Abstract
The surface alpha-case reaction of Ti casting using Ti powder-added investment molds was investigated. During the curing procedure, Ti powders (0, 10, and 50 mass%) were mixed with three types of investment mold materials (Al2O3, ZrSiO4, and ZrO2) to form an interstitial TiO2 phase, which is an alpha-case reaction compound. The microstructure and surface hardness profiles of Ti castings with Ti powder-added investment molds indicated that the alpha-case thickness was significantly reduced from approximately 350 to 50-100 μm, and a remarkable reduction in the maximum micro-Vickers hardness value of the Ti casting surface was also achieved. As observed from the experimental results, the alpha-case reduction mechanism suggested that the phase transformation from TiO2 to TiO not only acts as an effective barrier to O diffusion, but also reduces Al, Si, and Zr concentrations at the casting surface. This reduction might be caused by contact area reduction between the Ti powder-added investment molds and the Ti melts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Titanium (Ti) and its alloys have great potential in the aerospace, automotive, and biomedical industries owing to their desirable properties including light weight, high specific strength, biocompatibility, and corrosion resistance. However, the application of Ti casting is limited because of the higher cost of raw materials, melting and post machining processes. The reactivity of Ti in the molten state is the most important issue from some perspectives. Therefore, Ti has to be melted and cast in a vacuum or an inert atmosphere, which brings about an increase in total cost (Ref 1, 2). Moreover, the reaction between Ti melts and investment mold materials forms a brittle and hard “alpha-case” reaction layer on the surface of Ti castings that can lead to significantly reduced fatigue strength and ductility due to crack initiation and propagation by the applied load (Ref 3). The alpha-case is formed by interstitial oxygen and substitutional metallic elements dissolved from the oxide mold materials and binder. Heat treatment of a mill stock, at temperatures above about 700 K also results in the alpha-case formation at the surface because Ti is susceptible to reaction with oxygen and nitrogen in the surrounding atmosphere. Therefore, the thickness of the alpha-case and the cost due to complex processing steps must be considered in the initial design, since the alpha-case must be removed by chemical milling (Ref 4-6). According to the extensive research on the alpha-case of Ti casting with investment mold materials (Ref 7-10), the predominant reaction phases were interstitial Ti-O compounds and an alpha-Ti(O) solid solution, which were caused by the outward diffusion of oxygen elements from investment oxide mold materials and SiO2-containing binders to Ti melts. Substitutional compounds also contribute to the construction of an alpha-case, however, the reaction layer is thin because of the low diffusion rate of metallic elements (Ref 5, 11).
Even though thermodynamically stable investment mold materials, such as Al2O3, ZrO2, Y2O3, and rare-earth oxides (CeO2, Sm2O3, Gd2O3, La2O3, and ThO2), which are more stable than TiO2, were used to avoid an alpha-case reaction, the alpha-case of Ti castings were still formed in the range of 250-500 μm (Ref 8, 9, 12). Considering the harmful effect of alpha-case reaction layer on mechanical properties and cost affordability, the alpha-case reaction should be reduced by applying an appropriate mold material. In recent years, based on the alpha-case formation mechanism, Choi et al. (Ref 13) evaluated the feasibility of forming an Al2O3 mold containing interstitial Ti-O compounds as related to the alpha-case reaction for Ti investment casting. This study deals with the influence of Ti powder addition to different types of investment mold materials (e.g., Al2O3, ZrSiO4, and ZrO2) on the alpha-case reduction of Ti casting. Furthermore, a conceptual mechanism of alpha-case reduction of Ti casting by Ti powder-added investment mold materials will also be discussed.
Experimental Procedure
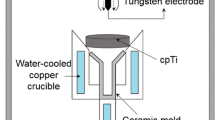
The investment mold was prepared by the lost-wax method. A wax pattern (Ø16*50 mm) was coated with a primary slurry composed of Al2O3 (99% purity, 325 mesh, Union Co., Republic of Korea), ZrSiO4 (98% purity, 325 mesh, OCI-Ferro Co., Republic of Korea), and ZrO2 (ZrO2 + HfO2 95% purity, 4 mass% CaO stabilized, 325 mesh, Unitech Ceramics Co., United Kingdom) mixed with 0, 10, and 50 mass% Ti (99% purity, 325 mesh, Sejong Materials Co., Republic of Korea) along with a colloidal SiO2 binder (30 mass% SiO2, mean particle size: 15 nm, S-Chemtech Co., Republic of Korea). The primary coating procedure was followed by the coating layer of a chamotte back-up. The investment molds were cured for 2 h at 1223 K in a furnace (pressure of 1 × 105 Pa) to strengthen and dehydrate the molds. For brevity, the Al2O3, ZrSiO4, ZrO2, and Ti designation will be indicated by AO, ZSO, ZO, and T, respectively. For example, the abbreviated designation of Al2O3 with 10 mass% Ti powder was defined as AOT91.
A mold was mounted in a plasma arc-melting furnace. The composition of the investment molds and designations are listed in Table 1. The furnace pressure was maintained at 1.33 × 10−1 Pa, and was subsequently charged with argon gas at a pressure of 4.9 × 103 Pa. Then, 120 g of a Ti button (99% purity, Gr. 2) was melted and poured into the mold.
Phases of the reaction compounds in the Al2O3, ZrSiO4, and ZrO2 molds were identified by x-ray diffraction (MAC SCIENCE M18XHF-SRA), x-ray fluorescence (BRUKER S4), and transmission electron microscopy (JEOL JEM-2100F). The microstructure of the metal-mold interfacial reaction region was examined using an optical microscope (OLYMPUS PME3), a field emission scanning electron microscope (JEOL JSM-7600F), and an electron probe microanalyzer (SHIMADZU EPMA 1600). The hardness was measured using a micro-Vickers hardness tester (MITUTOYO MVK-H2) under a 100-g load at 50-μm intervals.
Results and Discussion
Phase Identification of Ti Powder-Added Investment Molds
The x-ray diffraction patterns for investment molds after curing for 2 h at 1223 K are shown in Fig. 1. In all cases, the TiO2 content increased with the increasing Ti content. X-ray diffraction results show that no intermetallic compounds formed during curing for ZSOT and ZOT molds. This was due to the reaction between the oxides and Ti powder or oxides and colloidal SiO2. A small amount of the Ti5Si3 phase was detected in the AOT55 mold. According to a study by Jones, a colloidal SiO2 binder existed in the amorphous state, which was <5% crystallized after curing. In the results of x-ray diffraction, the crystallized SiO2 phase in the investment mold after curing was not detected because of the detection limitation (Ref 14). Quantitative results of the existing phases in the investment molds after curing are shown in Table 1, and the data were analyzed by x-ray fluorescence on the condition that the existing phases were solely oxides. TiO2 was formed in the investment molds, and the content of TiO2 was comparable to the amount of added Ti powder.
TiO2 particles were observed on the mold surface, as shown in Fig. 2(a)-(c), and the morphologies of TiO2 differed according to the types of investment mold materials. TiO2 of the AOT mold (Fig. 2a) had a needle shape and showed agglomeration while maintaining the size of the added Ti raw powder. Conversely, the TiO2 of ZSOT and ZOT molds (Fig. 2b and c) had a block-like shape. The difference in the morphologies of TiO2 could be caused by the differences in the reactivities between the Ti powder and investment oxides. Specifically, the Gibbs free energy change of ZrO2 is more negative than Al2O3 (ZrO2: −871.337 kJ mol−1, Al2O3: −863.364 kJ mol−1 at 1200 K) (Ref 15); therefore, it is plausible that Ti powder more easily reacts with Al2O3 than with ZrO2, which can lead to the differences in the TiO2 morphologies. In particular, Table 2 shows reasonable evidence to explain the effect of elemental Al on the TiO2 morphology, where Al was detected at approximately 5 at.% in the TiO2 in AOT91 mold. In contrast, elemental Zr was not detected in TiO2 in the ZSOT91 and ZOT91 molds. The bright image and spot diffraction patterns of the TiO2 phase in the investment molds are shown in Fig. 3. Based on the results of the diffraction pattern analysis, the TiO2 in the AOT91, ZSOT91, and ZOT91 molds after curing had a tetragonal structure originating from the [00\(\bar{1} \)], [\( \bar{1}\)01], and [020] zone axes, respectively.
Thus, based on the x-ray diffraction, x-ray fluorescence, morphology observation of the mold surface, and transmission electron microscopy results, TiO2 should be formed in investment molds, and it is reasonable to conclude that the Ti powder could react with the oxides (Al2O3, ZrSiO4, and ZrO2) or the colloidal SiO2 binder. However, the reaction between Ti powder and atmospheric oxygen was the dominant phenomenon.
Alpha-case Reaction of Ti Castings with Ti Powder-Added Investment Molds
The interface structures between the Ti castings and investment molds are shown in Fig. 4. AO, ZSO, and ZO molds showed a similar serious alpha-case reaction layer that was approximately 350 μm with a coarse acicular microstructure on the casting surface. However, it was difficult to observe the reaction layer in the AOT, ZSOT, and ZOT molds.
The micro-Vickers hardness profiles of the Ti castings poured into various investment molds used in this study are given in Fig. 5. Compared with the average hardness value, 159 HV, of the base material, the alpha-case thicknesses of AO, ZSO, and ZO molds were approximately 350 μm. The AOT, ZSOT, and ZOT molds reacted with Ti melts, but less than the AO, ZSO, and ZO molds did. In all cases, the reaction layers of AOT, ZSOT, and ZO molds in the micro-Vickers hardness profiles were less than 50-100 μm, except for the Ti casting with a ZOT91 mold of 350 μm, which was same as that of the ZO mold. However, no specific interface reaction layer of the ZOT91 mold is observed, as shown in Fig. 4(f), and it is confirmed that the maximum micro-Vickers hardness was reduced in comparison with the ZO mold. The alpha-case thickness, maximum hardness of the surface region, and average hardness of the inner region are given in Table 3. In comparison, the maximum micro-Vickers hardness value of the Ti casting was reduced from 318, 324, and 261 HV for AO, ZSO, and ZO to 179, 194, and 221 HV for the AOT55, ZSOT55, and ZOT55 molds, respectively. The alpha-case thicknesses of AOT molds were smaller than the ZSOT and ZOT molds. This difference might result from the TiO2 morphology. The reason for this difference in alpha-case reactions is uncertain; however, Al containing TiO2 in AOT molds with a dissimilar morphology compared with the TiO2 in ZSOT and ZOT molds could enhance the refractory behavior or reduce the reactivity.
The O concentrations at the Ti casting surface are shown in Fig. 5. In the case of Ti casting with AO and AOT molds, micro-Vickers hardness profiles corresponded to the O concentrations with depths of 200 μm and 30 μm, respectively. In addition, the metallic element concentrations from each oxide and colloidal SiO2 binder clearly increased by approximately 40-70 μm on the casting surface, as shown in Fig. 6. In earlier results, the elemental O at the casting surface was analyzed through several analytic methods (Ref 6, 10). Since alpha-case thickness depends on the casting scale and conditions, its values corresponded to the O concentrations, which had a range of about 100-500 μm. In addition, elemental O existed in the Ti casting surface as Ti-O compounds and an alpha-Ti(O) solid solution. Al and Zr elements diffused from the oxide molds into Ti melts, and the presence of Si element shown in Fig. 6(d)-(f) was caused by the oxide and colloidal SiO2 binder.
A number of researchers examined the intermetallic compounds or oxides at the casting surface. According to Sung et al., the substitutional Ti3Al compound was formed at the casting surface because of the reaction between Al2O3 mold and Ti melts (Ref 5). Saha et al. (Ref 6) identified the formation of a Ti5Si3 phase as a consequence of the reduction of SiO2 in ZrSiO4 mold by Ti melts. Lin and Lin (Ref 8) reported that a Ti2ZrO ternary phase and Ti5Si3 were induced by the outward diffusions of Zr and Si elements from a ZrO2 mold to Ti melts. It is reasonable to note that a Ti2ZrO phase can be formed at the casting surface of a complete solid solution binary system of Ti and Zr. Alternatively, the characterized results of the AOT, ZSOT, and ZOT, are differed considerably, as shown in Fig. 5 and 7. The thicknesses of the layers of O and metallic elements such as Al, Zr, and Si were reduced at the casting surface.
The quantitative spot analysis of compositional changes in the mold surfaces after casting is given in Table 2, and the morphologic changes are shown in Fig. 2(d)-(f). The compositions of reacted regions of the Ti melts with investment molds showed that Al2O3, ZrSiO4, and ZrO2 were transformed to oxygen-deficient oxides such as AlO, ZrSiO2, and ZrO. TiO2 particles in AOT, ZOT, and ZSOT molds were also transformed into TiO. However, the alpha-case reaction layer of the AOT, ZOT, and ZSOT molds was reduced more significantly than AO, ZO, and ZSO molds. Therefore, the TiO2 phase played a key role in alpha-case reduction of Ti casting.
In addition, it should be noted that the AOT mold with Ti melts has three kinds of reaction systems, namely, Ti melts can react with Al2O3, SiO2 and TiO2. In case of the Ti melts reacting with Al2O3 and SiO2, the diffusion rate of interstitial O from Al2O3 and SiO2 was predominant, and the diffusion of substitutional Al and Si elements was slow. In the case of Ti melts reacting with TiO2 in investment molds, the oxygen from TiO2 also diffused in Ti melts. Chang and Lin (Ref 16) investigated the reaction of 5 mol% CaO stabilized ZrO2 with Ti at 1823 K for 2 h. CaO released O atoms, which induced a thin (~2 μm) TiO phase at the interface, which in turn functioned as a diffusion barrier of metallic elements (Ti and Zr). In terms of the phase formation and diffusion barrier effect on the alpha-case reaction, the earlier results on the role of the TiO phase corresponded with this study. Notably, Ti has less O solubility of O-deficient Ti oxides than those of O-rich Ti oxides, considering the alpha-case reduction by the TiO2 in the investment molds (TiO2: 1.73 at.%, and TiO: 0.13 at.%) (Ref 17). Therefore, TiO2 in the investment mold was changed to TiO, which acted as an effective barrier to O diffusion in Ti melts.
Detailed analysis of the phase formation at metal-mold interface is in progress. From the experimental results of the Ti melts with Ti powder-added investment molds, we suggest a conceptual drawing of the alpha-case reduction mechanism, as shown in Fig. 8 and 9. The reaction of Ti melts with investment molds results in the alpha-case formation, which comprised the interstitial phases, i.e., the Ti-O compounds and alpha-Ti(O) solid solution, as well as the substitutional phases, i.e., Ti-M (M: Al, Si, and Zr) intermetallics or Ti-M-O compounds. On the other hand, when the Ti melts came into contact with Ti powder-added investment molds, the TiO2 phase in the investment mold decreased the continuous diffusion of oxygen because of the intermediate TiO phase. The phase transformation from TiO2 to TiO not only acts as an effective barrier to O diffusion, but also reduces Al, Si, and Zr concentrations at the casting surface, which might be caused by the contact area reduction between the Ti powder-added investment mold and the Ti melts. From the findings of this study, it is easy to emphasize the direct addition of TiO2 powder rather than Ti powder in Al2O3 because of its cost efficiency, considering the alpha-case reduction effect of the TiO2 phase. However, as pointed out in Choi et al.’s study, premature gellation between TiO2 and colloidal SiO2 binder occurred, which can be an obstacle to applying the mold material for Ti investment casting (Ref 13).
The experimental results of this study reveal that Ti powder-added investment molds enhance the Ti casting quality because of the alpha-case reaction, which can provide good mechanical properties enhancement, dimensional accuracy, environment-friendliness, and economic feasibility.
However, it should be noted that the dissolution temperature of TiO2 is only 180 K higher than the Ti (TiO2: 2113 K and Ti: 1933 K). TiO2 is likely to be melted in contact with the superheated Ti melts; thus, TiO2 is difficult to use alone considering the dissolution temperature of commercial oxide mold materials (Al2O3: 2322 K, ZrO2: 3123 K, and ZrSiO4: 2823 K) (Ref 18, 19).
To apply the TiO2 phase as an investment mold, the dissolution temperature as well as the reactivities of investment oxide mold materials must be compensated for with regard to the high melting temperature of molten Ti. Therefore, considering the refractory property and chemical reactivity of the investment mold, it is reasonable to say that TiO2 should be introduced by mixing with other heat-resistant investment oxide molds.
Summary
The alpha-case contains mainly interstitial compounds related to oxygen and metallic substitutional compounds, which are dissolved from the investment mold. Interstitial TiO2 compounds in the investment oxide mold materials (Al2O3, ZrSiO4, and ZrO2) were manufactured by Ti powder addition. TiO2 should be formed in investment molds, and the Ti powder is expected to react with investment oxides (Al2O3, ZrSiO4, and ZrO2) and the colloidal SiO2 binder. However, the reaction between Ti powder and atmospheric oxygen was the dominant phenomenon. The difference in the morphology of TiO2 could be caused by the difference in reactivity between the Ti powder and investment oxides, and it might be plausible that Ti powder more easily reacted with Al2O3 than ZrO2, which can lead to different TiO2 morphologies.
The alpha-case reaction layer of Ti casting is significantly reduced by the TiO2-containing investment mold materials. The interface structures between the Ti casting with AO, ZSO, and ZO molds showed a similar serious alpha-case reaction layer that was approximately 350 μm with a coarse acicular microstructure on the casting surface. However, it was difficult to observe the reaction layer in the AOT, ZSOT, and ZOT molds. The surface hardness profiles of Ti castings with Ti powder-added investment molds indicated that the alpha-case thickness was significantly reduced from approximately 350 to 50-100 μm. The alpha-case thickness follows the sequence AO = ZSO = ZO > ZOT > ZSOT > AOT.
The reaction of Ti melts with investment molds results in alpha-case formation, which is composed of the interstitial phases, Ti-O compounds, and an alpha-Ti (O) solid solution, as well as the substitutional phases, Ti-M (M: Al, Si, and Zr) intermetallic or Ti-M-O compounds. In case of Ti melts reacting with TiO2 in investment molds, the oxygen from TiO2 also diffused in the Ti melts; however, the TiO2 decreased the continuous diffusion of oxygen because of the intermediate TiO phase. The phase transformation from TiO2 to TiO can not only act as an effective barrier to O diffusion, but can also reduce Al, Si, and Zr concentrations at the casting surface, which might be caused by contact area reduction between the Ti powder-added investment mold and Ti melts.
References
F.H. Froes, M.N. Gungor, and M.A. Imam, Cost-Affordable Titanium—The Component Fabrication Perspective, JOM, 2007, 59, p 28–31
C. Leyens and M. Peters, Titanium and Titanium Alloys, WILEY-VCH, Weinheim, 2003, p 1–35
M.J. Donachie, Jr., Titanium—A Technical Guide, 2nd ed., ASM International, Ohio, 2000, p 55–63
K. Suzuki, The High-Quality Precision Casting of Titanium Alloys, JOM, 1998, 50, p 20–23
S.Y. Sung and Y.J. Kim, Alpha-Case Formation Mechanism on Titanium Investment Castings, Mater. Sci. Eng. A, 2005, 405, p 173–177
R.L. Saha, T.K. Nandy, R.D.K. Misra, and K.T. Jacob, Evaluation of the Reactivity of Titanium with Mould Materials During Casting, Bull. Mater. Sci., 1989, 12, p 481–493
S.Y. Sung, M.G. Kim, and Y.J. Kim, A Study on the Identification of Metal/Mold Reaction Product of Ti Investment Casting, J. Kor. Inst. Met. Mater., 2003, 41, p 557–561 (in Korean)
K.F. Lin and C.C. Lin, Interfacial Reactions between Ti-6Al-4V Alloy and Zirconia Mold During Casting, J. Mater. Sci., 1999, 34, p 5899–5906
C. Frueh, D.R. Poirier, and M.C. Maguire, The Effect of Silica-Containing Binders on the Titanium-Face Coat Reaction, Metall. Mater. Trans. B, 1997, 28, p 919–926
W.J. Boettinger, M.E. Williams, S.R. Coriell, U.R. Kattner, and B.A. Mueller, Alpha Case Thickness Modeling in Investment Castings, Metall. Mater. Trans. B, 2000, 31, p 1419–1427
Z. Liu and G. Welsch, Literature Survey on Diffusivities of Oxygen, Aluminum, and Vanadium in Alpha Titanium, Beta Titanium, and in Rutile, Metall. Trans. A, 1988, 19(4), p 1121–1125
R.L. Saha, T.K. Nandy, R.D.K. Misra, and K.T. Jacob, On the Evaluation of Stability of Rare Earth Oxides as Face Coats for Investment Casting of Titanium, Metall. Trans. B, 1990, 21(6), p 559–566
B.J. Choi, S. Lee, and Y.J. Kim, Influence of TiO2 on Alpha-Case Reaction of Al2O3 Mould in Ti Investment Casting, Mater. Sci. Technol., 2013, 29(12), p 1453–1462
S. Jones and P.M. Marquis, Role of Silica Binders in Investment Casting, Brit. Ceram. Trans., 1995, 94, p 68–73
M.W. Chase, C.A. Davies, J.R. Downey, D.J. Frurip, R.A. McDonald, and A.N. Syverud, JANAF Thermochemical Table, 4th ed., American Chemical Society and American Institute of Physics, New York, 1998, p 154, 1769
Y.W. Chang and C.C. Lin, Compositional Dependence of Phase Formation Mechanisms at the Interface Between Titanium and Calcia-Stabilized Zirconia at 1550°C, J. Am. Ceram. Soc., 2010, 93, p 3893–3901
L.N. Belyanchikov, Thermodynamics of Titanium-Based Melts: II. Oxygen in Liquid Titanium, Russian Metall., 2010, 2010(12), p 1156–1163
J.F. Shackelford and W. Alexander, CRC Materials Science and Engineering Handbook, 3rd ed., CRC Press LLC, New York, 2001, p 1344–1370
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, BJ., Lee, S. & Kim, YJ. Alpha-Case Reduction Mechanism of Titanium Powder-Added Investment Molds for Titanium Casting. J. of Materi Eng and Perform 23, 1415–1423 (2014). https://doi.org/10.1007/s11665-013-0859-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-013-0859-6