Abstract
Litter production and decomposition are critical to forest productivity, nutrient cycling, and carbon sequestration in tropical woody ecosystems. However, nutrient release and leaf litter stoichiometry in tropical legume tree plantations over the long term after outplanting are poorly understood or even unknown. Toward improving our understanding of the pattern of changes in the decomposition of N-fixing leaf litters and their possible impact on carbon storage, we measured litter production, mass loss and nutrient release for 240 d during litter decomposition for two tropical legume tree species (Plathymenia reticulata and Hymenaea courbaril), in Rio de Janeiro, Brazil. Litter production for P. reticulata was 5.689 kg ha−1 a−1 and 3.231 kg ha−1 a−1 for H. courbaril. The patterns of mass loss rates were similar; however, nutrient release was greater for P. reticulata, while H. courbaril showed immobilization of nutrients, especially for N, which increased by almost 20% in the early phase of decomposition followed by gradual release. Litter from the N-fixing species did differ in nutrient chemistries over time, which was not surprising given that initial nutrient concentrations varied broadly, except for C and P. Most of the nutrient concentrations increased as the remaining litter mass decreased in both species, except for C and K. The C:N and N:P ratios differed between the species, but N:P did not correlate to mass loss. Both species had N-rich leaves, but P. reticulata decomposition was very likely P-limited, while H. courbaril seemed to be co-limited by N and P. The results showed different patterns in nutrient release and the stoichiometry involved in the decomposition dynamics of the two tropical N-fixing species, even though they have similar litter decay rates. Both species, but especially P. reticulata, may help re-establish nutrient cycling in disturbed ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Atlantic Forest biome has been identified as one of the world’s hotspots of biodiversity (Myers et al. 2000). However, only a small part of this tropical diversity has been studied. As a consequence, the lack of information and limited experience have led to logging of native species in natural forests rather than new plantations.
Among tropical N-fixing species belonging to the Fabaceae family, Plathymenia reticulata Benth and Hymenaea courbaril Linnaeus have, for instance, high growth rates and good timber production (Barroso et al. 2018), which can be of interest for forestry plantations, reclamation of degraded areas and/or intercropping systems. In addition, both species have medicinal properties (Cartaxo et al. 2010; Nicole et al. 2011).
The multiple purpose potential of P. reticulata and H. courbaril and the necessity of mitigating the impacts of natural forest exploitation highlight the importance of exploring the ecology of these species in relation to nutrient cycling (Cornwell et al. 2008; Martins et al. 2020). In highly weathered, low-fertility soils such as tropical soils, litter decomposition supplies most of the nutrients through the soil–plant system, especially N-fixing species (Tripathi et al. 2006; Waring et al. 2014; Ludvichak et al. 2016). Litter decomposition also plays a major role in maintaining forest productivity and the soil fauna (Barantal et al. 2014; Liu et al. 2019).
Several factors drive organic matter mineralization and nutrient release in woody ecosystems, but recent studies have shown that the chemical quality of litter is the major influencer of decomposition rates (Bradford et al. 2016; Bhatnagar et al. 2018; Lanuza et al. 2018; Vivanco and Austin 2019; Bo et al. 2020). Determining the necessary balance of various nutrients and how this balance is affected by the environment (Ågren and Weih 2020; Yu et al. 2020) is critical to better understanding litter decomposition (Zhang et al. 2018).
In this sense, C, N and P stand as the main drivers of the decomposition dynamics and are good predictors of litter decay (Tripathi et al. 2006). Positive correlations between N and P concentrations with litter decay rates were found by Cornwell et al. (2008) and Hobbie (2015) at a global scale. However, leaf litter decomposition in tropical forest ecosystems has been reported to be more regulated by P limitation rather than other nutrients, which may lead to limited C assimilation (Bo et al. 2020; Cassart et al. 2020).
C compounds have also been reported to control decomposition rates in tropical forest ecosystems (Hättenschwiler and Jørgensen 2010). Leaf litter rich in recalcitrant compounds (such as lignin) was found to have lower leaf litter decomposition rates (Duarte et al. 2013; Butenschoen et al. 2014). It is noteworthy that C turnover is more rapid in tropical ecosystems, which is directly related to C storage and global climate warming (Villela et al. 2012; Martinelli et al. 2017; Ochoa-Hueso et al. 2019).
The relationships among C, N and P (expressed as C:N:P ratios) are utilized to determine which nutrient is limiting litter decomposition rates based on optimal or critical ratios for maintaining populations of decomposers (Tessier and Raynal 2003; Bachega et al. 2016; Bo et al. 2020). High critical C:P ratios were found in tropical conditions driven by low P availability (Manzoni et al. 2010) and a low N:P ratio (Hättenschwiler and Jørgensen 2010).
Better knowledge of litter decomposition and nutrient release, especially in the threatened Atlantic Forest biome, will inform the design strategies for forest plantations and restoration projects and inform land managers about the long-term consequences of planting certain species (Duarte et al. 2013; Austin et al. 2014; Caldeira et al. 2019; Vitória et al. 2019). The main objectives of this work were therefore to (1) assess the pattern of decomposition and nutrient release of two important tropical legume species, and (2) understand the ecological stoichiometry involved in the decomposition of each species. This study quantifies these variables for the first time for two important tropical N-fixing species over the long-term after outplanting.
Material and methods
Site description
The study was carried out in the mountain region of Rio de Janeiro, Brazil, within the municipal limits of Trajano de Moraes. The site is 720 m a.s.l., and rainfall averages 1000 mm a−1. For monthly precipitation, humidity, and air temperature, see Fig. S1. At 0–10 cm depth, the soil comprises 340 g kg−1 sand, 119 g kg−1 silt, and 541 g kg−1 clay and has a bulk density of 1.07 g cm−3. Previously a pasture, the site had experienced erosion and unplanned fires, and in 1992, Atlantic Forest species were planted to restore the area. The plantation was done as minimum tillage, with manual weeding, 0.40 m × 0.40 m × 0.40 m planting pits, with cattle manure at 10 L per pit and 10-28-06 NPK fertilizer (100 g per pit) applied only at planting. A total of 49 seedlings per tree species were planted in single plots and spaced at 3 m × 3 m (21 m × 21 m each plot). The seedlings were produced from seeds collected in the region that contained no description of the matrices collected. In total, the site has 23 species planted in single plots (at the same age), and during the first year after planting, ants were controlled with baits. There is no long-term record of these Atlantic Forest plantations after outplanting.
Among the species planted and evaluated by our research group (Barroso et al. 2018), we selected two that had higher survival rates and belong to the Fabaceae family: P. reticulata and H. courbaril. The study site map, additional planting details, and the topsoil chemical properties were detailed by Barroso et al. (2018).
Litter collection and decomposition
Fresh leaf litter was collected in each plantation for 1 year. In each plantation, 20 litter traps (0.75 m × 0.75 m) were fixed to the ground at 1.5 m above the ground to collect litter. The traps were emptied monthly, and the leaves from each forest plantation were hand-sorted from the other fractions (unknown leaves from understory vegetation, branches, fruits, flowers, and miscellanea) and oven-dried for 72 h at 65 °C.
To evaluate leaf decomposition, we used the litterbag methodology described by Bocock and Gilbert (1957). The experiment was installed in September 2018 for P. reticulata (time 0) and in December 2018 for H. courbaril (time 0), according to the natural peak of leaf litter productivity of each species. The 20 cm × 20 cm (2-mm nylon mesh) litterbags containing 10 g of leaf litter were randomly placed on the soil. Leaves with disease symptoms and/or herbivory were avoided. A total of 96 litterbags (48 in each species) were installed and six bags each were extracted after 10, 30, 60, 90, 120, 150, 180, and 240 d.
The collected litterbags were cleaned by eliminating insects, soil particles, and other undefined materials. Then, the samples were oven-dried for 72 h at 65 °C, weighed (0.01 g), and ground (to pass through 1 mm mesh) to determine chemical concentrations. The C concentrations were measured according to Tedesco et al. (1995), N was measured using the Kjeldahl method after sulphuric acid digestion, P using colorimetry (Braga and Defelipo 1974), K using fusion-flame photometry, and calcium (Ca), magnesium (Mg), and manganese (Mn) using atomic absorption spectroscopy (Bataglia et al. 1983). Lignin and cellulose were measured only at time 0 and determined using the acid detergent fiber method (Van Soest and Wine 1967).
Calculations and data analyses
For the lignin and cellulose data, we calculated confidence intervals using Students t-test to illustrate the initial variation in the leaf litter data. Nutrient release patterns for C, N, P, K, Ca, Mg, and Mn were calculated as the percentage loss for each nutrient at each sampling time based on the original content (nutrient concentration multiplied by dry mass at each sampling time and multiplied by 100).
The mass loss data (expressed as a percentage; dry mass at the sampling time multiplied by 100 and divided by 10 as the initial dry mass) was submitted to regression adjustments and analysis of variance (ANOVA) of the adjusted models. To verify whether the data met ANOVA assumptions, they were submitted to diagnostic plots (normality, linearity, and homoscedasticity of residuals) and maximum likelihood function (Box–Cox test) using the MASS package (Venables and Ripley 2002) in R environment (R Core Team 2019).
The confidence intervals (P < 0.05) of the predicted adjusted models were calculated. We calculated the decomposition rate constant (k) based on the model proposed by Olson (1963):
where Wo is initial mass of the litter, Wt is mass remaining after time t (d), and k is a decomposition constant.
When investigating the ecological stoichiometry relationships as a function of mass lost over the decomposition period, the same procedure was applied before the regression adjustments, but only phosphorus concentration from H. courbaril required log-transformation.
Principal component analysis (PCA) was conducted to evaluate variability among the nutrient concentrations and litter decay in each forest species. For this analysis, data was standardized through z-scale transformation to avoid scale influence and plotted using the ggbiplot package (Vu 2011) in R environment (R Core Team 2019).
Results
Initial litter quality
Annual litterfall productivity varied from 5.689 kg ha−1 in the P. reticulata plot to 3.231 kg ha−1 in the H. courbaril plot, 43% lower than in the P. reticulata plot. For initial litter quality, P. reticulata had higher lignin content compared to H. courbaril. However, cellulose and lignin:N ratio were almost similar between the species (Table 1).
Litter decay and nutrient release
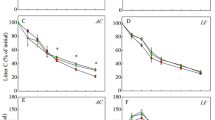
The nutrients were seemingly not exhausted (Fig. 1) up to 240 days, the end of the experiment. Over the 240 days, the remaining mass of litter linearly decreased for both species (Fig. 2a), as confirmed by the overlapping adjusted decay models. At the end of the experiment, the remaining mass was similar between the species; 59% for P. reticulata and 61% for H. courbaril litters. The decomposition rate constant (k d−1) also was similar between P. reticulata and H. courbaril, 0.0021 and 0.0020, respectively.
Changes in the concentration of C, N, P, K, Ca, Mg, and Mn as a function of mass lost during the decomposition of leaf litter from Plathymenia reticulata and Hymenaea courbaril over 240 days in Southeast Brazil. Confidence interval of the adjusted model for P. reticulata is in blue and in purple for H. courbaril
During the first 10 days, mass and all nutrients except Ca in the H. courbaril litter decreased (Fig. 1a, b). Potassium, P, and Mn showed a greater drop from their original contents in P. reticulata, while the release of the other nutrients was not as steep. Nutrient accumulation was observed, especially for H. courbaril, where N content was increased by almost 20% in the early stage of decomposition followed by gradual release.
Carbon, N, Ca, and Mg had a more pronounced decrease in P. reticulata leaves 180 days after the start of the experiment, whereas H. courbaril did not show a strong pattern for nutrient release up to 240 days.
Nutrient relations in litter
Linear correlations with similar slopes were observed when H. courbaril nutrient concentrations were plotted against the mass lost for all nutrients, except C. However, P. reticulata did not show correlations for C, Ca, and Mg, while K had a nonlinear correlation (Fig. 2b–h).
Linear relationships for C:N and C:P ratios were observed, and both ratios decreased over the decomposition period. The C:P slope was similar in both species, whereas H. courbaril had a higher C:N drop. No correlations were found when plotting the N:P ratio as a function of the mass lost for both species (Fig. 3a–c).
The PCA for P. reticulata (explaining 78.9% of the data variability) showed a direct association between litter decay and N concentrations with PC1 (Fig. 4a), and all nutrients had a negative correlation to PC1. Phosphorus, Mn, K, Mg, and Ca showed a positive correlation to PC2, but C and C:N, C:P, and N:P ratios had negative correlations.
Principal component analysis of the litter decay; C, N, P, K, Ca, Mg and Mn concentrations; and C:N, C:P, and N:P ratios for Plathymenia reticulata (a) and Hymenaea courbaril (b) and a comparison between both species (c) in Southeast Brazil. The area of the ellipses was determined according to the normal distribution of the data (ellipses level = 68%)
The PCA for H. courbaril (explaining 79% of the data variability) revealed an indirect correlation among litter decay, nutrient concentrations, and ratios with PC1 (indicated by the arrows in opposite directions), except for C and N:P ratio, which were negatively related to PC2 (Fig. 4b). On the other hand, none of the variables in the PCA for P. reticulata were directly negatively correlated to mass loss.
With respect to the relation of species with nutrient concentrations, the species were organized in different groups in the PCA (accounting for 78.9% of the variability), where P. reticulata was more associated with N, C, N:P, and C:P and H. courbaril with C:N, K, Mn, Ca, Mg and P (Fig. 4c).
Discussion
Litter decay and nutrient release
Litter mass loss was not significantly affected by the species, regardless of the litter traits and sampling date. The microbial communities decomposing the litter might adapt to leaf litter chemistry of each species (Pandey et al. 2007) and may help explain the lack of differences between the litter decay rates. The decomposition rate constant (k) was similar to another tropical N-fixing species (Inga subnuda), but other tropical leguminous species (Erythrina verna and Senna macranthera) had higher decay rates (Duarte et al. 2013).
In our site, average air temperature and humidity did vary slightly over time (Fig. S1). Low temperatures may strongly affect decomposition rates, but physical and chemical traits of leaves also play a role in regulating decomposition rates at higher temperatures (Bradford et al. 2016).
Physical leaf traits, e.g., specific leaf area and toughness, also influence mass loss (Pérez-Harguindeguy et al. 2000; Güsewell and Verhoeven 2006). Although we did not assess these traits, P. reticulata clearly has a higher leaf specific area and tenderer leaves compared with H. courbaril (see Fig. S2 for an overview of the leaves). Therefore, faster decomposition could be expected for P. reticulata, but was not found, mainly due to the maintenance of the decomposers through resource availability (Lanuza et al. 2018; Ochoa-Hueso et al. 2019; Bo et al. 2020).
With respect to chemical traits, litter decomposers produce enzymes to assimilate C from complex organic matter and use it as an energy source (Waring et al. 2014). Energy deprivation may also drive decomposition processes (Hättenschwiler and Jørgensen 2010). Carbon showed a pattern similar to mass loss across both species, and C concentration was stable over time. Declines in decomposition rates are expected when recalcitrant C compounds are present in litter (Duarte et al. 2013), which suggests that probably the only easily accessible C (such as cellulose and hemicellulose) was released jointly with the mass loss during the experiment.
Nitrogen accumulation during litter decomposition might be due to external factors such as atmospheric deposition. The N accumulation peaks occurred in the months with higher precipitation records at our site (November for P. reticulata, and March and May for H. courbaril, Fig. S1). In addition, other factors such as biological N fixation and decomposers (fungi and bacteria) that assimilate the required nutrients and remain stuck in the leaves can also contribute to N accumulation; the latter factor can explain the P accumulation in both species (Fanin et al. 2013). The same pattern for N and P accumulations was already reported by Parsons and Congdon (2008), Bachega et al. (2016) and Lanuza et al. (2018). On the other hand, N and P accumulation may contribute to nutrient conservation in nutrient-poor sites (Singh et al. 1999).
The fast K decrease, also reported by others (Osono et al. 2008; Lanuza et al. 2018), can be explained by its high solubility, which makes interpretation complex. K is an important nutrient because it might influence organic matter degradation and ecosystem productivity (Fay et al. 2015; Ochoa-Hueso et al. 2019).
Calcium release occurs mainly in the late decomposition phases because it is a major component of cell structures, which are degraded last (Osono and Takeda 2004). Evaluating decomposition for longer periods would clarify this point, because this pattern was not clearly revealed for both species.
Mn, P, Mg, and K were released quickly in the first 10 days of P. reticulata decomposition. Mg tends to be released faster (Osono and Takeda 2004) and is essential for soil fauna (Makkonen et al. 2012). The same pattern was not observed for H. courbaril, which varied more over time. Additionally, the presence of cations is related to litter acidity and decomposers consumption (Cornelissen et al. 2003).
Nutrient relations in litter
Carbon leaf concentrations did not correlate to mass lost for either tree species because variations in C levels were small over the decomposition period, supporting our prior assumption that only easily accessible C such as cellulose is released after the mass loss (Moorhead et al. 2013; Bachega et al. 2016), providing the decomposers with energy. The lack of correlation between C and litter decay means that elements other than C exerted a major influence over decomposition.
Both species had high initial lignin concentration, especially P. reticulata, when compared to other species (Mendonça and Stott 2003; Tripathi et al. 2006; Duarte et al. 2013), which could lead to energy starvation in late stages of decomposition when it regulates the processes more (Berg and Matzner 1997; Chapin et al. 2002). The recalcitrance of this compound, which few organisms can degrade (Sigoillot et al. 2012) may almost stop decomposition rates (Marchante et al. 2019), but we did not find any decreases in the decomposition rate. On the other hand, a large recalcitrant fraction in the litter can be important for long-term C sequestration (Leblanc et al. 2006), especially in P. reticulata leaf litter.
Our species differed broadly in their N:P ratio; P. reticulata ranged from 40 to 60, H. courbaril from 20 to 30. This ratio reflects which variable could be limiting litter decomposition: N-limited when litter has low N:P ratio (< 14), and P-limited when high N:P ratio (> 16) (Tessier and Raynal 2003). Thus, for both species, litter decomposition was likely P-limited, but apparently less so in H. courbaril. P-limitation in plant litter is reportedly common in tropical sites (Aerts 1997; Vivanco and Austin 2006; Cassart et al. 2020) including tropical N-fixing species plots (Mendonça and Stott 2003; Duarte et al. 2013).
Both species had C:P ratios above 300, where as a general rule, litter decomposition is P-limited (Stevenson and Colle 1999; Duarte et al. 2013). Duarte et al. (2013) reported the same pattern for another tropical legume tree species (Inga subnuda), which suggests a broad N-fixing effect that needs to be investigated more closely. Although we considered the critical N:P and C:P ratios proposed by Tessier and Raynal (2003) and Stevenson and Colle (1999), the nutrient limitations may vary depending on the microbial populations in each site (Güsewell and Gessner 2009), which must be further investigated in our stands. N-limitation may occur with N concentrations below 11.3 g kg−1 (Güsewell and Verhoeven 2006); both studied species had greater initial concentrations than that threshold, which could increase the mass loss during the early stages of decay (Hättenschwiler and Jørgensen 2010; Hobbie 2015). However, this pattern was not observed probably due to the available P regulating the process. Indeed, the PCA for P. reticulata (Fig. 4a) indicated a weak correlation between litter decay and P concentrations, but in H. courbaril the negative relation between the variables was more robust (Fig. 4b).
The critical role exerted by C, N and P in litter decomposition can commonly lead to co-limitation of these elements during the process (Fanin et al. 2013). A co-limitation may also explain the high N accumulation in H. courbaril (Fig. 1b), given that N-limited microbes immobilize N more efficiently (Güsewell and Gessner 2009).
For the C:N ratio, 25:1 is considered ideal for microbial maintenance and organic matter decomposition; lower microbial activities could thus be associated with lower (Bachega et al. 2016) or higher C:N ratios (Berg and Matzner 1997; Hättenschwiler and Jørgensen 2010), leading to N mineralization or limitation, respectively.
Our results did not indicate N starvation for P. reticulata litter, so N mineralization may be occurring, and the species might be a good choice to reestablish N cycling in forest ecosystems. However, H. courbaril had a higher initial C:N ratio (about 30:1), which suggests that decomposition is N-limited even with N concentrations above the threshold as mentioned by Güsewell and Verhoeven (2006). In addition, Vivanco and Austin (2019) noted that N can be trapped in complex plant polymers, but other nutrients, such as manganese, might facilitate N release.
Little attention has been addressed to micronutrients such as manganese, which is directly involved in the lignin degradation through the enzyme manganese peroxidase and thus very important in the decomposition of lignified litters (Berg et al. 2007, 2015) such as P. reticulata leaf litter. H. courbaril had almost fourfold more Mn than P. reticulata did, and lower lignin, but the C concentration remained similar in both over the incubation period. Since easily degradable C remained available, Mn did not play a key role until that point.
This study reports for the first time the decomposition rates and nutrient release of two tropical N-fixing species and may help us better understand the ecological role of each species. However, some topics remain unclear and need further tests to provide a greater ecological overview of tropical N-fixing species. This study also leads us to a new question: Do stoichiometrically distinct litters affect soil microbiota (e.g., fungi to bacteria ratios) and their metabolic capabilities (e.g., enzymatic activities)?
Conclusion
Both tropical N-fixing species had similar litter decay rates, but P. reticulata litter released more nutrients compared to H. courbaril litter. N and P concentrations, rather than other elements, in the litters controlled the decomposition processes because P. reticulata decomposition was primarily limited by P and H. courbaril was co-limited by N and P. P. reticulata is a good choice to reestablish N cycling in forest ecosystems.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79(3):439–449
Ågren GI, Weih M (2020) Multi-Dimensional plant element stoichiometry—Looking beyond carbon, nitrogen, and phosphorus. Front Plant Sci 11:1–23
Austin AT, Vivanco L, González-Arzac A, Pérez LI (2014) There’s no place like home? An exploration of the mechanisms behind plant litter-decomposer affinity in terrestrial ecosystems. New Phytol 204:307–314
Bachega LR, Bouillet JP, Piccolo MC, Saint-André L, Bouvet JM, Nouvellon Y, Gonçalves JLM, Robin A, Laclau JP (2016) Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the home field advantage hypothesis. For Ecol Manage 359:33–43
Barantal S, Schimann H, Fromin N (2014) C, N and P fertilization in an Amazonian rainforest supports stoichiometric dissimilarity as a driver of litter diversity effects on decomposition Ecosystems. Proc R Soc B 281:20141682
Barroso DG, Souza MGOS, Oliveira TPF, Siqueira DP (2018) Growth of Atlantic forest trees and their influence on topsoil fertility in the Southeastern Brazil. Cerne 24(4):352–359
Bataglia OC, Furlani AMC, Teixeira JPF, Furlani PR, Gallo JR (1983) Métodos de análise química de plantas. Campinas, Instituto Agronômico, p 48
Berg B, Matzner E (1997) Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ Rev 5(1):1–25
Berg B, Erhagen B, Johansson MB, Nilsson M, Stendahl J, Trum F, Vesterdal L (2015) Manganese in the litter fall-forest floor continuum of boreal and temperate pine and spruce forest ecosystems–a review. For Ecol Manage 358:248–260
Berg B, Steffen KT, McClaugherty C (2007) Litter decomposition rate is dependent on litter Mn concentrations. Biogeochemistry 82:29–39
Bhatnagar JM, Peay KG, Treseder KK (2018) Litter chemistry influences decomposition through activity of specific microbial functional guilds. Ecol Monogr 88(3):429–444
Bo F, Zhang Y, Chen HYH, Chen HYH, Wang P, Ren X, Guo J (2020) The C:N: P Stoichiometry of planted and natural Larix principis-rupprechtii stands along altitudinal gradients on the Loess Plateau. China for 11:363
Bocock KL, Gilbert OJW (1957) The disappearance of leaf litter under different woodland conditions. Plant Soil 9(2):179–185
Bradford MA, Berg B, Maynard DS, Weider WR, Wood SA (2016) Understanding the dominant controls on litter decomposition. J Ecol 104:229–238
Braga JM, Defelipo BV (1974) Determinação espectrofotométrica de fósforo em extratos de solos e plantas. Rev Ceres 1(1):73–85
Butenschoen O, Krashevska V, Maraun M, Marian F, Sandmann D, Scheu S (2014) Litter mixture effects on decomposition in tropical montane rainforests vary strongly with time and turn negative at later stages of decay. Soil Biol Biochem 77:121–128
Caldeira MVW, Godinho TO, Moreira FL, Campanharo IF, Castro KC, Mendonça AR, Trazzi PA (2019) Litter as an ecological indicator of forest restoration processes in a dense ombrophylous lowland forest. Floresta e Ambient 26(1):20180411
Cartaxo SL, Souza MMA, Albuquerque UP (2010) Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J Ethnopharmacol 131(2):326–342
Cassart B, Basia AA, Jonard M, Ponette Q (2020) Average leaf litter quality drives the decomposition of single-species, mixed-species and transplanted leaf litters for two contrasting tropical forest types in the Congo Basin (DRC). Ann for Sci 77:1–20
Chapin F, Matson P, Vitousek P (2002) Principles of terrestrial ecosystem ecology. Springer, New York, p 536
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, Steege HT, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust J Bot 51(1):335–380
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Hanguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diáz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westtoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Duarte EMGG, Cardoso IM, Stijnen T, Mendonça MAFC, Coelho MS, Cantarutti RB, Kuyper TW, Villani EMA, Mendonça ES (2013) Decomposition and nutrient release in leaves of Atlantic Rainforest tree species used in agroforestry systems. Agrofor Syst 87:835–847
Fanin N, Fromin N, Buatois B, Hättenschwiler S (2013) An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter-microbe system. Ecol Lett 16:764–772
Fay PA, Prober SM, Harpole WS, Knops JMH, Bakker JD, Borer ET, Lind EM, MacDougall AS, Seabloom EW, Wragg PD, Adler PB, Blumenthal DM, Buckley YM, Chu C, Cleland EE, Collins SL, Davies KF, Du G, Feng X, Firn J, Gruner DS, Hagenah N, Hautier Y, Heckman RW, Jin VL, Kirkman KP, Klein J, Ladwig LM, McCulley RL, Melbourne BA, Mitchell CE, Moore JL, Morgan JW, Risch AC, Shutz M, Stevens CJ, Wedin DA, Yang LH (2015) Grassland productivity limited by multiple nutrients. Nat Plants 1:15080
Güsewell S, Gessner MO (2009) N: P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct Ecol 23:211–219
Güsewell S, Verhoeven JTA (2006) Litter N: P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 287:131–143
Hättenschwiler S, Jørgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30(6):357–363
Lanuza O, Casanoves F, Delgado D, van den Meersche K (2018) Leaf litter stoichiometry affects decomposition rates and nutrient dynamics in tropical forests under restoration in Costa Rica. Restor Ecol 27(3):549–558
Leblanc HA, Nygren P, McGraw RL (2006) Green mulch decomposition and nitrogen release from leaves of two Inga spp. in an organic alley-cropping practice in the humid tropics. Soil Biol Biochem 38:349–358
Liu Y, Shen X, Chen Y, Wang L, Chen Q, Zhang J, Xu Z, Tan B, Zhang L, Xiao J, Zhu P, Chen L (2019) Litter chemical quality strongly affects forest floor microbial groups and ecoenzymatic stoichiometry in the subalpine forest. Ann for Sci 76:1–15
Ludvichak AA, Schumacher MV, Dick G, Momolli DR, Souza HP, Guimarães CC (2016) Nutrient return through litterfall in a Eucalyptus dunnii Maiden stand in sandy soil. Rev Árvore 40(6):1041–1048
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80(1):89–106
Marchante E, Marchante H, Freitas H, Kjøller A, Struwe S (2019) Decomposition of an N-fixing invasive plant compared with a native species: Consequences for ecosystem. Appl Soil Ecol 138:19–31
Martinelli LA, Lins SRM, Silva JCS (2017) Fine litterfall in the Brazilian Atlantic Forest. Biotropica 49(4):443–451
Martins TGV, Reis GG, Reis MGF, Telles LAA, Lage MR, Mendes GGC, Pinto DL, Castro NLM, Lorenzon AS, Silva RS, Gonzáles DGE (2020) Potential planting areas for native tree species in Minas Gerais state, Brazil, based on environmental variables and wood demand. Ecol Modell 432:109211
Mendonça ES, Stott DE (2003) Characteristics and decomposition rates of pruning residues from a shaded coffee system in Southeastern Brazil. Agrofor Syst 57(2):117–125
Moorhead DL, Lashermes G, Sinsabaugh RL, Weintraub MN (2013) Calculating co-metabolic costs of lignin decay and their impacts on carbon use efficiency. Soil Biol Biochem 66:17–19
Myers N, Mittermeier R, Mittermeier C, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 16(4):853–858
Nicole MF, Gleidy AS, Karine NC, Magali GS, Cogo JC, Belo CAD, Santos MG, Groppo FC, Oshima-Franco Y (2011) Inhibition of Bothrops jararacussu venom activities by Plathymenia reticulata Benth extracts. J Venom Res 2(4):52–58
Ochoa-Hueso R, Delgado-Baquerizo M, An King PT, Benham M, Arca V, Power SA (2019) Ecosystem type and resource quality are more important than global change drivers in regulating early stages of litter decomposition. Soil Biol Biochem 129:144–152
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44(2):322–331
Osono T, Takeda H (2004) Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J for Res 9(1):23–31
Osono T, Takeda H, Azuma JI (2008) Carbon isotope dynamics during leaf litter decomposition with reference to lignin fractions. Ecol Res 23:51–55
Pandey RR, Sharma G, Tripathi SK, Singh AK (2007) Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in Northeastern India. For Ecol Manage 240:96–104
Parsons SA, Congdon RA (2008) Plant litter decomposition and nutrient cycling in north Queensland tropical rain-forest communities of differing successional status. J Trop Ecol 24(3):317–327
Pérez-Harguindeguy N, Díaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218:21–30
R Core team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Sigoillot J-C, Berrin J-G, Bey M, Lesage-Meessen L, Levasseur A, Lomascolo A, Record E, Uzan-Boukhris E (2012) Fungal strategies for lignin degradation. Adv Bot Res 61:263–308
Singh KP, Singh PK, Tripathi SK (1999) Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoil at Singrauli. India Biol Fertil Soils 29(4):371–378
Stevenson FJ, Cole MA (1999) Cycles of soil. Wiley, Hoboken, p 427
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais. Porto Alegre, p 174
Tessier JT, Raynal DJ (2003) Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J Appl Ecol 40:523–534
Tripathi SK, Sumida A, Shibata H, Ono K, Uemura S, Kodama Y, Hara T (2006) Leaf litterfall and decomposition of different above- and belowground parts of birch (Betula ermanii) trees and dwarf bamboo (Sasa kurilensis) shrubs in a young secondary forest in Northern Japan. Biol Fertil Soils 43(2):237–246
Van Soest P, Wine RH (1967) Development of a comprehensive system of feed analysis and its applications to forages. J Anim Sci 26(2):119–128
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Villela D, de Mattos E, Pinto A, Vieira SA, Martinelli LA (2012) Carbon and nitrogen stock and fluxes in coastal Atlantic forest of southeast Brazil: potential impacts of climate change on biogeochemical functioning. Brazilian J Biol 72(3):633–642
Vitória AP, Alves LF, Santiago LS (2019) Atlantic forest and leaf traits: an overview. Trees 33(6):1535–1547
Vivanco L, Austin AT (2006) Intrinsic effects of species on leaf litter and root decomposition: a comparison of temperate grasses from North and South America. Oecologia 150:97–107
Vivanco L, Austin AT (2019) The importance of macro- and micro-nutrients over climate for leaf litter decomposition and nutrient release in Patagonian temperate forests. For Ecol Manage 11:1–14
Vu QV (2011) ggbiplot: a ggplot2 based biplot. R package version 0.55
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117:101–113
Yu MF, Tao Y, Liu W, Xing W, Liu G, Wang L, Ma L (2020) C, N, and P stoichiometry and their interaction with different plant communities and soils in subtropical riparian wetlands. Environ Sci Pollut Res 27:1024–1034
Zhang J, Zhao N, Liu C, Yang H, Li M, Yu G, Wilcox K, Yu Q, He N (2018) C:N:P stoichiometry in China’s forests: from organs to ecosystems. Funct Ecol 32:50–60
Acknowledgements
The authors thank Floresta Estadual José Zago administrators for logistical support and colleagues from the Department of Geosciences and Natural Resources Management for suggestions on the data and Senhao Wang for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project Funding: This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (141513/2017-9), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E26/200.84/2019), and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (88881.361830/2019-01).
Corresponding editor: Yanbo Hu
The online version is available at http://www.springerlink.com
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siqueira, D.P., de Carvalho, G.C.M.W., de Souza Silva, J.G. et al. Litter decomposition and nutrient release for two tropical N-fixing species in Rio de Janeiro, Brazil. J. For. Res. 33, 487–496 (2022). https://doi.org/10.1007/s11676-021-01383-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01383-z