Abstract
To understand the effect of refractory on the evolution of inclusions in the secondary steelmaking, steel samples were taken at different stages of the process train. Laboratory experiments were carried out using different refractories (alumina, spinel, and MgO). In the laboratory study, the types of inclusions considered were alumina, spinel, and calcium aluminate. The focus was given to Al-killed steel. The results showed that alumina refractory and spinel refractory had little effect on all the three types of inclusions, while the effect of MgO refractory depended on the activity of dissolved oxygen in liquid steel. With lower oxygen activity, alumina inclusions could transform into spinel inclusions. No evident change could be found for spinel and calcium aluminate inclusions. When the oxygen activity was high enough, spinel inclusions could not be generated from alumina inclusions. The laboratory results helped in understanding the evolution of the inclusions in the industrial process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In steelmaking, alumina, spinel, and calcium aluminate inclusions are the common types of inclusions in Al-killed steels.[1–4] Alumina and spinel inclusions are irregular, and hard to deform, which may cause defects in final products,[1,5,6] while most of calcium aluminate inclusions are globular and sometimes are considered harmful for the fatigue property of steel.[7,8] Therefore, the control of these inclusions becomes extremely important for steelmaking industry.
Many studies have been conducted aiming at a better control of inclusions in steelmaking process. A number of publications have pointed out that refractory has great impact on the formation and evolution of inclusions during refining process.[2,3,9–23] Three types of refractories—alumina, spinel, and MgO—are widely used in industrial production.
For MgO refractory, Brabie et al.[9,10] proposed that carbon in refractory could reduce MgO into Mg gas, and then Mg gas would lead to the formation of spinel inclusions. A number of studies[2,3,11–18] also indicated that some of the dissolved Mg in Al-killed steel is supplied from the reduction of MgO refractory by dissolved Al. Some researchers[13,19] suggested that the carbon in high-carbon steels, e.g., bearing steels, could also reduce MgO refractory, resulting in the dissolution of Mg into the liquid metal.
MgO inclusions have also been found in liquid steel. The MgO inclusions may come from refractory. Some researchers[20–22] considered that the MgO refractory was also one of the sources of the inclusions with MgO islands inside the calcium aluminate liquid phase.
In fact, the effects of alumina and spinel refractories on the inclusions have rarely been reported, though a few studies have briefly touched this area.[23] Moreover, the slag-steel-refractory system has usually been chosen to study the effect on inclusions. Hence, the individual effects of slag and refractory have not been clearly identified. Further systematical investigation is needed to understand the effects of different refractories on different types of inclusions.
The present work focuses on the effect of refractory on inclusions in Al-killed steel. Steel samples were taken from industry to identify the types of inclusion. Laboratory experiments were carried out to study the effects of different refractories on different inclusions. Based on the comparison of the results of laboratory experiments with industrial findings, the effect of refractory on different types of inclusions in real production was discussed.
Experimental
Industrial Sampling
The steel grade was produced by the process route: Hot metal predesulphurization → BOF steelmaking (80 t) → LF (80 t) refining → RH (80 t) refining (calcium treatment) → Bloom (280 mm × 325 mm) continuous casting. The process was described in detail in a previous publication.[3] During tapping from the BOF, most of aluminum, alloys, and slag formers were added into the ladle. After tapping, the dissolved oxygen activity was 2–4 ppm (by Celox measurements). In LF refining, some aluminum and refining fluxes were also added. The treating times for LF and RH refining were about 40 and 25 minutes, respectively. Note that all the ladles were built with carbon bearing MgO refractory.
The general composition of experimental steel grade is presented in Table I. Steel samples were taken at different stages during the process, viz. (1) at the end of BOF, (2) after tapping, (3) at the middle stage of LF refining, (4) after LF refining, and (5) after RH refining. The samples were prepared for inclusion analysis. A scanning electron microscope (SEM, HITACHI S-3700N) with energy-dispersive spectrometer (EDS) was employed to study the morphologies and phases of inclusions.
Laboratory Experiments
Materials and sample preparation
Three different refractories were considered in the present study, viz. alumina, spinel (mole ratio MgO/Al2O3 = 1:1), and MgO. The types of inclusions studied were alumina, spinel, and calcium aluminate.
All the refractory plates (diameter 18 mm) were made from chemical agents (reagent grade). To make a plate, the oxide powders were well mixed and then pressed into a plate by a mold with a pressure of 20 MPa. Thereafter, the plate was sintered (on a piece of Pt sheet) in a muffle furnace at 1773 K (1500 °C) for 8 hours. Besides, MgO crucibles (inside diameter 20 mm) were also used to further investigate the formation of spinel inclusions in steel.
In view of the difficulties in finding a specific inclusion in the steel sample, thin oxide sticks were made. To make the stick, the corresponding type of oxide plate was cut into small pieces and polished into thin oxide sticks (diameter <1.5 mm). In order to keep the stick in liquid steel without melting at 1873 K (1600 °C), solid calcium aluminate CaO·2Al2O3 (simplified as CA2 in the following text) was chosen in the study of calcium aluminate inclusions.
Two steel grades were employed in the experiments: Steel-I and Steel-II. Steel-I is Al-killed microalloyed steel, the composition of which is given in Table II, while Steel-II is almost pure iron (Fe > 99.9 pct). The steels were cut into small pieces (around 30 g per piece). A small hole was drilled in the middle of each steel piece in order to put an oxide stick in.
Experimental procedure and analysis
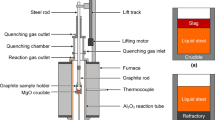
The experimental setup is presented in Figure 1. A resistance furnace was employed. The alumina reaction tube and the quenching chamber were internally connected and sealed by O-rings. A vacuum pump could evacuate the reaction tube. The quenching chamber was cooled by water. High-purity argon with high flow rate could be injected on to the sample to enhance the quenching. A sample holder (graphite/molybdenum) was hung on a suspension steel rod. The suspension rod could be moved up and down by a lifting motor. O-rings were used to seal the whole system.
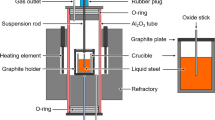
The experimental conditions are presented in Table III. In a typical run, a sintered refractory plate (for Runs A1 to D3 only) was placed at the bottom of the crucible (inside diameter 20 mm). An oxide stick was put in the hole of the steel piece, before placing them into the crucibles. In order to keep the stick in the middle of the liquid steel bath, the position of the thin stick was fixed by an alumina plate placed on the top of the crucible (see Figure 1(b)). Thereafter, the crucibles were positioned in a graphite or Mo sample holder. In general, three crucibles were placed in the sample holder in each run. The sample holder was positioned in the quenching chamber by the steel rod. The alumina reaction tube and the quenching chamber were sealed, evacuated, and filled with high-purity argon. The procedure of evacuating and argon filling was repeated three times. Thereafter, the furnace was heated up, and the gas was switched to the reaction gases with a total gas flow rate of 0.2 NL/min. When the furnace reached the target temperature, the sample holder with crucibles was lowered into the reaction tube and kept at the position of 1573 K (1300 °C) to preheat for 15 minutes. After that, the sample holder was lowered to the even temperature zone of the reaction tube. This time was considered the time zero (t = 0 minutes) of the run. After the reaction time, the sample holder was quickly lifted into the quenching chamber by the motor. The argon gas with high flow rate was immediately injected into the quenching chamber to enhance quenching.
In the experiments, the activity of dissolved oxygen in liquid steel is evaluated by Eq. [1]. For Run A1 to E2, the graphite crucible was used. The oxygen partial pressure in Eq. [1] can be calculated using Eq. [2]. In the case of Mo crucible (Run F1 to F2), the oxygen partial pressure can be obtained from Eq. [3]. The thermodynamic data for Reactions [1] through [3] are obtained from literature:[24,25]
The activity of dissolved oxygen, a [O] in Run A1 to E2 was 1 ppm, while in Run F1 to F2, a [O] was 456 ppm. The experiments F1 and F2 were carried out to investigate the effect of oxygen potential on the dissolution of Mg in liquid steel from MgO refractory.
The steel sample with the oxide stick inside was cut and prepared for microscopic analysis. The morphology and phase(s) in the stick were also investigated by SEM-EDS. Special attention was paid to the boundary between steel and oxide stick.
Results
Inclusions in Industrial Samples
The identified types of inclusions and their specification are given in Table IV. Figures 2(a) through (h) show the morphologies of these types of inclusions. Totally, eight types of inclusions are found in the industrial samples. Type 1 inclusions are calcium silicate with high FeO content and low Al2O3, MgO, and MnO contents, while Type 2 inclusions are a combination of Type 1 and some (Mg, Fe, Mn)O islands. The inclusions of Type 3 are (Fe, Mn)O inclusions. Types 1 to 3 inclusions are only observed in the crude steel at the end of BOF. The amount of Type 4 inclusions (alumina clusters) are very limited, and they are only found after tapping from the BOF. Singular alumina particles (Type 5) are mainly detected in liquid steel after tapping. A few of them are also observed at the middle stage of LF and after LF refining. Spinel is considered Type 6 inclusions, and they are mainly seen at the middle stage of LF refining. Besides, a few of them are also found after tapping and after LF refining. Calcium aluminate inclusions, namely Type 7, contain also low contents of MgO and SiO2. Type 8 is the combination of Type 6 and Type 7. In a typical Type 8 inclusion, spinel phase is surrounded by a calcium aluminate outer layer. Type 7 and Type 8 are the main types of inclusions after LF and RH refining. Even though, a small amount of these two kinds of inclusions were also detected after tapping and at the middle stage of LF. The composition range of each type of inclusions is presented in Table V. Earlier study[26] has already reported that Type 1 and Type 2 are from BOF slag, and Type 3 is formed during oxygen blowing.
As shown in Figure 2, the inclusions of Types 1, 2, 3, 7, and 8 are in globular shape, while other types are irregular inclusions. The nature of globular shape and the composition shown in Table V imply that Types 1, 2, 3, 7, and 8 are liquid inclusions (or the outer layer is liquid). Note that Type 1 and Type 2 inclusions have a wide size range (1 to 50 μm, Figures 2(a) and (b) just give two examples). Type 4 inclusions are relatively small, and their sizes are less than 10 μm (see Figure 2(c)). As shown in Figures 2(d) and (e), the size of alumina clusters is much bigger than that of singular alumina inclusions (<10 μm). Meanwhile, inclusions of Types 7 and 8 are also smaller than 10 μm.
Effect of Refractory on Inclusions in Laboratory Experiment
Effect of refractory on alumina inclusions
Figure 3 presents the elemental mappings of alumina sticks in steel kept with different refractories. It is easy to understand that alumina refractory could not affect alumina inclusions, and therefore, the elemental mappings of the stick kept with alumina refractory are not shown in this figure. In order to keep it brief, O element is not shown in the figures, though it is still one of the elements in the sticks. Also note that all the elemental mappings are obtained from the samples at low oxygen partial pressure. As shown in this figure, after 180 minutes of reaction, the alumina stick in steel still remains alumina phase using spinel refractory (see Figure 3(a)). When the alumina stick is put into liquid steel with MgO refractory, some spinel particles are formed at the boundary between liquid steel and alumina stick. The spinel particles are very small and irregular. Their sizes are usually less than 10 μm (see Figure 3(b)). It implies that MgO refractory helps alumina inclusions to generate spinel inclusions, but spinel refractory could not influence evidently.
The experimental result of each run is summarized in Table VI. As shown in Table VI, the effect of MgO refractory on alumina sticks (inclusions) depends also on the activity of dissolved oxygen. When the oxygen activity is very low, spinel phase is formed on the alumina sticks (Run C1, D1, and E1 to E2). On the other hand, if the oxygen activity is too high (Run F1 to F2), even with MgO refractory, no new phase could be found on the alumina sticks.
Effect of refractory on spinel inclusions
Figure 4 shows the elemental mappings of spinel sticks in steel kept with different refractories. All the elemental mappings are acquired from the samples at low oxygen activity. As shown in this figure, after 180 minutes of reaction, the spinel sticks in steel do not have any evident change, no matter what refractory is used. Even after 360 minutes (Run D2), the spinel stick in liquid steel does not show any change, in the case of both spinel and MgO refractory. It means that these refractories have very little effect on spinel inclusions in liquid steel.
Effect of refractory on calcium aluminate inclusions
The elemental mappings of calcium aluminate sticks are shown in Figure 5. They are also acquired from the samples in the CO + C atmosphere. As shown in this figure, Al element and Ca element are mainly distributed in the sticks. At the boundary between calcium aluminate and liquid steel, no element enrichment can be clearly seen. Besides, almost no Mg element is distributed in the calcium aluminate sticks, no matter whether the refractory is spinel or MgO (see Figures 5(b), (c) respectively). It is also necessary to mention that the result of Run D3 (MgO refractory, t = 360 minutes, see Table VI) is almost the same as shown in Figure 5(c) (t = 180 minutes). This indicates that the three refractories have no obvious effect on the calcium aluminate inclusions in liquid steel.
Discussion
Effect of Refractory on Inclusions
Effect of alumina refractory
As shown in Table VI, and Figures 4(a) and 5(a), alumina refractory has little effect on the three types of inclusions in Al-killed steel. According to Eq. [4], the existence of alumina may influence the activities of dissolved aluminum and oxygen:
In the case of alumina refractory, the activity of alumina can be considered as unity; meanwhile, the activity of dissolved oxygen is around 1 to 2 ppm (see Table VI). Therefore, the activity of dissolved aluminum in liquid steel would not change according to Eq. [4]. In other words, the activities of the dissolved elements in liquid steel would maintain the same, and the inclusions in liquid steel would not be affected. The little effect of alumina on the inclusions would confirm that the use of alumina crucibles in present study has negligible effect on the experimental results.
Effect of MgO refractory
The alumina stick does not contain MgO as shown in Figure 3(a). On the other hand, Table VI (Run C1, D1, E1 to E2) and Figure 3(b) reveal that spinel particles are formed at the boundary between liquid steel and alumina stick, when MgO refractory is used at low oxygen activity. Thus, the Mg element in spinel inclusions should be related to MgO refractory. Since MgO is solid, the only possible explanation for this effect is the dissolution of MgO in the steel according to Eq. [5]. In fact, the thermodynamic data of Eq. [5] are very scattered (see Table VII[6,27–36]). Therefore, to avoid using inaccurate values, the reactions are discussed on a qualitative base. Despite the big scatter of the data, the mechanism could still be discussed.
The dissolved Mg in liquid steel can be transferred to the alumina stick and react with it to form spinel phase using Eq. [6]:
Since the refractory is pure solid MgO, the activity of MgO can be considered as unity. According to Eq. [5], the activity of dissolved Mg would decrease with the increase of dissolved oxygen activity.
It is reported that the Mg content in steel is only a few ppm (even less) with an oxygen activity lower than 5 ppm in the steel during ladle treatment.[3,37] In the present work, when MgO refractory is used (Run C1, D1, E1 to E2) with a low oxygen activity (around 1 ppm), the amount of dissolved Mg should be very tiny. Due to the strong affinity of dissolved Mg with oxygen, when the dissolved Mg generated from refractory meets alumina, spinel phase would be formed. Since the alumina stick is still much bigger than real inclusions, the amount of dissolved Mg transferred to the boundary is not enough to form a spinel layer surrounding the alumina stick, but only some small particles on the stick as shown in Figure 3(b). When the oxygen activity increases to around 500 ppm (Run F1 to F2), the dissolved Mg in liquid steel should be extremely low. The mass transfer of Mg would be extremely small, which can be ignored. Therefore, no spinel phase could be detected in Run F1 to F2 as shown in Table VI.
In Run E2, even the steel is pure iron, spinel phase is still generated at low dissolved oxygen level. It means that the formation of spinel is due to Reaction [6], but not the following reaction:
The present results indicate that lower oxygen activity is one of the key factors for the formation of dissolved Mg from MgO refractory. The dissolution of the MgO refractory into Al-killed steel could be due to both the reduction of MgO by dissolved Al[2,3,11–18] and reduction of MgO by dissolved carbon in steel.[13,19]
As shown in Table VI and Figure 4(b), MgO refractory has almost no effect on spinel inclusions in liquid steel. In fact, the formation of spinel inclusions has been discussed by many researchers.[2,3,6] It is found that when there is trace of dissolved Mg, spinel inclusions could be stable in liquid steel at a certain dissolved oxygen level. In the present study, the formation of spinel particles on alumina stick in Run C1, D1, and E1 to E2 actually supports the argument that spinel inclusions could be stable at the experimental oxygen level. Therefore, the effect of MgO refractory on spinel inclusions should be very limited.
As shown in Table VI and Figure 5(c), the effect of MgO refractory on calcium aluminate inclusions is not obvious. Because of the decomposition of MgO refractory, the Reaction [8] may take place:
According to this reaction, spinel phase may form on the calcium aluminate stick. However, there is no spinel phase at the boundary between liquid steel and calcium aluminate stick (see Figure 4(b), Run C3), even after 360 minutes of reaction (Run D3, see Table VI). This implies that the experimental conditions do not favor Eq. [8].
On the other hand, according to the CaO-MgO-Al2O3 phase diagram,[38] a small amount of MgO could dissolve into CaO·2Al2O3 solid solution. Although some MgO is dissolved, the phase is still solid calcium aluminate. As discussed above, the amount of dissolved Mg in steel is tiny. Consequently, the amount of Mg transferred to calcium aluminate should be also very small. In fact, no obvious element enrichment is found at the boundary in Figure 5(c) (Run C3), even after 360 minutes of reaction (Run D3). Note that EDS analysis has uncertainties. Due to the difficulty of detecting trace elements, it is also hard to see the mapping difference of Mg element as shown in Figure 5(c).
Some oxide sticks were used to simulate inclusions in the present study. When MgO refractory was employed at low oxygen activity, spinel particles were detected at the boundary between alumina stick and liquid steel. In fact, this result indicates that the tiny amount of dissolved Mg in liquid steel could make some phase transformation for the stick, although the stick is much bigger than real inclusions, and the new phase is small particles, not a phase layer. On the other hand, at the same oxygen level, no new phase was detected on spinel stick and calcium aluminate stick, even no local element enrichment was found. It implies the reactions for phase evolution are not favorable for spinel and calcium aluminate inclusions on the experimental conditions. However, it should be mentioned that both spinel phase and calcium aluminate phase can pick up small amount of MgO. The uncertainties associated with EDS would make it difficult to detect the tiny increase of MgO in these two phase.
Effect of spinel refractory
The results in Table VI, and Figures 3(a) and 5(b) show that spinel refractory has little impact on all the three types of inclusions. As known, spinel is a solid solution composed of Al2O3 and MgO. Hence, the activities of alumina and MgO in spinel refractory would play important role. Fujii et al.[39] measured the activities of these two components. According to their results, the activity of MgO in spinel refractory is very low (around 0.06), while the activity of alumina is around 0.5. As discussed above, it is easy to understand that spinel refractory is difficult to generate dissolved Mg in liquid steel due to the low activity of MgO. Therefore, spinel refractory has little influence on inclusions in steel.
Comparison of the Laboratory Results with Industrial Finding
At the end of BOF, a certain amount of calcium silicate inclusions were found. Some of them contain only liquid phase (Type 1), while some of them have (Mg, Fe, Mn)O islands embedded in the liquid calcium silicate phase (Type 2). Earlier study[26] indicates that they are small droplets of BOF slag. The paper[26] also indicates that after deoxidation calcium silicate is transformed into calcium aluminates. It is reasonable to conclude that inclusions of Type 7 and Type 8 found just after deoxidation (after tapping and at the middle of LF refining in Table IV) are, respectively, the products of the reduction of Type 1 and Type 2 by dissolved aluminum. However, the amount of these inclusions is very small as presented in Table IV.
The dissolved oxygen activity in liquid steel in the ladle was very low after tapping. After deoxidation, (Fe, Mn)O inclusions (Type 3) in BOF crude steel would be reduced by dissolved Al to form alumina inclusions (Type 5). The reduction of FeO and MnO by dissolved Al can very well explain the vanishing of Type 3 inclusions after tapping (see Table IV).
Table IV indicates that a small amount of alumina clusters (Type 4) were detected after tapping. It is well known that alumina clusters could be formed by homogenous nucleation when the supersaturation is high enough. The big alumina clusters (see Figure 2d) would float up and join the top slag easily. Therefore, the alumina clusters could only be detected just after the main deoxidation step. Although some amount of aluminum was also added into ladle in LF refining, homogenous nucleation could not take place due to the low supersaturation of aluminum. The present finding is in good accordance with earlier studies.[21,37]
While the main inclusions are singular alumina (Type 5) after tapping, spinel (Type 6) becomes the main type of inclusions at the middle stage of LF refining. This indicates that most of alumina inclusions have been transformed into spinel phase. The industrial results are in line with the laboratory results. Both of them confirm that MgO refractory could help alumina inclusions to transform into spinel inclusions. In the ladle, MgO refractory has a large contact area with liquid steel. Although the top slag could also supply Mg to liquid steel in the ladle, MgO refractory is easier to decompose due to its high activity (unity) and the large contact area with liquid steel at low oxygen level. It is very important to mention that the dissolved oxygen level in Al-killed steel is very low (measured to be 2 to 4 ppm), then spinel inclusions could be formed from alumina incisions due to the decomposition of MgO refractory. On the other hand, if the dissolved oxygen in liquid steel is very high, the formation of spinel inclusions from alumina would be very difficult. This could be explained by the experimental results of Run E1 to F2 as presented in Table VI.
As revealed in Table IV, calcium aluminates (Types 7 and 8) exist in liquid steel during the whole period of refining processes. This finding is well explained by the present laboratory results. In the laboratory experiments, no appreciable Mg enrichment is found at the boundary between steel and calcium aluminate (see Figure 5(c)). Note that only CaO·2Al2O3 phase is considered in the present work. However, according to the CaO-MgO-Al2O3 phase diagram,[38] the solubility of MgO in the other calcium aluminates (3CaO·Al2O3, 12CaO·7Al2O3 and CaO·Al2O3) is larger than that in CaO·2Al2O3. It is expected that the formation of spinel phase on these calcium aluminates is even more difficult than that on CaO·2Al2O3, since the dissolved Mg is not enough. The present results suggest that MgO refractory would have negligible effect on calcium aluminate inclusions.
A lot of studies[2,3,6] show that calcium aluminate is the most stable phase in liquid steel with trace of dissolved Ca. It is believed that top slag is the source of dissolved Ca in liquid steel. As shown in Table IV, the ladle spinel phase is mainly detected at the middle stage of LF refining, while after LF and RH refining, calcium aluminates (Types 7 and 8) become the main types of inclusions. As mentioned above, the amount of calcium aluminates transformed from Type 1 and Type 2 is very mall, so most of the calcium aluminates should be from other types of inclusions. The vanishing of spinel phase after LF and RH refining in fact indicates that the most of the inclusions of Type 7 and Type 8 are from the transformation of spinel phase. On the other hand, in the present laboratory study, spinel phase was found to be stable (see Figure 3(b), Run C1, D1 and E1 to E2) without slag. The comparison between industrial and laboratory results suggests strongly that the refining slag helps spinel inclusions to transform to calcium aluminate inclusions, and MgO refractory could not have an evident impact on this evolution.
Table IV also reveals that after LF refining, a small amount of Type 5 inclusions (alumina) are still found. This can be explained by the additional deoxidation using Al during LF refining. After RH treatment, this type of inclusions has vanished. The constant supply of Ca from the slag would react with alumina resulting in calcium aluminate inclusions (Type 7), though the solubility of Ca is very low. The effect of slag on the transformation of spinel inclusions and alumina inclusions has been reported by many researchers.[2,3,6]
It is worthwhile to mention that the mole ratio of MgO/Al2O3 is around 1:1 in the present laboratory study, while in some steel plants, alumina-saturated and MgO-saturated spinel refractories are used. According to the experimental results by Fujii et al,[39] the activity of MgO is unity in MgO-saturated spinel refractory, while the activity of alumina is almost zero. It is expected that MgO-saturated spinel refractory would behave similar as MgO, possibly helping alumina inclusions to generate spinel phase. Similarly, when the alumina-saturated spinel refractory is used, the effect would be negligible.
Summary
In order to study the effect of refractory on different types of inclusions in Al-killed steel, steel samples were taken at different stages of the process train, and laboratory experiments were carried out. Refractories of alumina, spinel, and MgO and inclusions of alumina, spinel, and calcium aluminate were considered in the experiments. The results showed that alumina refractory and spinel refractory had little impact on all the three types of inclusions, while the effect of MgO refractory depended on the activity of dissolved oxygen in liquid steel. When the oxygen activity was very low, alumina inclusions could transform into spinel inclusions, but no evident change could be found for spinel and calcium aluminate inclusions. On the other hand, when the oxygen activity is high enough, spinel inclusions could not be generated from alumina inclusions due to the extremely low dissolved Mg in liquid steel. Lower oxygen activity is one of the key factors for the formation of dissolved Mg from MgO refractory.
References
L. Zhang and B. G. Thomas: ISIJ Int., 2003, vol. 43, pp. 271–91.
M. Jiang, X.H. Wang, B. Chen and W.J. Wang: ISIJ Int., 2008, vol. 48, pp. 885–90.
Z. Y. Deng and M. Y. Zhu: ISIJ Int., 2013, vol. 53, pp. 450–58.
K. Beskow and D Sichen: Ironmaking Steelmaking, 2004, vol. 31, pp. 393–400.
H. Todoroki and S. Inada: Bull. Iron Steel Inst. Jpn., 2003, vol. 8, pp. 575–79.
J.H. Park and H Todoroki: ISIJ Int., 2010, vol. 50, pp. 1333–46.
M. Larsson, A. Melander, and A. Nordgren. Mater. Sci. Technol., 1993, vol. 9, pp. 235–45.
Y. Wang and R. Akid. Corros. Sci., 1996, vol. 52, pp. 92–102.
V Brabie. ISIJ Int., 1996, vol. 36, pp. s109–12.
V Brabie. Steel Res., 1997, vol. 66, pp. 54–60.
J. W. Kim, K. S. Kim, D. S. Kim, D. Y. Lee and K. P. Yang: ISIJ Int., 1996,vol. 36, pp. s140–43.
T. Nishi and K. Shimme: Tetsu-to-Hagané, 1998, vol.84, pp. 97–102.
H. Matsuno and Y. Kikuchi: Tetsu-to-Hagané, 2002, vol. 88, pp. 48–50.
H. Todoroki and K. Mizuno: ISIJ Int., 2004, vol. 44, pp. 1350–57.
W. Y. Cha, D. S. Kim, Y. D. Lee and J. J. Pak: ISIJ Int., 2004, vol. 44, pp. 1134–39.
J. H. Park and D. S. Kim: Metall. Mater. Trans. B, 2005, vol. 36B, pp. 495–502.
Y. Ehara, S. Yokoyama and M. Kawakami: Tetsu-to-Hagané, 2007, vol. 93, pp. 208–14.
J. H. Park, S. B. Lee and H. R. Gaye: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 853–61.
Y. Kato and Y. Nuri: Sanyo Tech. Rep., 1997, vol. 4, pp. 63–70.
G. Okuyama, K. Yamauchi, S. Takeuchi and K. Sorimachi: ISIJ Int., 2000, vol. 40, pp. 121–28.
Y. J. Kang, M. Nzotta and D. Sichen: Steel Grips, 2007, vol.5, pp.18–34.
M. H. Song, M. Nzotta and D. Sichen: Steel Res. Int., 2009, vol. 80, pp. 753–60.
Z.Y. Deng and M.Y. Zhu: 8th Pacific Rim Int. Conference on Advanced Materials and Processing (PRICM 8). Hawaii, 2013, pp. 753–60.
B. Deo and R. Boom: Fundamentals of steelmaking metallurgy, 1993, New York, Prentice Hall International.
E. T. Turkdogan: Physical chemistry of high temperature technology; 1980, New York, Academic Press.
Y.G. Chi, Z.Y. Deng and M.Y. Zhu: Formation and evolution of non-metallic inclusions during deoxidation by Al addition in BOF crude steel. Sent for publication.
G. K. Sigworth and J. F. Elliot: Met. Sci., 1974, vol. 8, pp. 298–310.
J. D. Seo, S. H. Kim and K. R. Lee: Steel Res., 1998, vol. 69, pp. 49–53.
H. Itoh, M. Hino and S. Ban-ya: Tetsu-to-Hagané, 1997, vol. 83, pp. 623–28.
E. T. Turkdogan, P. J. Spencer and D. Janke: Steel Res., 1991, vol. 62, pp. 379–84.
R. Inoue and H. Suito: Metall. Mater. Trans. B, 1994, vol. 25B, pp. 235–244.
M. Nadif and C. Gatellier: Process Technol. Proc., Iron & Steel Society, 1986, vol.6, pp. 741–50.
I.S. Kulikov: Izv. Akad. Nauk SSSR, Met., 1985, pp. 9–15.
A.P. Gorobetz: Metall. Koksokhim, 1980, vol. 69, pp. 34–37.
Q. Han, D. Zhou and C. Xiang: Steel Res., 1997, vol. 68, pp. 9–14.
J. Gran and D. Sichen. Metall. Mater. Trans. B, 2011, vol. 42, pp.921–24.
K. Beskow, J. Jia, C.H.P Lupis and D Sichen: Ironmaking Steelmaking, 2002, vol. 31, pp. 427–35.
Verein Deutscher Eisenhüttenleute (VDEh), Slag Atlas, 2nd Ed., 1995, Düsseldorf, Verlag Stahleisen GmbH.
K. Fujii, T. Nagasaka and M. Hino: ISIJ Int, 2000, vol. 40, pp.1059–66.
Acknowledgments
The authors are very thankful to Dr. Fan Li and Mr. Yunguang Chi for their help in the experiments. China Scholarship Council (CSC) is acknowledged for supporting Zhiyin Deng’s study at KTH Royal Institute of Technology. The authors are also grateful for the support of the National Natural Science Foundation of China (U1560208) and National Natural Science Foundation of Liaoning Province, China (2014029101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted April 7, 2016.
Rights and permissions
About this article
Cite this article
Deng, Z., Zhu, M. & Sichen, D. Effect of Refractory on Nonmetallic Inclusions in Al-Killed Steel. Metall Mater Trans B 47, 3158–3167 (2016). https://doi.org/10.1007/s11663-016-0746-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0746-2