Abstract
Aiming to support the use of native species from the Atlantic Rainforest in local agroforestry systems, we analysed chemical and biochemical components related to leaf decomposition of Inga subnuda, Senna macranthera, Erythrina verna, Luehea grandiflora, Zeyheria tuberculosa, Aegiphila sellowiana, and Persea americana. These tree species are native (except for P. americana) and commonly used in agroforestry systems in the Atlantic Rainforest. For the three first species (Fabaceae), we also analysed the remaining dry matter and released nutrients from leaves, using litter bags, and biological nitrogen fixation, using Bidens pilosa and Brachiaria plantaginea as references of non-N2-fixing plants. Leaves from I. subnuda, L. grandiflora, and P. americana had a lower decomposition rate than the other species, exhibiting negative correlations with lignin/N and (lignin+polyphenol)/N ratios. The percentages of remaining dry matter after 1 year were 69 % (I. subnuda), 26 % (S. macranthera) and 16 % (E. verna). Higher nutrient release was found in decreasing order from residues of E. verna, S. macranthera, and I. subnuda. The percentages of nitrogen fixation were 22.6 % (E. verna), 20.6 % (I. subnuda) and 16.6 % (S. macranthera). Diversification of tree species in agroforestry systems allows for input of diversified organic material and can contribute to maintaining and improving soil functions resulting in improvements of soil quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Only a small part of the huge tropical plant biodiversity is used in agro-ecosystems. Those plants that are used have been systematically overlooked by science (Leakey et al. 2005). For these reasons, agro-ecosystems lose important ecosystem services associated with biodiversity, and they do not contribute as much as possible to environmental preservation. For instance, in the Atlantic Rainforest biome, which is one of the five biodiversity hotspots of the world (Myers et al. 2000), the agricultural matrix of coffee (Coffea arabica L.) in full sun and monoculture pasture (Cardoso et al. 2001) does not contribute to preservation of the numerous forest fragments that still exist in the biome. A more diversified agricultural matrix using agroforestry where native trees are intercropped with coffee and pasture has been regarded as one of the best options for conservation of this biome, whose natural habitats have been largely converted for agricultural or other use (Perfecto and Vandermeer 2008).
Atlantic Rainforest tree species are not widely used in agro-ecosystems, and little is known about their functions. Despite the importance of intrinsic biodiversity values, the trees by themselves do not justify monetary and political efforts in the study and conservation of biodiversity in most cases (Moonen and Bárberi 2008). These efforts are only fruitful if biodiversity has a role in the functioning of agro-ecosystems and if these systems directly or indirectly benefit human society. Such benefits are known as ecosystem services (Costanza et al. 1997).
However, the trees of the Atlantic Rainforest can be used in agroforestry systems, where they benefit nature conservation and provide ecosystem services. For instance, agroforestry systems can serve as corridors, linking forest fragments, which contribute to their preservation (Perfecto and Vandermeer 2008) and the use of native tree species in agroforestry systems can improve soil quality, an important ecosystem service (Souza et al. 2012). The organic material (above- and below-ground litter) produced by trees in agroforestry systems protects the soil against erosion, serves as food for soil organisms, improves soil structure, and contributes to nutrient cycling, thus increasing nutrient availability to plants (Beddy et al. 2010).
The use of native trees in agroforestry systems in the tropics could be prioritised. These trees usually have efficient mechanisms to deal with exchangeable acidity and low levels of available nutrients, especially N and P (Kanmegne et al. 1999). Species of the Fabaceae (legumes) are of particular importance because many of them are associated with N2-fixing bacteria, which contribute to the continuous incorporation of N in the system, thereby stimulating cycling of other nutrients (Sá and Vargas 1997). Positive interactions between N2-fixing and non-fixing tree species have been widely reported in diverse cropping systems, including coffee systems (Nyfeler et al. 2011).
Decomposition and nutrient cycling rates depend both on environmental conditions and quality of the plant material, which is determined by its (bio-) chemical composition. These characteristics are often used to predict decomposition rates and their effects on soil nutrient availability (Mendonça and Stott 2003; Silva et al. 2008). Nitrogen and lignin (LG) mass fractions, C/N ratios, polyphenol (PP)/N ratios, and (LG+PP)/N ratios are considered to be the best predictors of N release by species in agroforestry systems (Palm and Sanchez 1991).
Material with high contents of compounds with slow decomposition (LG and PP) and low content of N hinder action of microorganisms, leading to a lower rate of decomposition and nutrient release. Materials with a fraction of N lower than 1.74 % (Palm 1995), hence a C/N ratio larger than 28.7 (assuming 50 % C), typically have low decomposition rates. Decomposition rates also decrease as a function of LG+PP content (Palm 1995; Hadas et al. 2004). According to Palm (1995), N mineralisation rate decreases when N is smaller than 1.74 %, or when LG and PP are larger than 15 and 3 % respectively, and the (LG+PP)/N ratio is larger than 10. Plant materials with PP values lower than 0.5 % are considered of high quality and can be decomposed more rapidly (Tian et al. 1992). According to Silva et al. (2008), materials that have LG/N ratios larger than 5 (different from Palm 1995, LG/N >8.5) have low decomposition rates. Even though slow litter decomposition hinders nutrient release, litter accumulation can contribute to better soil protection, higher moisture content, and less soil loss through erosion (Silva et al. 2008).
For native tropical species that are currently used or have the potential for use in agroforestry systems, information about decomposition and nutrient release is scarce, which hampers to implement and manage agroforestry systems. To provide this information, the current study had the following aims: (i) to assess leaf decomposition of seven tree species used in agroforestry systems in the Atlantic Rainforest (ii) to test if the decomposition of the leaves of those trees is correlated with their (bio-) chemical quality (iii) to assess nutrient release from the leaves of three species of the Fabaceae and (iv) to quantify biological nitrogen fixation (BNF) of these three species.
Materials and methods
Experimental area
The plant material used in this study was collected from agroforestry systems in the municipalities of Araponga (20°48′S and 42°32′W) and Divino (20°33′S and 42°11′W). Both municipalities are located in Zona da Mata (Minas Gerais, Brazil) in the Atlantic Rainforest biome.
The average temperature of the region is 18 °C, and the annual rainfall varies from 1,200 to 1,800 mm with a dry period of 2–4 months. The terrain is mountainous with slopes ranging from 20° to 45° (Golfari 1975). Soils are predominantly oxisols, which are deep and well drained. These soils are acidic and have low nutrient availability, particularly phosphorus (P). The area is mainly used for pasture and full sun coffee (cash crop) production. Coffee is usually intercropped with beans, corn, and other subsistence crops (Cardoso et al. 2001).
In this region, subsistence family farmers are predominant, and they are considered of vital importance, especially with regard to food and coffee production. To improve soil quality, farmers began experimenting agroforestry coffee systems in 1994, with the support of the Center for Alternative Technologies of Zona da Mata (NGO), the Federal University of Viçosa and organisations composed of family farmers, especially rural worker unions (Cardoso et al. 2001). Along the experimenting process, the farmers selected various tree species to be used in the agroforestry coffee and pasture systems consisting of native species of the Atlantic Rainforest and some exotic species, which are adapted to the region and are often fruit-bearers (Souza et al. 2010, 2012).
Species studied
The species selected for this study are among the most widely used in agroforestry coffee systems in Araponga and Divino: Aegiphila sellowiana Cham. (Verbenaceae), Inga subnuda subsp. luschnathiana (Benth.) T. D. Penn. (Fabaceae), Erythrina verna Vell (Fabaceae), Luehea grandiflora Mart. (Malvaceae), Senna macranthera (DC. ex Collad.) HS Irwin & Barneby (Fabaceae), Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae), and Persea americana Mill. (Lauraceae). Except P. americana, all species are native to the Atlantic Rainforest.
Four randomly selected individuals (considered replicates) of each species were selected from four agroforestry systems located in the properties of family farmers. According to the farmers, the individually sampled trees were introduced as seedlings or spontaneously established on the farms in 1994, with the exception of E. verna, which was introduced to the system in 1998. Only A. sellowiana individuals were drastically pruned during the collection of material.
Leaf sampling
For the carbon dioxide fluxes, chemical and biochemical characterisation of plant material and decomposition and nutrient release, physiologically mature leaves of the four individuals (considered replicates), of each of the seven tree species were sampled. Physiologically mature leaves were characterised by a fully expanded limb and no signs of senescence or necrosis.
In October 2005, fresh leaf samples were collected at the middle canopy position from points located at the North, South, East, and West coordinates (~20 leaves from each coordinate). Most authors dealing with residue decomposition in agroforestry systems consider litterfall as basis for decomposition studies and there is a lack of information about the characteristics and decomposition dynamics of pruned material, a normal practice in agroforestry systems. Therefore, we studied freshly picked leaves more similar to pruned material than litterfall.
The collected material from each individual was pooled, forming a composite sample, dried in a forced-air circulation oven (72 h at 65 °C) and ground for further analysis. For the Fabaceae species, part of the fresh material was separated for remaining dry matter and released nutrients study.
Carbon dioxide fluxes from leaves
Carbon dioxide fluxes from leaves were analysed by respirometry in which CO2–C evolution was measured using a Stotzky respirometer as previously described by Curl and Rodriguez-Kabana (1972). The composed leaf samples of each individual of the seven species were used to determine total C and N by the dry combustion method using an elemental analyser (Perkin Elmer CHNS/O 2400).
In glass bottles (500 ml), plant material corresponding to 2 g of C from each composite sample was mixed with 100 cm3 of B horizon soil (Oxisol), and soil moisture content was increased to 70 % of field capacity. The soil was acidic (pH 4.5), had low available P (1.0 mg dm−3), K (13 mg dm−3), Ca and Mg (0.0 cmolc dm−3) and high exchangeable Al (1.4 cmolc dm−3). The carbon content was 20.9 g kg−1 and field capacity 0.32 kg kg−1 (Embrapa 1999).
The glass bottles containing soil and plant material were connected to the elemental analyser and subjected to continuous flow of free-air CO2. Evolved CO2 was then captured by a NaOH solution and quantified by 0.25 M HCl titration with a phenolphthalein indicator. Controls included four empty bottles and four bottles containing soil without addition of leaf litter. The bottles were prepared in duplicate, and randomized in the respirometer (a total of 72 bottles: four replicates of seven species and two controls, each replicate in duplicate). Fifteen CO2 measurements were taken for each flask over a period of 37 days. The total amount of CO2 produced was calculated from the sum of the values obtained during each sampling. The CO2 (mg C g−1 soil) calculation was performed using the formula:
where B is the volume of HCl in an empty vial (ml); V is the volume of HCl used in the sample (ml); M is the concentration of HCl; 6 is the atomic mass of carbon (12) divided by moles of CO2 that react with NaOH (2); V1 is the volume of NaOH used to capture CO2 (ml); and V2 is the volume of NaOH used in titration (ml).
Leaf chemical and biochemical characterisation
Chemical and biochemical characterisations were performed on composite samples (without replications) of each species in duplicate. N was determined with the Kjeldahl method after sulphuric acid digestion (Tedesco et al. 1995). P was measured with the colorimetric method in which the molybdate-phosphate complex turns blue in the presence of ascorbic acid (Braga and Defelipo 1974). For calculating C/N and C/P, we assumed that plant dry matter consists of 50 % carbon (Pribyl 2010).
Soluble polyphenols (PPs) were extracted with methanol (50 %) and determined by colorimetry using the Folin–Denis reagent (Anderson and Ingram 1993). Lignin (LG), cellulose (CL), and hemicellulose (HC) were determined with the neutral detergent fibre (NDF) and acid detergent fibre (ADF) methods, which are referred to as the van Soest method (Goering and van Soest 1970; van Soest et al. 1991). The HC values were determined by subtracting ADF from NDF. Lignin contents were obtained from the ADF fraction. CL contents were obtained by subtracting lignin from ADF. Cumulative CO2 flux during a period of 37 days was correlated with mass fractions of LG, CH, CL, PP, N, and P, and also with C/N, LG/N, LG/PP, PP/N, and (LG+PP)/N ratios.
Decomposition and released nutrients
Remaining dry matter and released nutrients were assessed for the three legume species I. subnuda, S. macranthera, and E. verna. Fresh leaves (75 g; in duplicate) from four trees (replicates) of each species were placed in litter bags (20 × 20 cm) with mesh size 2 × 6 mm, allowing most animals of mesofauna (and partly macrofauna) to pass through. Dry matter weight (dried at 65 °C in an oven) was determined in samples equivalent to 75 g of fresh material. The litter bags were randomly distributed in a shaded area located on the campus of the Federal University of Viçosa. The campus is situated in the same region, with the same class of soil and climate as the municipalities where the leaves were collected, thus, we assumed to have similar decomposer biota. Temperature and monthly rainfall data in Viçosa during the experiment are shown in Fig. 1.
Two litter bags of four replicates of each tree species were collected at 5, 15, 30, 60, 90, 120, and 150 days (24 litter bags each time). After collection, litter was cleaned and impurities, such as soil, insects, roots, and other materials, removed using brushes and tweezers. The material was then dried in an oven (65 °C) until constant weight and remaining dry matter was calculated.
To analyse decomposition and nutrient mineralisation rate, the one-component exponential decomposition model was applied:
where X is the amount of remaining dry matter or nutrients after a period of time (t; in days); X0 is the amount of dry matter or nutrients at t = 0; k is the decomposition constant (day−1).
Half-life was calculated as t1/2 = ln (2)/k, where t1/2 is half-life (days) for dry matter or nutrients, time required for half of these residues to disappear.
Total N content in the residues was determined using the Kjeldahl method after sulphuric acid digestion. After nitric/perchloric digestion, the P and K contents were determined by flame photometry, and the Ca and Mg contents were determined by atomic absorption spectrophotometry (Braga and Defelipo 1974).
Atmospheric N2 fixation
To assess N2 fixation of the legumes, the 15N natural abundance technique was used (Peoples et al. Peoples et al. 1989). The percentage of N2 fixed by legumes (BNF %) was estimated with the following formula:
where δ 15 N reference is the natural enrichment of the non-N2-fixing reference species; δ 15 N legume is the natural enrichment of the legume evaluated in the system; and B is the 15N natural abundance (δ 15 N) of the legume grown under exclusive dependence on N2. The B value used was −1.3 ‰ based on the shrub Prosopis glandulosa (Shearer et al. 1983); this value was also used by Teixeira et al. (2006). Despite having more shallow root systems compared to the legume trees, two herbaceous plants, Bidens pilosa (Asteraceae) and Brachiaria plantaginea (Poaceae), were used as non-N2-fixing reference plants. Mature leaves of five plants of B. pilosa and entire shoots of B. plantaginea were collected nearby the trees. The samples were grouped to form one composite sample per reference species.
In January 2006, physiologically mature leaves (fourth pair of leaves on the branch; leaf + petiole) were collected from four individuals (replicates) of each species of the Fabaceae (I. subnuda, S. macranthera, and E. verna). Sampling was performed at the middle canopy position at points located at the North, South, East, and West coordinates (replicates) and pooled to form a composite sample per individual.
Materials from legumes and reference plants were placed in paper bags and subsequently dried in a forced-air circulation oven at 65 °C for 72 h. The materials were then milled and ground in a ball mill followed by 15N analysis with a mass spectrometer (SERCON; model ANCA GLS 20.20).
Statistical analysis
Analyses of variance were performed for respirometry and decomposition followed by planned comparisons to test differences between means. Regression analyses were also performed between decomposition and time; we do not present parameter estimates when these regressions were not significant. Statistica (Statsoft 1997) was used for the statistical analyses.
Results
CO2 fluxes
The average and the standard errors (n = 4) of CO2 fluxes from leaf litter of the seven tree species over a period of 37 days are shown in Fig. 2. At the end of this period, there were differences among species (F 7,21 = 359.0; P < 0.001). Two groups of species that significantly (P < 0.05) differed in the amount of CO2 produced could be distinguished: species that produced more CO2, including S. macranthera, E. verna, Z. tuberculosa, and A. sellowiana; and species that produced less CO2, including P. americana, I. subnuda, and L. grandiflora.
Leaf chemical and biochemical characterisation
Table 1 summarises data on leaf (bio-)chemistry. LG ranged from 7.7 % (E. verna) to 27.3 % (I. subnuda); HC ranged from 13.4 % (P. americana) to 41.6 % (E. verna); CL ranged from 11.3 % (S. macranthera) to 21.3 % (I. subnuda); PP ranged from 4.4 % (Z. tuberculosa) to 8.3 % (L. grandiflora); N ranged from 2.0 % (L. grandiflora) to 3.82 % (A. sellowiana); and P ranged from 0.11 % (Z. tuberculosa) to 0.19 % (S. macranthera and A. sellowiana).
The three species with the lowest CO2 fluxes (Fig. 2) had the highest (LG+PP)/N ratio, and these species were P. americana (13.8), L. grandiflora (10.9), and I. subnuda (10.1). The species with the highest LG/N ratio were: P. americana (10.6) and I. subnuda (8.6). The highest CL contents were obtained for I. subnuda (21.3 %) and L. grandiflora (17.2 %). L. grandiflora, Z. tuberculosa, and P. americana had C/N ratios greater than 23, and A. sellowiana, and the three legumes had C/N ratios <16. Leaf C/P ratios were larger than 300 in L. grandiflora, Z. tuberculosa, P. americana and I. subnuda, and below 300 in the other species. Throughout the studied period, the CO2 flux was significantly negatively correlated with LG/N and (LG+PP)/N. At the end of the measurements, the cumulative CO2 flux was significantly negatively correlated with the LG/N ratio (r = −0.76; P < 0.05) and (LG+PP)/N ratio (r = −0.79; P < 0.05).
Decomposition and nutrient release
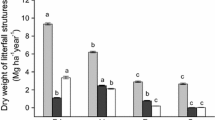
The percentages and standard errors (n = 4) of remaining dry matter from legume leaf litter during the 150 days of analysis are shown in Fig. 3. At 15 days, the remaining dry matter differed among species (F 2,63 = 56.9, P < 0.0001) and with time (F 6,63 = 65.02, P < 0.0001). The differences among species became more pronounced over time (interaction between time and species, F 12,63 = 3.42; P < 0.001). At 150 days, weight loss of I. subnuda was 31 %, S. macranthera 74 % and E. verna 84 %.
Table 2 shows the k values and half-life values (t 1/2) for dry matter (carbon), N, P, K, Ca, and Mg for leaves of the three legume species. For dry matter, k ranged from k = 0.012 day−1 (E. verna), followed by S. macranthera (0.008 day−1) and I. subnuda (0.003 day−1). K values for N and P were similar with the highest k values for E. verna (k N = 0.014 and k P = 0.012 day−1) and lowest k values for S. macranthera (k N = k P = 0.007 day−1) and I. subnuda (k N = 0.004 and k P = 0.007 day−1). After 150 days, approximately 80 % of N and P was released in E. verna litter and 60 % in S. macranthera. For I. subnuda, 35 % N and 50 % P was released.
K release occurred more rapidly in E. verna litter (k K = 0.027 day−1) than in I. subnuda litter (k K = 0.011 day−1). After 150 days, ~99 % of K was released from E. verna litter and 82 % from S. macranthera and I. subnuda litter. Ca and Mg release was slower than K release with KCa = 0.007 day−1 and KMg = 0.010 day−1 for E. verna (values corresponding to 75 % released Ca and 80 % released Mg) and KCa values of 0.004 day−1and KMg of 0.007 day−1 for S. macranthera (values corresponding to 35 % released Ca and 50 % released Mg). For litter of I. subnuda, Ca and Mg release did not follow the exponential model; the p value was not significant.
Biological nitrogen fixation
Table 3 shows the average and standard errors (n = 4) of δ 15N of legume (E. verna, S. macranthera, and I. subnuda) and reference species (B. pilosa and B. plantaginea). The difference between reference species and legume was less than 5 ‰. The highest percentage of BNF was found for E. verna (22.6 %) followed by I. subnuda (20.6 %) and S. macranthera (16.6 %), but these differences were not significantly different (P < 0.05).
Discussion
CO2 fluxes and leaf chemical and biochemical composition
The results of the present study indicated that the differences in C fluxes among species were due to the different compounds in the analysed leaves in which the LG+PP/N and LG/N ratios negatively correlated with CO2–C evolution, which explain the lower decomposition rates of L. grandiflora, I. subnuda, and P. americana leaves than those of A. sellowiana, E. verna, S. macranthera and Z. tuberculosa.
All species had N mass fraction larger than 1.74 % and C/N ratios smaller than 28.7, which could lead to a high decomposition rate, according to Palm (1995). The three species (L. grandiflora, I. subnuda, and P. americana, Table 1) with low decomposition rates (Fig. 2) had LG/N ratios in leaves greater than 6.8 (more in agreement with Silva et al. 2008, than Palm 1995). A. sellowiana, E. verna and S. macranthera with high decomposition rates (Fig. 2) had LG/N ratios smaller than 5; together with Z. tuberculosa (also with high decomposition rate) these species had (LG+PP)/N smaller than 10, thus indicating more rapid nutrient release (Palm 1995) with subsequent rapid nutrient recycling. PP mass fractions (Table 1) of all species were higher than 4.4 %, therefore only PP content did not explain the differences in decomposition rate (<0.5 % easy decomposition, Tian et al. 1992) among the species (Fig. 2), in disagreement with Mendonça and Stott (2003) who studied four native trees from the same region and found that high PP and low P contents controlled the decomposition rates of the pruned materials.
As a general rule, decomposition of leaf litter with C/P ratios >300 (L. grandiflora, Z. tuberculosa, P. americana and I. subnuda in the present study; Table 1) is P-limited according to Stevenson and Cole (1999). According to the same authors, the ratio of C to cations (K, Ca, Mg) is not a reliable guide for predicting litter decomposition rate.
According to Duarte (2007), senescent materials of L. grandiflora, I. subnuda, P. americana and S. macranthera had the highest content of nutrients. However, the three first species produced the most recalcitrant litter (highest (LG+PP)/N and LG/N ratios) and low decomposition rate. Pleguezuelo et al. (2009) also found a low decomposition rate for Persea americana, and Palm and Sanches (1990) showed that the decomposition rate of leaves of Inga edulis was lower than that of Erythrina sp. Fresh leaves of Senna spectabilis were rated as high quality litter and Partey et al. (2009) suggested their use as green manure.
We expect (as shown for I. subnuda, Table 2) that nutrients from L. grandiflora, I. subnuda and P. americana are not rapidly released because of the low decomposition rate. This can be a problem for annual crops (especially N-limited cereals), due to a lack of synchrony between N and P release and plant demand (Palm 1995), essential to ensure optimum crop yield and to avoid negative environmental impacts (Grant et al. 2002). However, in the case of perennial crops such as coffee, these nutrients can be slowly released and still used by the crops. There is also a continuous nutrient release due to a continuous supply of material by the trees. Moreover, slow litter decomposition can contribute to greater soil protection against erosion (Silva et al. 2008), which may be high in sloping soils, such as soils at Zona da Mata. Increased soil cover by litter will also reduce nutrient losses by erosion (Palm et al. 2001), leaching, and volatilisation. Therefore, faster decomposition is not always better.
Decomposition and nutrient release
Weight loss data obtained from legumes (Fig. 3) were similar to the CO2 fluxes (Fig. 2). Both data showed high decomposability of litter of E. verna and S. macranthera and low decomposability of litter of I. subnuda (Fig. 2). Similar results were found for Erythrina sp. and Inga edulis by Palm and Sanchez (Palm and Sanches 1990), in Peru. Other authors have also found that Inga sp. has lower decomposition rate and, consequently, lower nutrient release than other species used in tropical agroforestry systems (Leblanc et al. 2006; Schwendener et al. 2007).
Initially, there are greater amounts of easily decomposable materials, such as sugars, amino acids, and proteins, and there is a predominance of recalcitrant materials, such as LG and PP, as the process progresses (Hadas et al. 2004; Matos et al. 2008). Therefore, decomposition rates decline over time Materials with LG contents greater than 15 % and PP contents greater than 3 %, which are associated with N contents less than 2.5 % (Palm et al. 2001), indicate N immobilisation by the soil microbial biomass (Leblanc et al. 2006) during decomposition and a slower N release. Of the three legumes studied, N immobilisation and a slower N release may particularly be occurring with the I. subnuda residues. In agronomic terms, slower N release to the soil can be advantageous, especially in the case of perennial crops, as there is a supply of N throughout the crop cycle. Contrary, in annual crops that are N-limited, such as maize, temporary N immobilisation has a negative impact on crop yield.
The results of the present study indicated that the dynamics of P release were similar to that of N, which was expected because the behaviour of N and P are controlled by similar processes. However, K release was faster, which is due to the non-participation of K (unlike N, P, Ca and Mg) in plant organic compounds. K is an active element in the plant. It exists in an ionic form and is easily released. Calcium and magnesium help make up the middle lamella of the plant cell wall, thus becoming one of the most recalcitrant compounds in plant cells (Vitti et al. 2006; Marschner 2008). Perhaps this explains why the release of Ca and Mg did not follow the negative exponential model.
Biological nitrogen fixation
Overall, percentages of nitrogen fixation were low (17–23 %). This low estimate for N fixation is a consequence of small differences in δ 15N values between the legumes and the reference plant species. As Gehring and Vlek (2004) noted, in cases where the δ 15N values between legumes and reference plants are small, quantification of N2-fixation is difficult. Högberg (1997) suggested that the difference should at least differ by 5 ‰ in order to estimate biological nitrogen fixation with some accuracy. Thus, BNF may have been underestimated in the present study.
Moreover, the choice of reference plants is important for accuracy. It has been reported that δ 15N values vary with soil depth with lower values in the layers between 0–10 cm and higher values in deeper layers (Ledgard et al. 1984; Morais et al. 2006). Both reference species used in the present study are herbaceous and have more shallow root systems compared to the legume trees that have deeper root systems. It was observed in the field that fine roots of S. macranthera are mainly found below 60 cm. Thus, the difference in root systems could also contribute to underestimating the BNF.
According to the literature, species of Senna do not fix nitrogen (Giller 2001) and S. macranthera does not form nodules (De Faria et al. 1984; Barberi et al. 1998). If we treat S. macranthera as non-fixing, the δ 15N values of the reference plants would even be lower, hence even further lowering our estimate of BNF in these agroforestry systems. Using only S. macranthera as reference would have resulted in the conclusion that BNF is not taking place. However, the δ 15N values of the two other reference plants suggest BNF by S. macranthera. This species also had high N mass fractions, both in green and senescent leaves (Duarte 2007; Jaramillo-Botero et al. 2008), which were not different from those of the two other legume species (Table 1) for green leaves; for senescent leaves, for the three Fabaceae, N mass fractions varied between 21.4 and 24.6 mg g−1 (Duarte 2007). One explanation for high leaf N is the presence of different mechanism for N acquisition in S. macranthera (Bryan et al. 1996), for example N scavenging, such as is described for S. spectabilis, an African species (Livesley et al. 2002). Such scavenging might explain the high leaf N mass fractions of S. macranthera, but so far no mechanism for scavenging is known.
Hoewever, Senna, together with the genera Cassia, Melanoxylon and Chamaecrista, belongs to the monophyletic Cassia clade (Bruneau et al. 2008). The Brazilian Melanoxylon and several Amazonian species of Chamaecrista are known to nodulate and fix nitrogen (Naisbitt et al. 1992; De Faria et al. 2010) and it may be noteworthy that Roggy and Prévost (1999) mentioned the occurrence of nodule-like structures in S. quinquangulata. That latter species is very closely related to S. macranthera (Marazzi et al. 2006) and it would therefore be important to assess the capacity for BNF in Brazilian species of Senna.
When considering the annual litter production by these legumes, their contribution to the N cycle (even at low percentages of BNF) can be substantial, especially for S. macranthera and I. subnuda. According to Duarte (2007), the annual leaf litter production per tree of E. verna, S. macranthera, and I. subnuda is 4.0, 17.7, and 32.8 kg, respectively. Considering their N mass fractions, each S. macranthera tree and I. subnuda tree would contribute ~60 and 140 g N year−1, respectively, due to BNF. Family farmers apply ~40 g N year−1 plant−1 of coffee with a NPK formulation of 20-05-20.
Conclusion
Diversification of tree species in agroforestry systems enables input of diversified organic material. Leaf litter of different species has different characteristics of decomposition rate and nutrient release. Leaves of I. subnuda, L. grandiflora, and P. americana are more difficult to decompose than those of A. sellowiana, E. verna, S. macranthera, and Z. tuberculosa. These differences in decomposition correlate with and are likely caused by higher lignin/N ratios and higher (lignin+polyphenol)/N ratios of leaves of the first group of species. Among the legumes, there was greater nutrient release in leaves of E. verna and S. macranthera than leaves of I. subnuda. There were only small differences in BNF among the legumes with greater N fixation in E. verna and I. subnuda than in S. macranthera.
This diversification can contribute to the maintenance of different ecosystem services provided by litter and soil organic matter, resulting in potential improvements in physical, chemical, and biological soil quality and, consequently, in ecological interactions.
Knowledge of decomposition and nutrient release dynamics of species can contribute to decision making by farmer families about agroforestry system design as it allows for spatial arrangements where species that produce slower decomposing litter (important for better soil protection and organic matter formation) can be combined with species that produce litter of rapid decomposition (which is important for faster nutrient release).
References
Anderson JD, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods, 2nd edn. Wallingford, UK 221p
Barberi A, Carneiro MAC, Moreira FMS, Siqueira JO (1998) Nodulação em leguminosas florestais em viveiros no Sul de Minas Gerais. Cerne 4:145–153
Beddy TL, Snapp SS, Akinnifesia FK, Sileshi GW (2010) Impact of Gliricidia sepium intercropping on soil organic matter fractions in a maize-based cropping system. Agric Ecosyst Environ 138:139–146
Braga JM, Defelipo BV (1974) Determinação espectrofotométrica de fósforo em extratos de solos e plantas. Rev Ceres 1:73–85
Bruneau A, Mercure M, Lewis GP, Herendeen PS (2008) Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany 86:697–718
Bryan JA, Berlyn GP, Gordon JC (1996) Toward a new concept of the evolution of symbiotic nitrogen fixation in the Leguminosae. Plant Soil 186:151–159
Cardoso IM, Guijt I, Franco FS, Carvalho AF, Ferreira-Neto PS (2001) Continual learning for agroforestry system design: University, NGO and farmer partnership in Minas Gerais. Agric Syst 69:235–257
Costanza R, D’Arge R, De Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Curl EA, Rodriguez-Kabana R (1972) Microbial interactions. In: Wilkinson RE (ed) Research methods in weed science. Atlanta, Southern Weed Society, pp 162–194
De Faria SM, Franco AA, Jesus RM, Menandro MS, Baitelli JB, Mucci ESF, Dobereiner J, Sprent JI (1984) New nodulating legume trees from South-East Brazil. New Phytol 98:317–328
De Faria SM, Diedhiou AG, de Lima HC, Ribeiro RD, Galiana A, Castilho AF, Henriques JC (2010) Evaluating the nodulation status of leguminous species from the Amazonian forest of Brazil. J Exp Bot 61:3119–3127
Duarte EMG (2007) Ciclagem de nutrientes por árvores em sistemas agroflorestais na Mata Atlântica. MSc Thesis, Federal University of Viçosa, Viçosa
Embrapa (1999) Manual de análises químicas de solos, plantas e fertilizantes. Brasília: Embrapa Solos/Embrapa Informática Agropecuária/Embrapa. p 370
Gehring C, Vlek PLG (2004) Limitations of the N-15 natural abundance method for estimating biological nitrogen fixation in Amazonian forest legumes. Basic Appl Ecol 5:567–580
Giller KE (2001) Nitrogen fixation in tropical cropping systems, 2nd edn. CAB International, Wallingford
Goering HK, van Soest PJ (1970) Forage fiber analyses (Apparatus, reagents, procedures, and some applications). United States Department of Agriculture (Agriculture handbook No. 379), Washington, p 20
Golfari L (1975) Zoneamento Ecológico do Estado de Minas Gerais para reflorestamento. Série Técnica, 3. CPFRC. Belo Horizonte, Brasil
Grant CA, Peterson GA, Campbell CA (2002) Nutrient considerations for diversified cropping systems in the Northern Great Plains. Agron J 94:186–198
Hadas A, Kautsky L, Goek M, Kara EE (2004) Rates of decomposition of plant residues and available nitrogen in soil, related to residue composition through simulation of carbon and nitrogen turnover. Soil Biol Biochem 36:255–266
Högberg P (1997) 15N natural abundance in plant-soil systems. New Phytol 137:179–203
Jaramillo-Botero C, Santos RHS, Fardim MP, Pontes TM, Sarmiento F (2008) Produção de serapilheira e aporte de nutrientes de espécies arbóreas nativas em um sistema agroflorestal na Zona da Mata de Minas Gerais. Árvore 32:869–877
Kanmegne J, Dugma B, Henrot J, Isirimah NO (1999) Soil fertility enhancement by planted tree-fallow species in the humid lowlands of Cameroon. Agrofor Syst 46:239–249
Leakey RRB, Tchoundjeu Z, Schreckenberg K, Shackleton SE, Shackleton CM (2005) Agroforestry tree products (AFTPs): targeting poverty reduction and enhanced livelihoods. Int J Agric Sustain 3:1–23
Leblanc HA, Nygren P, McGraw RL (2006) Green mulch decomposition and nitrogen release from leaves of two Inga spp. in an organic alley-cropping practice in the humid tropics. Soil Biol Biochem 38:349–358
Ledgard SF, Freney JR, Simpson JR (1984) Variations in natural enrichment of 15N in the profiles of some Australian pasture soils. Aust J Exp Agric 22:155–164
Livesley SJ, Gregory PJ, Buresh RJ (2002) Competition in tree row agroforestry systems. 2. Distribution, dynamics and uptake of soil inorganic N. Plant Soil 247:177–187
Marazzi B, Endress PK, De Queiroz LP, Conti E (2006) Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: patterns in the evolution of floral symmetry and extrafloral nectaries. Am J Bot 93:288–303
Marschner H (2008) Mineral nutrition of higher plants, 2nd edn. Academic Press, London p 889
Matos ES, Mendonça ES, Lima PC, Coelho MS, Mateus RF, Cardoso IM (2008) Green manure in coffee systems in the region of Zona da Mata, Minas Gerais: characteristics and kinetics of carbon and nitrogen mineralization. Rev Bras Ci Solo 32:2027–2035
Mendonça ES, Stott DE (2003) Characteristics and decomposition rates of pruning residues from a shaded coffee system in Southeastern Brazil. Agrofor Syst 57:117–125
Moonen AC, Bárberi P (2008) Functional biodiversity: an agroecosystem approach. Agric Ecosyst Environ 127:7–21
Morais RF, Xavier RP, Alves BJR, Boddey R, Urquiaga S (2006) Uso da abundância natural de 15N (δ15N) no perfil do solo como suporte para a estimativa da FBN. In: FERTBIO: Em busca das Raízes, 2006, Bonito-MS. Anais. Bonito-MS: Sociedade Brasileira de Ciência do Solo. 4p (CD-ROM)
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Naisbitt T, James EK, Sprent JI (1992) The evolutionary significance of the legume genus Chamaecrista, as determined by nodule structure. New Phytol 122:487–492
Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Lüscher A (2011) Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric Ecosyst Environ 140:155–163
Palm CA (1995) Contribution of agroforestry trees to nutrient requirements of intercropped plants. Agrofor Syst 30:105–124
Palm CA, Sanches PA (1990) Decomposition and nutrient release patterns of the leaves of tree tropical legumes. Biotropica 22:330–332
Palm CA, Sanchez PA (1991) Nitrogen release from the leaves of some tropical legumes as affected by their lignin and polyphenolic contents. Soil Biol Biochem 23:83–88
Palm CA, Gachengo CN, Delve RJ, Cadisch G, Giller KE (2001) Organic inputs for soil fertility management in tropical agroecosystems: application of an organic resource database. Agric Ecosyst Environ 83:27–42
Partey ST, Quashie-Sam SJ, Thevathasan NV, Gordon AM (2009) Decomposition and nutrient release patterns of the leaf biomass of the wild sunflower (Tithonia diversifolia): a comparative study with four leguminous agroforestry species. Agrofor Syst 81:123–134
Peoples MB, Faizah AW, Rekasem B, Herridge DF (1989) Methods for evaluating nitrogen fixation by nodulated legumes in the field. Australian Center for International Agricultural Research, Bruce
Perfecto I, Vandermeer J (2008) Biodiversity conservation in tropical agroecosystems: a new conservation paradigm. Ann NY Acad Sci 1134:173–200
Pleguezuelo CRR, Zuazo VHD, Fernández JLM, Peinado FJM, Tarifa DF (2009) Litter decomposition and nitrogen release in a sloping Mediterranean subtropical agroecosystem on the coast of Granada (SE, Spain): effects of floristic and topographic alteration on the slope. Agric Ecosyst Environ 134:79–88
Pribyl DW (2010) A critical review of the conventional SOC to SOM conversion factor. Geoderma 156:75–83
Roggy JC, Prévost MF (1999) Nitrogen-fixing legumes and silvigenesis in a rain forest in French Guiana: a taxonomic and ecological approach. New Phytol 144:283–294
Sá NMH, Vargas MAT (1997) Fixação biológica de nitrogênio por leguminosas forrageiras. In: Vargas MAT, Hungria M (eds) Biologia dos Solos dos Cerrados. EMBRAPA-CPAC, Planaltina, pp 127–152
Schwendener CM, Lehmann J, Rondon M, Wandelli E, Fernandes E (2007) Soil mineral N dynamics beneath mixtures of leaves from legume and fruit trees in Central Amazonian multi-strata agroforests. Acta Amazonica 37:313–320
Shearer GB, Kohl DH, Virginia RA, Bryan BA, Skeeters JL, Nilsen ET, Sharifi MR, Rundel PW (1983) Estimates of N2 fixation from variation in the natural abundance of 15N in Sonoran desert ecosystems. Oecologia 56:365–373
Silva GTA, Matos LV, Nóbrega PO, Campello EFC, Resende ASR (2008) Chemical composition and decomposition rate of plants used as green manure. Sci Agric 65:298–305
Souza HN, Cardoso IM, Fernandes JM, Garcia FCP, Garcia VR, Santos AC, Carvalho AF, Mendonça ES (2010) Selection of native trees for intercropping with coffee in the Atlantic Rainforest biome. Agrofor Syst 80:1–16
Souza HN, Goede RGM, Brussaard L, Cardoso IM, Duarte EMG, Fernandes RBA, Gomes LC, Pulleman MM (2012) Protective shade, tree diversity and soil properties in coffee agroforestry system in the Atlantic Rainforest biome. Agric Ecosyst Environ 146:179–196
Statsoft INC. (1997) Statistica for Windows.5.1. Computer program manual. Tulsa, USA
Stevenson FJ, Cole MA (1999) Cycles of Soil, 2nd edn. Wiley, Hoboken p 427
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análises de solo, plantas e outros materiais. Departamento de Solos, UFRGS, Porto Alegre p 174
Teixeira FCP, Reinert F, Rumjanek NG, Boddey RM (2006) Quantification of the contribution of biological nitrogen fixation to Cratylia mollis using the 15N natural abundance technique in the semi-arid Caatinga region of Brazil. Soil Biol Biochem 38:1989–1993
Tian G, Kang BT, Brussaard L (1992) Biological effects of plant residues with contrasting chemical under humid tropical conditions decompositions and nutrients release. Soil Biol Biochem 24:1051–1060
van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and non starch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Vitti GC, Lima E, Cicarone F (2006) Cálcio, Magnésio e Enxofre. In: Fernandes MS (ed) Nutrição Mineral de Plantas. Sociedade Brasileira de Ciência do Solo, Viçosa, pp 299–325
Acknowledgments
The authors thank the Center of Alternative Technologies (CTA), the farmers and their organizations for the work in partnership, the Brazilian sponsors FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support and scholarships for E.M.G. Duarte, M.A.F·C. Mendonça, M.S. Coelho and E.M.A. Villani, and Arne Janssen for useful comments and corrections on an earlier version of this paper. Constructive comments by two anonymous referees on an earlier version are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duarte, E.M.G., Cardoso, I.M., Stijnen, T. et al. Decomposition and nutrient release in leaves of Atlantic Rainforest tree species used in agroforestry systems. Agroforest Syst 87, 835–847 (2013). https://doi.org/10.1007/s10457-013-9600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-013-9600-6