Abstract
Permeability is crucial for stable operation and strengthening of smelting in blast furnace ironmaking, especially for the current utilization of low-grade iron ore. The viscosity of the primary slag is an important factor affecting permeability. In this study, the roles of MgO and Al2O3 in the viscous and structural behavior of CaO-MgO-Al2O3-SiO2-10 mass pct FeO (CaO/SiO2 weight ratio of 1.4) primary slag were investigated. The results showed that slag viscosity initially decreased and subsequently increased when Al2O3 content was increased from 6 to 16 mass pct, having the lowest value at 10 mass pct Al2O3. The reduction in viscosity with increase in Al2O3 content from 6 to 10 mass pct was attributed to the effect of solid crystals dissolving into the liquid phase caused by the decrease in the liquidus temperature, while the increase in viscosity at higher Al2O3 contents up to 16 mass pct was caused by the network-forming role of Al2O3 owing to its acidic nature. The viscosity fluctuated with increase in MgO content from 6 to 16 mass pct, which was related to the variation in the equilibrated primary phase field. The MgO and Al2O3 in the molten slag network structure functioned as network modifier and network former, respectively, to change the silicate network, as verified by Fourier transform infrared (FTIR) and Raman spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, low carbon usage and low-grade iron ore utilization have been the natural trend for blast furnace (BF) ironmaking owing to environmental and resource issues. Under such conditions, the permeability of the BF, which is the key to stable operation and strengthening smelting, will deteriorate. The major pressure drop in a BF occurs across the cohesive zone, which consists of upward gas flow, downward molten primary slag, cohesive metallic layers, and solid coke. The thermal–physical properties of primary slag, such as liquidus temperature and viscosity, play significant roles in slag drainage across the active coke zone and the deadman zone and further affect the permeability and stable operation. Viscosity is an important property of primary slag that should draw much attention.
The primary slag contains not only the gangue component of iron ore but also unreduced FeO. It can be regarded as a quinary slag system of CaO-SiO2-MgO-Al2O3-FeO (CSMAF), which is more complex than BF final slag in hearths, commonly assigned to a quaternary slag system of CaO-SiO2-MgO-Al2O3 (CSMA). The viscosity of the final slag has been widely researched, considering the effects of CaO/SiO2 weight ratio (C/S), MgO content, and Al2O3 content. Some studies have been conducted on the effect of C/S on the viscosity of CSMA slag,[1,2,3] and the results showed that the minimum viscosity appeared at a C/S of about 1.0, based on slags containing 10 mass pct MgO with 15 and 20 mass pct Al2O3.[2] For a slag containing high amounts of Al2O3 (Al2O3>25 mass pct), the viscosity decreased when the C/S increased from 0.8 to 1.6[4] and from 0.7 to 3.4[5] in the temperature range above the liquidus temperatures. An increase in MgO content resulted in a decrease in the viscosity,[3, 6,7,8,9] while excess addition of MgO resulted in a viscosity increase.[10,11,12] Many researchers have pointed out that the effect of Al2O3 on the viscosity depends on the slag composition. The addition of Al2O3 increased the viscosity because of the role of Al2O3 in the polymerization of the network structure.[13,14] Alternatively, other studies showed that increasing the amount of Al2O3 resulted in the slag viscosity increasing at first and then decreasing at higher Al2O3 proportions, which was attributed to the amphoteric behavior of Al2O3.[15,16,17,18] However, reports on CSMAF slags are limited. Lee et al. observed that the viscosity of CSMAF slag first increased and then decreased with increasing C/S and decreased with increasing FeO content.[19] The transition point composition was C/S of 1.3. Kim et al. studied the effects of MgO and Al2O3 on CSMAF slags containing 5 mass pct FeO, and the results showed that the slag viscosity increased with increasing Al2O3 content and exhibited a minimum value at approximately 7 mass pct MgO.[20]
Currently, iron ore containing high Al2O3 content is widely available in India and Australia. As it is a low-grade iron ore resource with low cost, it is an economical choice for ironmaking.[21] Adjusting the MgO content in iron ore is a popular countermeasure to cope with the usage of high Al2O3 ores when managing slag properties.[22] The viscous and structural behaviors of blast furnace primary slag have not yet been fully characterized, especially for slags with high Al2O3 content. A study of the effects of MgO and Al2O3 content on the viscosity properties of primary slag is necessary to provide insights into adjusting the operational slag chemistry to manage the drainage properties of the primary slag across the coke column. Moreover, combined with the viscosity studies of BF final slag, the results will help the blast furnace operator extend the selection of low-grade ore and maintain stability.
The primary slag is generated from the softening-melting of iron ore, and its C/S is close to that of the integrated ferrous burden. The C/S range of the integrated burden is approximately 1.4–1.7, higher than the 1.0–1.3 value of the final slag. According to previous studies, the primary slag had a higher C/S of more than 1.4[19, 23] and approximately 5 to 20 mass pct FeO.[19] From the softening-melting test of the integrated burden, the MgO and Al2O3 contents in the primary slag are approximately 6 to 12 mass pct and 8 to 16 mass pct,[24,25,26,27] respectively. Thereby, we investigated the influences of MgO and Al2O3 content over a wide range on the viscosity of CaO-SiO2-MgO-Al2O3-10 mass pct FeO slag with C/S = 1.4. The re-quenched slag samples were subjected to X-ray diffraction (XRD) analysis to observe the phase change. The viscous behaviors of the slag were explained with the help of the slag network structure acquired using Fourier transform infrared (FTIR) and Raman spectroscopy and phase diagrams estimated by FactSage 7.2.[28]
Experimental
The chemical compositions of the designed primary slag samples are listed in Table I. The samples were prepared from reagent grade CaCO3, SiO2, MgO, Al2O3, and FeC2O4·2H2O powders, among which CaCO3 and FeC2O4·2H2O were used to produce CaO and FeO, respectively. In these samples, MgO and Al2O3 content are the variables, and both change from 6 to 16 mass pct, with a fixed C/S of 1.4 at 15 mass pct Al2O3 (Nos. 1 to 6) and 10 mass pct MgO (Nos. 7 to 12), respectively. Table I also shows the calculated liquidus temperatures and relative amounts of the solid phases at 1773 K for each designed-composition slag. The slags with increasing MgO content, slags No. 1 to No. 4, were completely liquid at 1773 K. The principal crystalline phase was periclase (MgO) for the slags with 14 and 16 mass pct MgO. At 1773 K, the relative amount of solid periclase was only 2.05 pct in slag No. 5, and was 4.74 pct for slag No. 6. For the slags with increasing Al2O3 content, only the slag with 6 mass pct Al2O3 had a liquidus temperature higher than 1773 K. Its principal crystalline phase was dicalcium silicate (2CaO·SiO2), the percentage of which was 6.45 pct.
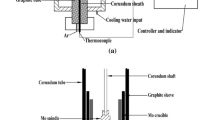
The viscosity of the primary slag was measured using a rotating cylinder method.[18,29] The information on the apparatus and procedure is described in detail in our previous work[30] and briefly here. The Mo crucible (inner diameter: 40 mm, height: 80 mm) and Mo rotating spindle that contact molten slag were used and calibrated before viscosity measurement using castor oil with known viscosities at room temperature. High-purity Ar gas and a graphite crucible, which coats the Mo crucible outside, were employed to prevent oxidation of FeO in the slag. For each slag sample, the chemical powders comprising the primary slag (140 g) were weighed separately after being dried. The four chemicals, CaO, SiO2, MgO, and Al2O3, were mixed and pre-melted in a graphite crucible at 1823 K under N2 flow, followed by quenching in water. Then, the pre-melted slag was calcined to remove carbon in a muffle furnace at 1473 K for 2 hours. The FeC2O4·2H2O powders and the pre-melted slag were successively added to the Mo crucible, accompanied by an elevated temperature up to 1823 K under an Ar atmosphere to obtain a molten slag. For 3 hours, 1823 K was maintained for slag homogenization, and then, the temperature was decreased from 1823 K to 1773 K. At this time, the spindle stirred the molten slag for 30 minutes at 1773 K, after which the viscosity measurement began. The measurement was carried out at intervals of 10 K during cooling with an equilibration time of 30 minutes at each temperature. After completing the viscosity measurement, the slag sample was reheated to 1773 K and kept for 60 minutes, and then, it was quenched in water, dried, and crushed into fine powder prior to determining its chemical composition. The viscosity measurement at each temperature point was performed five times to obtain an average value. In the case of slag No. 1, the viscosities at 1723 K and 1773 K were measured again during reheating with an equilibration time of 30 minutes to obtain a reproducible result. Table I also shows the FeO contents after the viscosity experiment, showing a small disparity.
In order to obtain the network structure of the slag at high temperature, a part of each sample powder was heated (1773 K for 60 minutes) and quenched again using a small Mo crucible (29 mm inner diameter, 4.5 mm depth, and 0.1 mm thickness). This is because the cooling effectiveness of bulk molten slag is not optimal for structural analysis using XRD (SmartLab SE, Rigaku, Japan), FTIR (Thermo Scientific Nicolet IS5, Nicolet), and Raman spectroscopy (XploRA PLUS, Horiba Scientific, France).
Results
Phase Changes
Figure 1 shows the phase changes of the slags with different MgO and Al2O3 contents at 1773 K, as investigated by XRD. For the slags with increasing MgO content, there were no characteristic peaks of crystals until 16 mass pct MgO was reached. The slag with 16 mass pct MgO contained a small amount of periclase (MgO). This broadly corresponded to the FactSage-calculated results for the slags with different MgO contents (see Table I). In the case of the slags with increasing Al2O3 content at a fixed MgO content of 10 mass pct, the slags with 6 and 8 mass pct Al2O3 had two kinds of crystalline phases: dicalcium silicate (2CaO·SiO2) and merwinite (3CaO·MgO·2SiO2), which was not fully consistent with the FactSage-calculated results shown in Table I. It was suggested that the percentage of solid phase decreased with the addition of Al2O3 from 6 to 8 mass pct, and then, the solid phase disappeared when the Al2O3 content was higher than 10 mass pct. The experimental slags, except No. 6 to No. 8, were completely liquid at 1773 K and maintained an amorphous state at room temperature.
Viscous Behavior
The viscosity of the slag containing 15 mass pct Al2O3 with increasing MgO content is shown in Figure 2. The measured viscosities of slag No. 1 at 1723 K and 1773 K during reheating are marked with circular symbols, showing acceptable repeatability. As can be seen, the viscosity fluctuated and presented a “W” shape with the increase in MgO content from 6 to 16 mass pct, exhibiting two minimal values at 8 and 12 mass pct MgO. It has been generally reported that MgO is a basic oxide which can provide free oxygen ions to simplify the slag network structure causing the decrease in viscosity, whereas MgO in excess of 7 mass pct results in an increase in the viscosity of the CaO-SiO2-MgO-10 mass pct Al2O3-5 mass pct FeO (C/S = 1.45) slag owing to the higher slag liquidus temperature.[19] For comparison, some viscosities[20] with increasing MgO content in blast furnace slags including CaO-SiO2-MgO-14 mass pct Al2O3-5 mass pct FeO (C/S = 1.45), CaO-SiO2-MgO-14 mass pct Al2O3-5 mass pct FeO (C/S = 1.35), and CaO-SiO2-MgO-13 mass pct Al2O3-0 mass pct FeO (C/S = 1.18) are depicted in Figure 2, which show partial similarity. The effect of MgO on the viscosity of the present slag appears in odd pattern and will be discussed in combination with the network structural investigation and the change of the primary equilibrium phase region.
The viscosity of the slag containing 10 mass pct MgO with increasing Al2O3 content is shown in Figure 3. The viscosity decreased with increase in Al2O3 content from 6 to 10 mass pct and then increased with increasing Al2O3 content up to 16 mass pct. In the study by Kim et al. on CaO-SiO2-10 mass pct MgO-Al2O3-5 mass pct FeO (C/S = 1.45) slag, the viscosity slightly increased with increase in Al2O3 content from 12 to 14 mass pct,[20] which is partially similar to the trend in present study. The viscosity values of the present slags were lower than those in the literature at 1773 K (1500 °C) because the FeO content in the present slag is higher. In the literature,[15,16,17] there was an inflection point at approximately 10 mass pct Al2O3, below which viscosity of the CSMA slag increased, but as Al2O3 content increased above 10 mass pct, the viscosity decreased. However, in the current study, the variation trend of the slag viscosity is inappropriately illustrated owing to the amphoteric behavior of Al2O3. The effect of Al2O3 between 6 and 10 mass pct on the decrease in viscosity will be explained on the basis of the decreasing proportion of solid phases. For Al2O3 content more than 10 mass pct, the effect of increasing viscosity could be ascribed to the acidic nature of Al2O3, which behaves as a network former. More detailed information is needed on the structural role of Al2O3 in the network structure via FTIR and Raman spectroscopy analysis.
The activation energy for viscous flow (Eη) is used to characterize the resistance of the liquid slag to shearing, the change in which also qualitatively reflects the variations in the slag structure.[31,32] The higher the Eη, the higher the energy barrier for the slag flow, and the higher the viscosity. Considering the logarithmic deformation of the Arrhenius-type equation, Lnη has a linear relationship with 1/T. The Eη is acquired from the linear slope multiplied by R (the gas constant, 8.314 J mol−1 K−1). The fitting results between Lnη and 1/T are shown in Figure 4. The change in Eη is depicted in Figure 5. The Eη value of the slags with 15 mass pct Al2O3 seemed to be similar for 6, 8, and 10 mass pct MgO, and the Eη for 8 mass pct MgO was slightly lower. In addition, a sharp decrease in Eη for 12 mass pct MgO was observed, while it increased rapidly for higher MgO content. The analysis revealed that the slags with 8 and 12 mass pct MgO may have some transitions of microstructure. For the slags with increasing Al2O3 content, the Eη showed an increasing trend overall, which conforms to the viscosity variation. This indicates that the Al2O3 addition beyond 10 mass pct results in a larger structural unit for viscous flow.
Network Structure
FTIR and Raman spectroscopy, which confirmed each other, were performed on the rapidly quenched slag to verify the effects of MgO and Al2O3 on the network structure. The FTIR transmitting spectra[2,33] of slag are observed between 1200 and 400 cm−1, which can be divided into four band groups: 1200 to 750, 750 to 630, 570 to 520, and 500 cm−1. The first band group[14] is assigned to [SiO4]4− tetrahedral symmetric stretching for the silicate structure consisting of monomers, dimers, chains, and sheets. These silicate structural units have bridging oxygen numbers of 0, 1, 2, and 3, and they are named Q0, Q1, Q2, and Q3, respectively. The latter three band groups related to the aluminate structure are assigned to [AlO4]5− tetrahedral asymmetric stretching, [AlO6]9- octahedral, and Si-O-Al rocking,[14,20] respectively. The intensity of Si-O-Al rocking represents the linkages between [SiO4]4− and [AlO4]5− tetrahedra.
Figure 6 shows FTIR results for the quenched slags with varied MgO and Al2O3 content. By increasing MgO and Al2O3 content, the trough of the [AlO4]5− asymmetric stretching band was changed little, and the peak for the [AlO6]9− octahedral was negligible. This indicates that Al2O3 in present slag exists completely in the form of [AlO4]5− tetrahedra and serves only as a network former. The addition of both MgO and Al2O3 resulted in little change to the aluminate structure. In Figure 6(a), as MgO content increased, the center of the band for silicate structure shifted to lower wave numbers (944 to 934 cm−1), which indicates that the number of relatively complex silicate structural units decreases, and the silicate structure depolymerizes. The intensity of Si-O-Al rocking appeared to be relatively weaker at 8 and 12 mass pct MgO and did not continuously decrease as expected owing to the decrease in the absolute amount of SiO2 as MgO content increased. This implies that the arrangement of [SiO4]4− and [AlO4]5− tetrahedra may suddenly transform, and the linkage between them is very weak when the slag composition passes through 8 and 12 mass pct MgO. For the slags with increasing Al2O3 content in Figure 6(b), the center of the band for silicate structure shifted to higher wave numbers (941 to 948 cm−1), and the intensity of Si-O-Al rocking decreased successively, indicating that the silicate structure polymerizes, and the linkage between [SiO4]4− and [AlO4]5− tetrahedra becomes weak.
The original Raman spectra of the rapidly quenched slags with varying MgO and Al2O3 contents are shown in Figure 7. The strong Raman bands were located at the 700 to 1100 cm−1 range, which is the signature of [SiO4]4− tetrahedral symmetry stretching. The shift in position of the highest peak to lower wave numbers as MgO content increased correlated well with the FTIR results. For the slags with increasing Al2O3 content, similar trends in FTIR and Raman results were noted. The asymmetric stretching peaks of [AlO4]5− tetrahedra between 530 and 610 cm−1 varied little, which agrees with the FTIR measurements.
The symmetric stretching bands of [SiO4]4− tetrahedra were deconvoluted into individual peaks at 840 to 860, 900 to 920, 960 to 1000, and 1050 to 1100 cm−1, giving a semi-quantitative assessment of the fraction of Qn (n = 0, 1, 2, 3). The deconvolution of the Raman spectra for the slags with different MgO and Al2O3 contents is shown in Figures 8 and 9, respectively. According to Eq. [1], the ratio of the integrated area of an individual peak to the sum of the integrated area of all individual peaks can provide the fraction of Qn. The average number of non-bridging oxygen (NBO/Si) represents the change in the silicate structure, and it can be calculated by the fraction of Qn multiplied by its non-bridging oxygen number, as shown in Eq. [2]. Figure 10 shows the abundance of silicate structural units with increasing MgO and Al2O3 contents. The variation in Q1 seemed to be relatively small compared to that in Q0 and Q2. As shown in Figure 10(a), with increase in MgO content from 6 to 16 mass pct, the addition of MgO gives rise to the depolymerization of the silicate network structure, indicated by the decrease in Q2 and increase in Q0 and NBO/Si. As shown in Figure 10(b), with an Al2O3 content greater than 10 mass pct, Q0 decreases, while Q2 increases significantly. In addition, NBO/Si decreases. This suggests that the silicate network structure becomes more polymerized, which is consistent with the FTIR results.
where Xn, An, and θn are the mole fraction, the individual peak area, and the Raman scattering coefficient of Qn (n = 0 to 3), respectively.
Discussion
In the slags with a completely liquid state, the silicate network consists only of Q0, Q1, and Q2 units, and the aluminate network structural unit is [AlO4]5− tetrahedral. The addition of MgO and Al2O3 predominantly altered the silicate network rather than the aluminate network structure, presenting little change in the [AlO4]5− stretching bands in the FTIR and Raman spectra.
Effect of MgO Content
Generally, MgO is a network modifier. As the MgO content increases, more O2− ions are introduced into the slag and simplify the silicate network in terms of higher Q0 and lower Q2 concentration, which should have resulted in a monotonous decrease in the slag viscosity. However, within the MgO range of 6 to 16 mass pct, the viscosity exhibited two minimal values at 8 and 12 mass pct MgO and increased above 12 mass pct MgO.
Some studies[6,34] reported that the slag compositional change caused the variation in the equilibrated primary phase field and correspondingly altered the ion arrangements in liquid, which implies combination of anion groups and cations in the micro-area, resulting in an unusual viscosity tendency. The phase diagram of the CaO-SiO2-MgO-15 mass pct Al2O3-10 mass pct FeO estimated using FactSage 7.2 is shown in Figure 11. The slag composition point is marked with solid black circles. The interesting features were noted as follows. On the one hand, by increasing the MgO content from 6 to 16 mass pct, the slag composition point passes through the melilite and spinel primary phase fields in sequence and then reaches the periclase primary phase field. The slag composition of 8 mass pct MgO occurs near the eutectic line of the melilite and spinel phase. A similar situation occurs for the slag composition of 12 mass pct MgO. On the other hand, the linkage between [SiO4]4− and [AlO4]5− tetrahedra is relatively weaker for the slags with 8 and 12 mass pct MgO, and the magnitude of the change in the NBO/Si with increase in MgO content from 6 to 8 mass pct and from 10 to 12 mass pct is relatively higher. It is proposed that the appearance of minimal viscosities at 8 and 12 mass pct MgO is a combined result of the ion rearrangement in liquid corresponding to the change in the equilibrated primary phase and the depolymerization of the silicate network structure. The variations in the combination form of anion groups and cations presumably loosened the Si-O-Al linkage and facilitated silicate depolymerization. When the MgO content exceeded 12 mass pct, NBO/Si increased slowly, which means that the silicate network structure depolymerized sufficiently and further dissociated slightly. From the phase diagram, the liquidus temperature tends to increase rapidly for MgO content over 12 mass pct. The formation of periclase-like clusters is thermodynamically favorable,[35] and the solid periclase phase precipitated from the slag with 16 mass pct MgO, as observed in Figure 1, which results in an increased viscosity. In this case, the dominant factor that increased the viscosity is suggested to be the higher liquidus temperature of the slag.
Phase diagram of the CaO-SiO2-MgO-15 mass pct Al2O3—10 mass pct FeO slag system estimated by FactSage 7.2[28] (Al2O3/Z = 15 pct and FeO/Z =10 pct, where Z = CaO + SiO2 + MgO + Al2O3 + FeO, mass fraction)
Effect of Al2O3 Content
In view of the phase change, the solid crystals, which should be responsible for the high viscosity, decreased until they disappeared in the slags with 6 to 10 mass pct Al2O3. In other words, the role of Al2O3 in decreasing the viscosity of the slag, which transforms the solid–liquid coexisting state to a completely liquid one, could be explained by the effect of the 2CaO·SiO2 and 3CaO·MgO·2SiO2 crystals dissolving into the liquid phase, which is somewhat similar to the findings by Park.[36] This may be ascribed to the decrease in liquidus temperature caused by addition of Al2O3.[25]
The [AlO4]5− tetrahedra in silicate melt require charge compensation from additional cations such as Ca2+ to preserve the electrical neutrality. The propensity of Al2O3 to form a network ([AlO4]5− tetrahedra) was suggested when the ratio of the molar fraction of Al2O3 to the sum molar fraction of basic oxides is less than 1.[31,37] The present slag fits the above situation, which means that sufficient Ca2+ in the primary slag favors the formation and stabilization of [AlO4]5− tetrahedra. Hence, Al2O3 existing as [AlO4]5− tetrahedra gets incorporated into the silicate network and reinforces its polymerization, as verified using FTIR and Raman spectroscopy. At the same time, the charge-compensating Ca2+ is confined by the [AlO4]5− tetrahedra in the liquid slag, thus losing its ability to affect the quantities of various polymer groups.[38] Therefore, the role of Al2O3 beyond 10 mass pct is as a network former because of the acidic nature of Al2O3, resulting in an increase in the viscosity.
Conclusions
In this work, the effects of MgO and Al2O3 content on the viscous and structural behavior of CaO-SiO2-MgO-Al2O3-10 mass pct FeO (C/S = 1.4) slag (blast furnace primary slag) were investigated, and the major findings have been summarized as follows.
-
1.
The viscosity of the slag containing 15 mass pct Al2O3 fluctuates with the increase in MgO content from 6 to 16 mass pct. It has two minimal values at 8 and 12 mass pct MgO, which could be explained by the ion rearrangement in the liquid corresponding to the change in the equilibrated primary phase and the depolymerization of the silicate network structure.
-
2.
Increasing the Al2O3 content from 6 to 10 mass pct causes a decrease in the viscosity of the slag with 10 mass pct MgO. This is due to the effect of the 2CaO·SiO2 and 3CaO·MgO·2SiO2 crystals dissolving into the liquid phase, which results from the decrease in the liquidus temperature caused by the addition of Al2O3. Furthermore, increasing the Al2O3 content to greater than 10 mass pct has the opposite effect on viscosity, as a result of the polymerization of the silicate network structure.
-
3.
From the structural information obtained from FTIR and Raman spectroscopy, MgO and Al2O3 in the molten slag predominantly alter the silicate network rather than the aluminate network, behaving as network modifier and network former, respectively.
References
[1] X.L. Tang, Z.T. Zhang, M. Guo, M. Zhang and X.D. Wang: J. Iron Steel Res. Int., 2011, vol. 18, pp. 1–17.
[2] H. Kim, H. Matsuura, F. Tsukihashi, W.L. Wang, D.J. Min and I. Sohn: Metall. Mater. Trans. B, 2013, vol. 44, pp. 5–12.
[3] Y.M. Gao, S.B. Wang, C. Hong, X.J. Ma and F. Yang: Int. J. Miner. Metall. Mater., 2014, vol. 21, pp. 353–62.
[4] T. Talapaneni, N. Yedla, S. Pal and S. Sarkar: Metall. Mater. Trans. B, 2017, vol. 48, pp. 1450–62.
[5] Y. Liu, X.W. Lv, B. Li and C.G. Bai: Ironmak. Steelmak., 2018, vol. 45, pp. 492–501.
[6] H. Kim, W.H. Kim, I. Sohn, and D.J. Min: Steel Res. Int., 2010, vol. 81, pp. 261–64.
[7] P.C. Li and X.J. Ning: Metall. Mater. Trans. B, 2016, vol. 47, pp. 446–57.
[8] Z.Y. Chang, J.L. Zhang, X.J. Ning, R.Z. Xu, K.X. Jiao and X.Q. Bai: Iron Steel, 2018, vol. 53, pp. 10–15. (In Chinese)
[9] X.D. Ma, M. Chen, J.M. Zhu, H.F. Xu, G. Wang and B.J. Zhao: ISIJ Int., 2018, vol. 58, pp. 1402–05.
[10] S.D. Mao and P. Du: J. Iron Steel Res., 2015, vol. 27, pp. 33–38. (In Chinese)
[11] X.F. Zhang, T. Jiang, X.X. Xue and B.S. Hu: Steel Res. Int., 2016, vol. 87, pp. 87–94.
[12] C.Y. Sun, X.H. Liu, J. Li, X.T. Yin, S. Song and Q. Wang: ISIJ Int., 2017, vol. 57, pp. 978–82.
[13] N. Saito, N. Hori, K. Nakashima and K. Mori: Metall. Mater. Trans. B, 2003, vol. 34, pp. 509–16.
[14] Y.Q. Sun, H. Wang and Z.T. Zhang: Metall. Mater. Trans. B, 2018, vol. 49, pp. 677–87.
[15] J.H. Park, D.J. Min and H.S. Song: Metall. Mater. Trans. B, 2004, vol. 35, pp. 269–75.
[16] J.H. Park, H. Kim and D.J. Min: Metall. Mater. Trans. B, 2008, vol. 39, pp. 150–53.
[17] J.F. Xu, L.J. Su, D. Chen, J.Y. Zhang, and Y. Chen: J. Iron Steel Res. Int., 2015, vol. 22, pp. 1091–97.
[18] Z.M. Yan, X.W. Lv, D. Liang, J. Zhang and C.G. Bai: Metall. Mater. Trans. B, 2017, vol. 48, pp. 1092–99.
[19] Y.S. Lee, D.J. Min, S.M. Jung, and S.H. Yi: ISIJ Int., 2004, vol. 44, pp. 1283–90.
[20] J.R. Kim, Y.S. Lee, D.J. Min, S.M. Jung, and S.H. Yi: ISIJ Int., 2004, vol. 44, pp. 1291–97.
[21] S.W. Kim, J.W. Jeon, I.K. Suh and S.M. Jung: Ironmak. Steelmak., 2016, vol. 43, pp. 500–07.
[22] F.M. Shen, X.G. Hu, H.Y. Zheng, X. Jiang, Q.J. Gao, H.S. Han and F. Long: Metals, 2020, vol. 10, pp. 784.
[23] M.Y. Kou, S.L. Wu, X.D. Ma, L.X. Wang, M. Chen, Q.W. Cai and B.J. Zhao: Metall. Mater. Trans. B, 2016, vol. 47, pp. 1093–1102.
X.M. Cheng, X.G. B, S.Z. Shi, Y.R. Ma, P. Li and H.X. Zhang: Iron Steel, 2014, vol. 49, pp. 9–14. (In Chinese).
[25] S.L. Wu, W. Huang, M.Y. Kou, X.L. Liu, K.P. Du, and K.F. Zhang: Steel Res. Int., 2015, vol. 86, pp. 550–56.
[26] S.L. Wu, B. Su, X.L. Liu and M.Y. Kou: Ironmak. Steelmak., 2018, vol. 45, pp. 50–57.
[27] T.L. Li, C.Y. Sun and Q. Wang: Iron Steel, 2019, vol. 54, pp. 12–18. (In Chinese)
Introduction to the calculation of phase diagram using FactSage thermodynamic software (CRCT-ThermFact Inc. & GTT-Technologies), http://www.factsage.com. Version 7.2 accessed on 15 Feb 2018
[29] I. Sohn and D.J. Min: Steel Res. Int., 2012, vol. 83, pp. 611–30.
[30] T.L. Li, C.Y. Sun, S. Song and Q. Wang: Metals, 2019, vol. 9, pp. 866.
[31] C. Feng, M.S. Chu, J.Tang, Y.T. Tang and Z.G. Liu: Steel Res. Int., 2016, vol. 87, pp. 1274–83.
[32] W.L. Li and X.X. Xue: ISIJ Int., 2018, vol. 58, pp. 1751–60.
[33] K.X. Jiao, J.L. Zhang, Z.Y. Wang, C.L. Chen, and Y.X. Liu: Steel Res. Int., 2017, vol. 88, pp. 1600296.
[34] H.S. Park, S.S. Park and I. Sohn: Metall. Mater. Trans. B, 2011, vol. 42, pp. 692–99.
[35] D. Siafakas, T. Matsushita, A.E.W. Jarfors, S. Hakamada, and M. Watanabe: ISIJ Int., 2018, vol. 58, pp. 2180–85.
[36] J.H. Park and D.J. Min: J. Non-cryst. Solids, 2004, vol. 337, pp. 150–56.
[37] X.J. Dong, H.Y. Sun, X.F. She, Q.G. Xue, and J.S. Wang: Ironmak. Steelmak., 2014, vol. 41, pp. 99–106.
[38] K. C. Mills: ISIJ Int., 1993, vol. 33, pp. 148–55.
Acknowledgments
We gratefully express our appreciation to Rio Tinto for supporting this work through The Rio Tinto–USTL (University of Science and Technology Liaoning) Joint Research Project and Liaoning Province Natural Fund Guidance Plan Project (20180550599).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted April 21, 2020; Accepted September 14, 2020.
Rights and permissions
About this article
Cite this article
Li, T., Zhao, C., Sun, C. et al. Roles of MgO and Al2O3 in Viscous and Structural Behavior of Blast Furnace Primary Slag with C/S = 1.4. Metall Mater Trans B 51, 2724–2734 (2020). https://doi.org/10.1007/s11663-020-01980-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01980-z