, Wetchasart N, Chimburut C (2017) Morphological characteristics and phylogenetic trends of trematode cercariae in freshwater snails from Nakhon Nayok Province, Thailand. Korean J Parasitol 55:47–54. https://doi.org/10.3347/kjp.2017.55.1.47
DeRosa M (2002) Photosensitized singlet oxygen and its applications. Coord Chem Rev 233–234:351–371. https://doi.org/10.1016/S0010-8545(02)00034-6
Elhadad HA, El-Habet BA, Azab RM et al (2018) Effect of Chlorophyllin on Biomphalaria alexandrina snails and Schistosoma mansoni Larvae. Int J Curr Microbiol Appl Sci 7:3725–3736. https://doi.org/10.20546/ijcmas.2018.703.431
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Elsareh F, Abdalla R, Abdalla E (2016) The effect of aqueous leaves extract of Solenostemma argel (Del Hayne) on egg masses and neonates of Biomphalaria pfeifferi snails. J Med Plants 4:271–274
Erzinger GS, Wohllebe S, Vollrath F et al (2011) Optimizing conditions for the use of chlorophyll derivatives for photodynamic control of parasites in aquatic ecosystems. Parasitol Res 109:781–786. https://doi.org/10.1007/s00436-011-2322-7
Erzinger GS, Souza SC, Pinto LH et al (2015) Assessment of the impact of chlorophyll derivatives to control parasites in aquatic ecosystems. Ecotoxicology 24:949–958. https://doi.org/10.1007/s10646-015-1437-5
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Ibrahim AM, Abdalla AM (2017) Impact of Moringa oleifera seed aqueous extract on some biological, biochemical, and histological aspects of Biomphalaria alexandrina snails. Environ Sci Pollut Res 24:28072–28078. https://doi.org/10.1007/s11356-017-0397-0
Ibrahim AM, Migazi MG, Dexian ES (1977) Histochemical localization of alkaline phosphatase activity in the alimentary tract of the snail Marisa cornuarietis (L). Zool Soc Egypt Bull 26:94–105
Jaiswal P, Kumar P, Singh VK, Singh DK (2010) Enzyme inhibition by molluscicidal components of Myristica fragrans Houtt. in the nervous tissue of snail Lymnaea acuminata. Enz Res. https://doi.org/10.4061/2010/478746
Kiros G, Erko B, Giday M, Mekonnen Y (2014) Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes 7:1
Kumar N, Singh VK (2016) Effect of chlorophyllin bait on acetylcholinesterase and cytochrome oxidase activities in the nervous tissue of Lymnaea acuminata with exposure of sunlight and red light. Eur J Biol Res 6:254–259. https://doi.org/10.5281/ZENODO.163653
Lee J-H, Quan J-H, Choi I-W, Park GM, Cha GH, Kim HJ, Yuk JM, Lee YH (2017) Fasciola hepatica: infection status of freshwater snails collected from Gangwon-do (Province), Korea. Korean J Parasitol 55:95. https://doi.org/10.3347/kjp.2017.55.1.95
Lowry O, Schagger H, Cramer WA, Vonjagow G (1994) Protein measurement with the folin phenol reagent. Anal Biochem 217:220–230. https://doi.org/10.1016/0304-3894(92)87011-4
Mahmoud M, Richter P, Kandil O et al (2013) Molluscicidal activity of chlorophyll extraction against the freshwater snails. J Coast Life Med 1:85–88. https://doi.org/10.12980/JCLM.1.2013C824
Matozzo V, Tomei A, Marin MG (2005) Acetylcholinesterase as a biomarker of exposure to neurotoxic compounds in the clam Tapes philippinarum from the Lagoon of Venice. Mar Pollut Bull 50:1686–1693. https://doi.org/10.1016/j.marpolbul.2005.07.011
Moema EBE, King PH, Baker C (2008) Cercariae developing in Lymnaea natalensis Krauss, 1848 collected in the vicinity of Pretoria, Gauteng Province, South Africa. Onderstepoort J Vet Res 75:215–223
Mohamed SH, Saad AA (1990) Histological studies on the hermaphrodite gland of Lymnaea caillaudi and Biomphalaria alexandrina upon infection with certain larval trematodes. Egypt J Histol 13:47–53
Ragheb M, El-Tayeb TA, El-Emam MA et al (2018) Fecundity, sex hormones and release of cercariae of Schistosoma mansoni in Biomphalaria alexandrina (Ehrenberg, 1831) treated with copper and magnesium chlorophyllin. Folia Malacol 26:17–24. https://doi.org/10.12657/folmal.026.003
Rahman AKMA, Islam SKS, Talukder MH et al (2017) Fascioliasis risk factors and space-time clusters in domestic ruminants in Bangladesh. Parasit Vectors 10:228. https://doi.org/10.1186/s13071-017-2168-7
Rahmani M, Csallany AS (1991) Chlorophyll and β-carotene pigments in moroccan virgin olive oils measured by high-performance liquid chromatography. J Am Oil Chem Soc 68:672–674. https://doi.org/10.1007/BF02662293
Richter PR, Strauch SM, Azizullah A, Häder D-P (2014) Chlorophyllin as a possible measure against vectors of human parasites and fish parasites. Front Environ Sci 2:18. https://doi.org/10.3389/fenvs.2014.00018
Singh DK, Agarwal RA (1991) Action sites of cypermethrin, a synthetic pyrethroid in the snail Lymnaea acuminata. Acta Hydrochim Hydrobiol 19:425–430. https://doi.org/10.1002/aheh.19910190415
Singh RN, Kumar P, Singh VK, Singh DK (2010) Available online through Toxic effects of Deltamethrin on the levels of biochemical changes in the snail Lymnaea acuminata. J Pharm Res 3:1739–1742
Soliman MFM (2008) Epidemiological review of human and animal fascioliasis in Egypt. J Infect Dev Ctries 2:182–189. https://doi.org/10.3855/jidc.260
WHO (1965) Molluscicide screening and evaluation. Bull World Health Organ 33(4):567–581
WHO (1983) Report of the Scientific working Group on Plant Molluscicide and guidelines for evaluation of plant molluscicides. Geneva: WHO (TDR/SCHSWE (4)/833)
WHO (2014) Schistosomiasis. Fact Sheet No. 115
WHO (2017) Schistosomiasis. Fact Sheet 115, updated Oct., 2017
Wohllebe S, Richter R, Richter P, Häder D-P (2009) Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol Res 104:593–600. https://doi.org/10.1007/s00436-008-1235-6
Wohllebe S, Richter P, Häder D-P (2012) Chlorophyllin for the control of Ichthyophthirius multifiliis (Fouquet). Parasitol Res 111:729–733. https://doi.org/10.1007/s00436-012-2893-y
Funding
The authors would like to thank the financial support of the internal project “103 M,” Theodor Bilharz Research institute, Giza, Egypt.
Author information
Authors and Affiliations
Contributions
AMI and FAB conceived and designed the study. AMI performed the experiments and analyzed the data. AMI wrote the first draft, and FAB revised and edited it. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Ethics approval and consent to participate
Ethical approval had been granted approval by the Ethics Committee of Theodor Bilharz Research Institute (TBRI).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, A.M., Bakry, F.A. Assessment of the molluscicidal impact of extracted chlorophyllin on some biochemical parameters in the nervous tissue and histological changes in Biomphalaria alexandrina and Lymnaea natalensis snails. Invert Neurosci 19, 7 (2019). https://doi.org/10.1007/s10158-019-0230-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10158-019-0230-1