Abstract
In many Japanese forests, the forest understory is largely dominated by dwarf bamboo (Sasa) species, which compete with overstory vegetation for soil nutrients. We studied the rate of leaf litterfall, and decomposition and mineralization of carbon (C) and nitrogen (N) from various components (leaf, root, wood, and rhizome) of overstory and understory vegetation in a young Betula ermanii forest from 2002 to 2004. Total litterfall was 377 g m−2 year−1, of which the overstory vegetation contributed about two thirds. A litter decomposition experiment conducted for 770 days indicated that mass loss of different litter components varied significantly, except for Sasa kurilensis wood and rhizome. Relative decomposition rates were significantly greater in the first growth period (June to October) than the dormant period (November to May) in most cases. Rainfall was the most important abiotic variable, explaining 75–80% of the variability in mass loss rates. Concentrations of ethanol soluble substances and N were significantly positively correlated (r=0.77 to 0.97, P<0.05) with mass loss at an early stage (41 days). The ratios of lignin/N and C/N were found to be negatively correlated with mass loss rates at all stages of litter decomposition. C stock loss was similar to that of mass loss, whereas N stock loss was slower, except for S. kurilensis fine root litter. The evergreen understory species S. kurilensis exhibited greater N use efficiency than B. ermanii, suggesting better competitive ability that might favor the production of a high biomass and invasion under tree species like B. ermanii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Japanese mountain birch (Betula ermanii Cham.), a light-demanding species (Koike 1987), occupies open spaces in forests or steep mountain slopes where few conifers are able to grow (Ohsawa et al. 1973). This species is also found growing with coniferous species and has a long life span of about 250 years (Watanabe 1979). In northern Japan, mountain birch is widely distributed in montane and subalpine belts up to 1,600 m (Ishizuka and Sugahara 1986). In Japanese forests, the understory is often densely covered with species of dwarf bamboo (mainly Sasa), which compete with overstory vegetation for resources such as sunlight, soil water, and nutrients (Takahashi et al. 2003; Tripathi et al. 2005).
The amount and seasonal patterns of litterfall and decomposition are important determinants of overall recycling of nutrients and maintenance of soil fertility in terrestrial ecosystems (Upadhyay and Singh 1989; Tripathi and Singh 1992a,b, 1995; Singh et al. 1999; Fioretto et al. 2003). Moreover, the impact of tree species on soil fertility depends on their litter chemical quality and decomposition rate. The decomposition of litter is primarily influenced by the physical environment in which decay takes place, the nature and abundance of decomposing organisms and the chemical quality of litter (Facelli and Pickett 1991; Heal et al. 1997; Sariyildiz et al. 2005).
Plant litter of varying substrate quality has been found to exhibit different mineralization potential and decomposition behavior (Mtambanengwe and Kirchman 1995). Litter decomposition is mainly governed by two factors, i.e., the climate and the initial substrate quality of the litter (Swift et al. 1979). Among the climatic variables, actual evapotranspiration (AET) is reportedly the major determinant of decomposition in a range of climatic conditions (Berg et al. 1993). However, in Indian dry tropical regions, precipitation and associated variables such as soil and litter moisture have also been found to be major factors influencing the rate of litter decomposition (Tripathi and Singh 1992a). Among the initial litter substrate quality variables, water or ethanol soluble substances, cellulose, lignin and nitrogen (N) content, and ratios of C/N and lignin/N have been shown to play a crucial role at different stages of litter decomposition (Taylor et al. 1989; Tripathi and Singh 1992a). Generally, the labile fraction of litter, which includes water soluble substances and free unshielded cellulose, decomposes rapidly within a few months (Berg et al. 1997), and as a result, the concentrations of lignin and nutrients like N increase in later stages (Berg and Staaf 1980; Berg 2000).
Although leaf and wood litter decomposition studies have been conducted in Japanese forests with respect to different forest tree species (Hardiwinoto 1991a,b; Kaneko and Salamanaca 1999; Osono and Takeda 2001, 2004; Salamanaca et al. 1998; Xu and Hirata 2005), the rate of decomposition and release of carbon (C) and nitrogen (N) from different above- and belowground components of overstory and understory vegetation have yet to be quantified. Therefore, the objectives of the present research are (1) to assess the seasonal variations in litter fall and C and N return to soil, (2) to evaluate the changes in litter decomposition rates and mineralization of C and N, and (3) to assess the role of abiotic and initial litter quality on the rates of decomposition and mineralization of C and N from various plant parts (above- and belowground) of overstory birch trees (B. ermanii) and understory dwarf bamboo shrubs (S. kurilensis Makino et Shibata). Furthermore, the N use efficiencies of the two species are compared.
Materials and methods
Site description
This study was carried out at the Uryu Experimental Forest of Hokkaido University in northern Japan (44° 23′ N, 142° 19′ E). The total annual rainfall ranged from 962 to 1,128 mm over 2 years (2002–2004) and most rainfall occurred in the second half of both years (Fig. 1). The mean annual temperature from 2002–2004 was 4.1°C and ranged from −10°C in January to 18°C in August. The mean relative humidity ranged from 63 to 82% during the course of the study. The soil is deep with a flat topography plus occasional rock outcrops. The site is characterized by a deep snowpack (about 2 m) and a long snow cover period (November to May). The active growth phase is restricted to about 5 months (June to October) and the remaining period is dormant due to snow cover. The total amount of snow equivalent to precipitation was about 800 mm in the years 1993–1994, accounting for about 53% of the total annual precipitation (Ishikawa et al. 1998).
Uryu Experimental forest is composed of coniferous (i.e., Abies sachalinensis Mast. and Picea glehnii Mast.) and deciduous broad-leaved species (e.g., Quercus crispula Blume, Acer mono Maxim., B. ermanii, Magnolia obovata Thunberg) (Takahashi et al. 2003). The leaf longevity of these deciduous broad leaf trees is short (ca. 4–5 months). Leaf emergence starts in late May to early June and complete leaf fall occurs by mid- or late October. The understory is dominated by evergreen dwarf bamboo (S. kurilensis).
In 1998, we selected an experimental plot (225 m2) within a large tract of dense secondary forest of B. ermanii. The forest floor was densely covered with dwarf bamboo. The forest was formed by scarification, in which existing vegetation and the O layer of soil including S. kurilensis rhizomes were completely removed using a bulldozer and mineral soil was exposed on the soil surface. After scarification, a pure stand of B. ermanii was established naturally. This is a common practice used in the artificial regeneration of forests in northern Hokkaido and has been conducted on a large scale since the 1970s (Umeki 2003). S. kurilensis invaded the scarified stand through vegetative growth from outside the area. The scarification procedure was carried out in 1984, and the age of the birch forest was 17 years in 2002.
When the experimental plot was established in 1998, the mean trunk height of B. ermanii was about 5 m. Total density and total basal cover varied from 14,090–17,420 tree ha−1 and 15.4–15.7 m2 ha−1, respectively. B. ermanii shared about 96% of the total tree density. The leaf area index of the dwarf bamboo was about 0.9 (Takahashi et al. 2003). The culm height and culm diameter of the dwarf bamboo ranged from 1.5–2.5 m and 0.5–1.5 cm, respectively. Culm density and biomass ranged from 19–31 m−2 and 2,200–7,700 g m−2, respectively (our unpublished data).
General information on soil chemistry in this site has been reported by Tripathi et al. (2005). The soil bulk density and gravimetric soil water content were 0.49±0.03 g cm−3 and 61 g 100 g−1, respectively. The soil pH (1:5, soil to water ratio) was 4.4±0.1. The total C and N contents and C/N ratio of the soil were 9.2±0.2%, 0.9±0.03%, and 10, respectively. Microbial biomass C, N, and inorganic N in the soil were 2,602±263, 570±62, and 15±1.7 mg kg−1, respectively. Microbial biomass C and N were estimated by chloroform fumigation-extraction (Inubushi et al. 1984, 1991). Inorganic N (NO3 − determined using the cadmium reduction method and NH+ using the indophenol blue color method) was analyzed with a flow injection auto analyzer (FI-5000V, Aqua-Lab, Tachikawa, Japan). The net N-mineralization rate of the soil was 17 mg kg−1 month−1, calculated based on the increase in the sum of ammonium-N and nitrate-N during field incubation in buried bags.
Measurement of litterfall and litter decomposition
Litterfall was collected using 10 randomly placed circular litter traps (each 0.5 m2 area) with a perforated nylon net bottom in the growth periods of 2002 and 2003. The height of the litter traps was about 1 m. Litterfall was collected in October 2002 and at monthly intervals from June to October in 2003. The litter material was transported to the laboratory and separated into leaf and nonleaf categories of B. ermanii and S. kurilensis. S. kurilensis litterfall was also measured by establishing five permanent plots, each measuring 1×1 m, on the ground in June 2003. S. kurilensis litterfall was also estimated by litter collection from these permanent plots at monthly intervals because S. kurilensis leaf litterfall was underestimated in the above litter traps because of the trap height of 1 m. All litter material was oven dried at 80°C for 24 h then weighed. In 2003, green and mature leaves were also collected from the branches of about 10–15 individuals of B. ermanii and S. kurilensis scattered throughout the plot to determine the C and N concentration.
Freshly fallen leaf litter samples of B. ermanii and S. kurilensis were collected from the ground in May 2002 within the experimental plot (about 15×15 m) from 60 to 80 individual trees/culms. Recently dead wood branches still attached to the culm were also collected in May 2002 from 30 to 40 S. kurilensis individuals within the stand. In addition, in May 2002, S. kurilensis fine roots (≤2 mm in diameter), rhizomes and B. ermanii fine roots (≤2 mm), and coarse roots (≤5–10 mm) were collected by digging out soil monoliths. These belowground roots/rhizomes were washed using a sieve system then dried in an oven at 35°C for 3 days to a constant weight. Samples of each category were mixed thoroughly and stored in separate polythene bags. After adjusting for the initial moisture content, all litter samples (equivalent to 3 g d.w.) were enclosed in nylon net (mesh size: 1 mm 2) bags (10×10 cm).
A total of about 115 bags were prepared for different litter categories, about 20 bags each for leaves of B. ermanii and S. kurilensis and about 15 each for the remaining litter categories. Litter bags containing leaf and wood litter were placed on the forest floor just above the soil surface at different places in the first week of June 2002. Bags containing roots and rhizomes were buried in the soil to a depth of 10 cm on the same date at different places. Generally, 3–4 bags from each category were collected at each time point from each location. For statistical comparison, three replications of each litter type were used. Within the 2-year experimental period, a total of five samplings (two each in 2002 and 2003 and one in 2004) were made for each litter type. Litter bags collected on each sampling date were kept in individual polythene bags and transported to the laboratory where adhering soil particles were removed. All litter samples were weighed fresh then oven dried at 80 °C for 24 h to a constant weight. Litter moisture was calculated as the difference between the fresh and dry weights.
Chemical analysis
Initial samples of the different litter categories were powdered then analyzed in triplicate for litter quality. The ash content of a portion of the litter samples was determined as the ignition loss at 500°C (in a Muffle furnace) for 5 h. The concentration of ethanol soluble substances and lignin in different litter material was analyzed according to the procedure outlined by van Vuuren and van der Eerden (1992). First, the ethanol soluble fraction was extracted from 500 mg of powdered sample with 50 ml ethanol (3×30 min). Acid-soluble components were then removed from the residual sample using two consecutive digestions with 72 and 2.5% H2SO4, respectively. The lignin content was calculated by subtracting the ash content (estimated from the residual of acid digestion) from the mass of the residual of the two digestions. The percent cellulose content was then estimated as follows (Taylor et al. 1989): 100-(% ash+% ethanol soluble+% lignin). Using the powdered samples, C and N concentrations were determined initially and at each retrieval date using an automatic analyzer (CN-Corder, Yanaco, Japan).
Calculations and statistics
The N retranslocation efficiency (NRE) was calculated according to Finzi et al. (2001):
The N use efficiency (NUE) was calculated according to Vitousek (1984):
The mean relative decomposition rate (RDR) in decomposing litter material was calculated using the formula:
where W 0 is the mass of litter present at the beginning of each growth period (i.e., at time t 0), W 1 is the mass of litter present at the end of each growth period (i.e., at time t 1), and t 1−t 0 is the sampling interval (days) during each growth period.
The mass loss over time was fitted to a simple negative exponential model (Olson 1963):
where x 0 is the original mass of litter, x t is the amount of litter remaining after time t, t is the time (year) and k is the decomposition rate (year−1). The time required for 50% and 95% mass loss was calculated as t 50=0.693/k and t 95=3/k, respectively. The significance of differences among the various litter categories was tested by analysis of variance (ANOVA) followed by the Tukey test when the differences were significant.
Results and discussion
Litterfall and N use efficiency
Total litterfall in the study site was 377 g m−2 year−1 (Table 1), of which B. ermanii contributed about two thirds and S. kurilensis one third. The contribution of nonleaf portions in the total litterfall was only about 6%. Leaf litterfall showed seasonal variations (Fig. 2). Litterfall in different forests around the world has been reported as being strongly seasonal and driven by air temperature and precipitation patterns (Bray and Gorham 1964; Hiraizumi et al. 1996; Xu et al. 2004b). In this study, litterfall of both species occurred from June to October; however, most of the leaf litterfall (about 66% of the total) of B. ermanii was recorded in October while the same proportion of S. kurilensis was noted in September and October. The leaf litterfall of S. kurilensis, an evergreen species, is likely to have been underestimated, as this species is covered with snow for a large part of the year. Although this study focused on litterfall during the growing season, litterfall might also occur in the dormant season even in the snowpack period (Hardiwinoto 1991a). Further study, including investigation during the dormant season, is therefore necessary to accurately quantify the annual litterfall in this region.
In the present study, litterfall was relatively smaller than reported values (390–490 g m−2 y−1) in subalpine and cool-temperate forests (Iwatsubo and Nishimura 1977) and was about half than that of the values reported in other forests of Japan and China (Tsutsumi 1987; Liu et al. 2002; Xu et al. 2004b). Using C and N concentrations (Table 2) and litterfall data, C and N return in this forest were quantified as 168 g C m−2 year−1 and 4.7 g N m−2 year−1, respectively. The amount of N return to the soil in the present study was about 1.5 times greater than in the report of Hardiwinoto (1991a) in four forest types in Uryu Experimental forest dominated by Quercus, Betula, Abies, and Picea, respectively. N return in litterfall was within the range of previously reported values in Japan (Iwatsubo and Nishimura 1977), but about half of that reported in forests in the Okinawa Islands, south Japan, and moist evergreen broad-leaved forest in southwest China (Liu et al. 2002; Xu et al. 2004b).
Efficient use of N is generally characterized by litterfall with a high C/N ratio (or low N concentration) (Vitousek 1984; Grubb 1989). In the present study, as a result of the lower N concentration of S. kurilensis leaf litter (10.06 mg g−1) compared to that of B. ermanii (14.5 mg g−1), the N use efficiency of S. kurilensis leaf litter (96 g g−1) was also higher than that of B. ermanii (69 g g−1, Table 1). This higher N use efficiency indicates greater N withdrawal (retranslocation) from senescing leaves to permanent storage organs (i.e., rhizomes), which means that the contribution of S. kurilensis litterfall to N return to the soil is relatively small. This withdrawal mechanism makes the plant partly independent of N from the soil because N is available by remobilization when absorption from the soil is impossible under stressful conditions. In this study, the concentration of N in S. kurilensis litter was lower than the reported concentration in litter (2.3%) of the bamboo species S. senanesis growing in this region (Hardiwinoto 1991b).
The significantly lower N content in abscised leaves relative to green leaves in both species (Table 2) suggests retranslocation of N from senescent leaves to the plant body before abscission. The NRE of B. ermanii and S. kurilensis were 34 and 51%, respectively. The NRE calculated from Hardiwinoto (1991a) for B. platiphylla (37%) was comparable to that for B. ermanii in the present study. Though the N content of green leaves of both species studied was nearly comparable (Table 2), the NRE of S. kurilensis was higher because of the considerable decrease in N content in the respective litter. This probably occurred because S. kurilensis is an invader understory species and has evolved an N conservation mechanism to compete with overstory species for limiting nutrients such as N. Permanent storage organs like the rhizome of S. kurilensis might help to conserve N retranslocated from the leaves and remobilize it when required. The C content of green and abscised leaves of both species was not significantly different.
Mass loss during decomposition
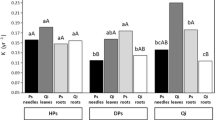
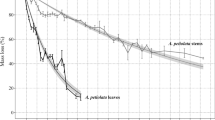
Litterfall mass remaining after the 2-year study period varied considerably among different categories and between species (Table 3). The maximum weight loss was recorded in B. ermanii leaf and S. kurilensis fine root litter and the minimum in S. kurilensis wood litter. The RDR of different litter categories ranged from 0.2 to 1.8% day−1 (Fig. 3). The RDR in the first year of decomposition differed significantly between the growth (June to October) and dormant period (November to May). In the first growth period, the RDR was significantly (P<0.05) higher than in the second growth period for all components except S. kurilensis fine root and B. ermanii leaf litter. Xu and Hirata (2005) reported a rapid initial mass loss in litter of different forest species followed by lower rates at later stages, except in a few cases. Instantaneous annual decay rates (k) calculated at the end of the study period ranged from 0.21 for S. kurilensis leaf and wood litter to 0.37 for B. ermanii leaf litter (Table 3). Relative mass loss rate trends observed among species and litter categories during the first year continued through the second year (Fig. 4a,b).
Periodical changes in relative decomposition rates (RDR; see Eq. 3 in the text) of different components of Betula ermanii and Sasa kurilensis in a young secondary forest in northern Japan. Bars with different superscript letters denote a significant difference (P<0.05; Tukey’s HSD). L leaves, CR coarse root, FR fine root, W wood, and Rh rhizome
The significantly higher litter decomposition rates of most components (except S. kurilensis fine root and B. ermanii leaf litter) in the first growth period (i.e., from June to October) reflect the favorable effects of increased precipitation and temperature, which help accelerate the rate of decomposition. However, low temperatures due to heavy snow in the dormant period (i.e., from November to May) likely decreased the activity of decomposer organisms causing a consequent decrease in the rate of litter decomposition. The annual decay constants of the different components in the present study were broadly comparable to the annual k values reported in previous forest leaf litter decomposition studies in Japan (Salamanaca et al. 1998; Xu and Hirata 2005).
Initial litter chemical quality
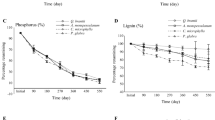
The initial chemical composition of different litter categories and species varied substantially (Table 4). The concentration of N in woody components, including B. ermanii coarse roots and S. kurilensis rhizomes and wood, was low (6.9 to 8.1 mg g−1), while the lignin content was high (417 to 447 mg g−1) compared to other litter categories. On the other hand, the concentration of N in leaf and fine root litter was high (9.8 to 15.1 mg g−1) and lignin concentration was low (265 to 308 mg g−1) in both species. Concentrations of lignin and N in the present study were broadly comparable to those of litter of seven other tree species including Castanopsis sieboldii and Schima wallichii in a Japanese forest (Xu and Hirata 2005). Significant correlations were observed among some chemical quality parameters. For example, as shown in Table 4, ethanol soluble content was negatively correlated with lignin content (r=0.82, P<0.05) and the C/N and lignin/N (r=0.87, P<0.05) ratios and positively with N content (r=0.87, P<0.05). Lignin and ash contents were significantly negatively correlated with C and N (r=0.85–0.91, P<0.05) contents, respectively.
Relationship between mass loss and abiotic variables
To evaluate the effect of prevailing environmental factors on litter mass loss of the different litter categories in this B. ermanii forest, we selected precipitation, air temperature, relative humidity (Fig. 1), and litter moisture as abiotic variables. The rates of litter mass loss of different litter categories were respectively correlated with mean values (for corresponding intervals between litterbag collection) of air temperature, relative humidity, and cumulative rainfall, and litter moisture at each retrieval date. Rainfall was significantly and positively correlated (r 2=0.75–0.80, P<0.05) with litter mass loss, except for S. kurilensis fine root litter (P>0.05) (Fig. 5). However, correlations with the other abiotic variables were not significant. Forward step-wise multiple regression analysis was conducted to evaluate the combined effect of different abiotic variables on mass loss, but none of the variables entered the equation other than rainfall, which explained 75–80% of the variability in mass loss. Similarly, the RDR was also correlated with the above abiotic variables, but not significantly.
Tripathi and Singh (1992a) previously found a significant correlation between mass loss and air temperature, litter moisture and rainfall in India. They also found an interactive effect of litter moisture with both air temperature and rainfall. These two factors combined explained about 56–59% of the variability in mass loss of bamboo leaf and root, and grass shoot litter. However, in the present study, we found no combined effects of abiotic variables, suggesting the need for more frequent retrieval of litter bags.
Effect of litter quality on mass loss and N release
The effect of initial litter quality on decomposition rates was evaluated by correlating mass loss of different components at various sampling dates with their initial chemical composition (i.e., ethanol soluble substances, cellulose, lignin, ash, C and N contents, and the ratios of lignin/N and C/N). The concentrations of ethanol soluble substances and N were strongly positively correlated (r=0.77 to 0.86 and 0.87 to 0.97, respectively, P<0.05) while lignin was negatively correlated (r=0.78–0.88, P<0.05) with mass loss at early (41 days) and late stages (520 and 770 days) of decomposition, respectively. However, ratios of C/N and lignin/N were significantly negatively correlated (r=0.78–0.96, P<0.05) with mass loss at all stages of litter decomposition. Cellulose was weakly correlated with mass loss. The best predictors of early stage litter mass loss were shown to be the C/N ratio, N content and lignin/N ratio, which explained about 85, 81, and 81% of the variability, respectively, followed by lignin (61%) and ethanol soluble substances (59%). At the end of the study, these variables respectively accounted for about 67 to 81% of the variability in mass loss.
Lignin, N and the C/N, and lignin/N ratios were the best predictors of C loss, accounting for about 72 to 81% of the variability at the end of the study. However, the N loss rate was not significantly correlated with any variables except cellulose, which explained about 69% of the variability at the end of the study.
The significant role of initial substrate quality in mass loss has been observed in various ecosystems and species in the field and microcosm studies. For example, Melillo et al. (1982) showed a strong negative correlation between the initial lignin to N ratio and mass loss of leaf litter from northern hardwood areas in the US. Cornelissen (1996) also reported a negative correlation between mass loss and the initial lignin content and lignin/N ratio in a variety of species. Moreover, Taylor et al. (1989) found the C/N ratio to be a better predictor of mass loss than the lignin/N ratio in a microcosm study. In the present study, the C/N ratio seems to be a better predictor of mass loss than the lignin/N ratio or N content. Decreasing rates of dry mass loss were reported to be significantly correlated with the initial C/N ratio during litter decomposition in a subtropical forest in Japan (Xu et al. 2004a). Tripathi and Singh (1992b) found a combined role of lignin and the C/N ratio, which were shown to explain about 93% of the variability in mass loss. However, the same authors also found a combined effect of lignin and N, accounting for about 84% of the variability in annual N mineralization. We attempted forward step-wise multiple regression to assess the combined effect of different litter quality parameters but none of the variables entered the equation.
Carbon and N dynamics
Concentration of N in residual materials (%) increased significantly in B. ermanii leaf and fine root and S. kurilensis leaf and rhizome litter with progressing decomposition (Table 5). In contrast, the C concentration of leaf litter of both species decreased while it increased in S. kurilensis rhizome litter during the course of decomposition; in other components no significant relationships existed. During the course of litter decomposition, increased concentrations of N in litter have been reported due to the rapid loss of labile fractions, i.e., water soluble substances as well as free unshielded cellulose (Berg et al. 1997; Berg 2000).
Generally, the pattern of C stock loss in the present study was similar to that of mass loss (Fig. 4c,d). However, the change in N stock showed an initial increase followed by a gradual decrease (Fig. 4e,f), differing from both mass loss and C stock loss, probably as a result of N immobilization by microorganisms during the initial stage of decomposition followed by N mineralization. S. kurilensis fine root litter showed a relatively greater release of N than other components. Litter components with a high C/N ratio and/or low initial N content showed slow release or high N immobilization (Rutigliano et al. 1998). S. kurilensis fine root litter exhibited an initially high N concentration and low C/N ratio and greater N release than the other components. At the end of the experiment, the release of N was shown to be slower than the mass loss and C loss in all components, except S. kurilensis fine root litter. Litter with high ratios of initial C/N and lignin/N showed greater N immobilization than litter with a low C/N ratio (Tripathi and Singh 1992b; Osono and Takeda 2004). Nitrogen immobilization during the course of litter decomposition has also been reported by Berg and Soderstrom (1979) and Upadhyay and Singh (1989) with forest tree species such as Pinus ruxburghii, Rhododendron arboreum, and a number of species of Quercus, namely, Quercus glauca, Quercus floribunda, Quercus leucotrichophora, and Quercus lanuginose. Moreover, Tripathi and Singh (1992b) reported strong N immobilization during the decomposition of different parts of bamboo and grasses.
Conclusion
The findings show that litter decomposition in the study forest is mainly regulated by rainfall, initial concentrations of lignin and N, and ratios of C/N and lignin/N. N conservation mechanisms like the greater N use efficiency of dwarf bamboo (S. kurilensis) (Table 1) likely serve to strengthen competitive ability, favoring establishment under the birch canopy and sustaining high productivity even in N-limited conditions. Furthermore, the pattern of N release regulated by microbial processes such as immobilization and mineralization of N (Fig. 4e,f) varied between different litter components of the two species during decomposition, ensuring N availability for plant growth in B. ermanii forests of Northern Japan where S. kurilensis forms dense undergrowth.
References
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133:13–22
Berg B, Soderstrom B (1979) Fungal biomass and nitrogen in decomposing Scots pine needle litter. Soil Biol Biochem 11:339–341
Berg B, Staaf H (1980) Decomposition rate and chemical changes of Scots pine needle litter. II. Influence of chemical composition. In: Persson T (ed) Structure and function of northern coniferous forests—an ecosystem study. Swedish Research Council, Stockholm. Ecol Bull (Stockholm) 32:373–390
Berg B, Berg M, Bottner P, Box E, Breymeyer A, Calvo de Anta R, Couteaux M, Gallardo A, Escudero A, Kratz W, Madeira M, Malconen E, Meentemeyer V, Munoz F, Piussi P, Remacle J, Virzo De Santo A (1993) Litter mass loss in pine forests of Europe and Eastern United States as compared to actual evapotranspiration on a European scale. Biogeochemistry 20:127–153
Berg B, McClaugherty C, Johanasson MB (1997) Chemical changes in decomposing plant litter can be systemized with respect to the litter’s initial chemical composition. Reports from the Departments of Forest Ecol and For Soils. Swed Univ Agric Sci report 74
Bray JR, Gorham E (1964) Litter production in the forests of the world. Adv Ecol Res 2:101–157
Cornelissen JHC (1996) An experimental comparisons of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol 84:573–582
Facelli JM, Pickett STA (1991) Plant litter: its dynamics and effects on plant community structure. Bot Rev 57:1–32
Finzi AC, Allen AS, DeLucia EH, Ellsworth DS, Schlesinger WH (2001) Forest litter production, chemistry and decomposition following two years of free-air CO2 enrichment. Ecology 82:470–484
Fioretto A, Papa S, Fuggi A (2003) Litterfall and litter decomposition in a low Mediterranean shrubland. Biol Fertil Soils 39:37–44
Grubb PJ (1989) The role of mineral nutrients in the tropics: a plant ecologist view. In: Proctor J (ed) Mineral nutrients in tropical forest and savanna ecosystems. Blackwell, Oxford, pp 153–159
Hardiwinoto S (1991a) Nutrient elements in the litterfall of deciduous broad-leaved forests and evergreen coniferous forests in Northern Hokkaido, Japan. Res Bull Coll Exp For 48:307–323
Hardiwinoto S (1991b) Decomposition process and dynamics of nutrient elements in deciduous broad-leaved and evergreen coniferous forests of Northern Hokkaido, Japan. Res Bull Coll Exp For 48:325–353
Heal OW, Anderson JM, Swift MJ (1997) Plant litter quality and decomposition: an historical overview. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, UK, pp 3–45
Hiraizumi S, Kawaguchi J, Tadaki Y (1996) The return of nutrients with litterfall in some secondary forests in Nagoya. Bull Nagoya Univ For 15:123–140
Inubushi K, Wada H, Takai Y (1984) Determination of microbial biomass-nitrogen in submerged soil. Soil Sci Plant Nutr 30:455–459
Inubushi K, Brookes PC, Jenkinson DS (1991) Soil microbial biomass C, N and ninhydrin-N in aerobic and anaerobic soils measured by the fumigation-extraction method. Soil Biol Biochem 23:737–741
Ishikawa N, Nakabayashi H, Ishi Y, Kodama Y (1998) Contributions of snow to the annual water balance in Moshiri watershed, Northern Hokkaido, Japan. Nordic Hydrology 29:347–360
Ishizuka M, Sugahara S (1986) Composition and structure of natural mixed forests in central Hokkaido I. Composition differences and species characteristics by elevation and from disturbances. J Jpn For Soc 68:79–86
Iwatsubo G, Nishimura T (1977) Water, Carbon and Mineral Cycling. In: Shidei T, Kira T (eds) Primary productivity of Japanese forests: JIBP synthesis, vol 16. University of Tokyo Press, Tokyo, pp 115–168
Kaneko N, Salamanaca EF (1999) Mixed leaf litter effects on decomposition rates and soil microarthopod communities in an oak-pine stand in Japan. Ecol Res 14:131–138
Koike T (1987) The growth characteristics in Japanese mountain birch (Betula ermanii) and white birch (Betula platyphylla var. japonica), and their distribution in the northern part of Japan. In: Fujimori T, Kimura M (eds) Human impacts and management of mountain forests. FFPRI, Ibaraki, Japan, pp 189–200
Liu W, Fox JED, Xu Z (2002) Litterfall and nutrient dynamics in a montane moist evergreen broad-leaved forest in Ailao Mountains, SW China. Plant Ecol 164:157–170
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Mtambanengwe F, Kirchman H (1995) Litter from a tropical savanna woodland (Miombo): chemical composition and C and N mineralization. Soil Biol Biochem 27:1639–1651
Olson JS (1963) Energy storage and balance of producers and decomposers in ecological systems. Ecology 44:322–331
Ohsawa M, Tezuka T, Numata M (1973) Altitudinal distribution of forest vegetation on Mt. Poroshiri, the Hidaka range, Hokkaido, Japan. Mem Nat Sci Mus 6:183–198
Osono T, Takeda H (2001) Organic chemical and nutrient dynamics in decomposing beech leaf litter in relation to fungal ingrowth and succession during 3-year decomposition process in a cool temperate deciduous forest in Japan. Ecol Res 16:649–670
Osono T, Takeda H (2004) Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res 19:593–602
Rutigliano FA, Alfani A, Bellini L, Vizo De Santo A (1998) Nutrient dynamics in decaying leaves of Fagus sylvalica L. and needles of Abies alba Mill. Biol Fertil Soils 27:119–126
Salamanaca EF, Kaneko N, Shigeo K, Nagayama Y (1998) Nutrient dynamics and lignocellulose degradation in decomposing Quercus serrata leaf litter. Ecol Res 13:199–210
Sariyildiz T, Anderson J M, Kucuk M (2005) Effects of tree species and topography on soil chemistry, litter quality, and decomposition in Northeast Turkey. Soil Biol Biochem 37:1695–1706
Singh KP, Singh PK, Tripathi SK (1999) Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoil at Singrauli, India. Biol Fertil Soils 29:371–378
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Blackwell, Oxford, UK
Takahashi K, Uemura S, Suzuki J, Hara T (2003) Effects of understory dwarf bamboo on soil water and growth of overstory trees in a dense secondary Betula ermanii forest, Northern Japan. Ecol Res 18:767–774
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Tripathi SK, Singh KP (1992a) Abiotic and litter quality control during the decomposition of different plant parts in dry tropical bamboo savanna in India. Pedobiologia 36:109–124
Tripathi SK, Singh KP (1992b) Nutrient immobilization and release patterns during plant decomposition in a dry tropical bamboo savanna, India. Biol Fertil Soils 14:191–199
Tripathi SK, Singh KP (1995) Litter dynamics of recently harvested and mature bamboo savannas in a dry tropical region in India. J Trop Ecol 11:403–417
Tripathi SK, Sumida A, Shibata H, Uemura S, Ono K, Hara T (2005) Growth and substrate quality of fine root and soil nitrogen availability in a young Betula ermanii forest of Northern Japan: Effects of the removal of understory dwarf bamboo (Sasa kurilensis). For Ecol Manag 212:278–290
Tsutsumi T (1987) Nutrient cycling in forest ecosystems. Tokyo University Press, Tokyo
Umeki K (2003) The regeneration of natural forests on Hokkaido, Northern Japan: Results of scarification and problems remaining to be solved. J Jpn For Soc 85:246–251
Upadhyay VP, Singh JS (1989) Patterns of nutrient immobilization and release in decomposing forest litter in central Himalaya, India. J Ecol 77:127–146
van Vuuren MMI, van der Eerden LJ (1992) Effects of three rates of atmospheric nitrogen deposition enriched with 15N on litter decomposition in a heathland. Soil Biol Biochem 24:527–532
Vitousek PM (1984) Litter fall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Watanabe S (1979) The subarctic summer green forest zone in the northeastern Asia. Bull Yokohama Phytosoc Soc Jpn 16:101–111
Xu X, Hirata E (2005) Decomposition pattern of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 273:279–289
Xu X, Hirata E, Enoki T, Tokashiki Y (2004a) Leaf litter decomposition and nutrient dynamics in a subtropical forest after typhoon disturbance. Plant Ecol 173:161–170
Xu X, Hirata E, Shibata H (2004b) Effects of typhoon disturbance on fine litterfall and related nutrient input in a subtropical forest on Okinawa Island, Japan. Basic Appl Ecol 5:271–282
Acknowledgements
We thank Dr. Keita Arakawa for providing laboratory facilities and Dr. Meghraj Bhandari for helping with analysis. We also thank the staff of Uryu Experimental Forest of Hokkaido University for their support in the field. The Institute of Low Temperature Science, Hokkaido University and JSPS authorities are thankfully acknowledged for providing financial support as part of a project entitled “Interaction through soil resource utilization between trees and undergrowth Sasa species in sub-boreal forests in Japan”. This research was also supported by CREST of JST (Japan Science and Technology Agency), under “Parameterization of the Relationships between the Water Cycle System and Plant Eco-physiological Properties in Boreal Forest Areas (R & D of Hydrological Modeling and Water Resources)”. SKT is thankful to the Council of Scientific and Industrial Research, New Delhi, for granting leave to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tripathi, S.K., Sumida, A., Shibata, H. et al. Leaf litterfall and decomposition of different above- and belowground parts of birch (Betula ermanii) trees and dwarf bamboo (Sasa kurilensis) shrubs in a young secondary forest in Northern Japan. Biol Fertil Soils 43, 237–246 (2006). https://doi.org/10.1007/s00374-006-0100-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-006-0100-y