Abstract
The thermodynamics of magnesium in liquid iron was determined at 1823 K (1550 °C). For this purpose, liquid iron was equilibrated with Ag-Mg alloys in a semienclosed molybdenum vessel. From the partition of magnesium between iron and silver, the activity coefficient of Mg and the self-interaction parameter \( \varepsilon_{\text{Mg}}^{\text{Mg}} \) were determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Magnesium and other alkaline earth metals, especially their oxides, are used widely in the production of high-quality steels. Magnesium metal is used mostly as a desulfurization agent of hot metal, whereas its oxide serves as the main component in refractory linings for different kinds of steelmaking reactors. The use of MgO lining and MgO-containing slag result in a certain amount of dissolved Mg in the liquid metal. The content of Mg dissolved in the metal has great impact on the stability of MgO containing inclusions. In addition, the carbothermic reduction of MgO in MgO-C linings is suggested to be one of the mechanisms behind formation of spinel type nonmetallic inclusions in molten steel,[1–3] leading to an increase in the total magnesium content in ladle processing.
Because of the its crucial role, the deoxidation equilibrium for the reaction Mg(wt pct Fe) + O(wt pct Fe) = MgO(s) has been studied by several research groups.[4–8] Unfortunately, the results from different research groups show a poor consistency. The reported equilibrium constant of this reaction varies even three orders of magnitude. Zhang et al.[9] investigated the dissolution equilibrium of magnesium vapor in liquid iron, trying to exclude oxygen from the system. Their data treatment was later discussed by Hillert[10] and Tarby.[11] The self-interaction parameter of magnesium, derived by Tarby[11] from the data of Zhang et al.[9] was, as described by the author, of “unusual magnitude.”
To have better process control and optimization toward clean steelmaking, reliable thermodynamic data for magnesium in liquid iron is essential. Therefore, the objective of the current work is to determine the activities of magnesium in liquid iron by measuring experimentally the distribution ratio of magnesium between silver and iron.
The principle of the experiment is to use a semienclosed vessel that would allow gas to escape when there is a pressure difference between the inside and outside of the vessel, but not allow extensive gas exchange with the outside at mechanical equilibrium. For this purpose, a Mo-vessel equipped with a conical lid was used.
To check the reliability of the setup to maintain 1 atmosphere inside the container and at the same time avoid any gas entering from the surrounding into the vessel, a series of tests were made at room temperature. The Mo-vessel was filled with a well-weighed amount of ethanol. The conical Mo lid was put on the vessel by hand and a small hammer, using slight force not to seal the chamber completely. The Mo vessel with ethanol was placed in the water bath of a thermostat kept at predetermined temperature. After a certain period of time, the vessel was withdrawn from the bath and dried carefully on the outside. The whole vessel was thereafter weighted. For each temperature, four different holding times in the water bath were employed. The normalized weights are plotted as function of time in Figures 1 for 3 temperatures. The normalized weight is defined as
where W and W o stand for the instant and initial weights of the sample, respectively.
The two curves at 350.6 K and 352.6 K (77.6 °C and 79.6 °C) in Figure 1 indicate that the weight loss of ethanol below and around its boiling temperature (351.6 K [78.6 °C]) is negligible. In contrast, the curve obtained 5 K above the boiling point shows a considerable ethanol loss. It should be mentioned that although the curve of 352.6 K (79.6 °C) (1 K above the boiling temperature) shows negligible weight change, it still indicates a slow weight decrease. These results evidently indicate that the vessel setup would allow overpressures to escape but would not allow extensive gas exchange with the surrounding gas.
The iron and silver powders (both 99.99 pct supplied by Alfa Aesar) were heat treated for at least 3 hours at 973 K and 773 K (700 °C and 500 °C), respectively, in a hydrogen atmosphere to remove traces of oxygen on the surfaces of the particles. The hydrogen gas had oxygen content less than 2 ppm (HiQ, Hydrogen 5.0). Because the same gas had been used successfully for the reduction of iron oxides and molybdenum oxides, no special cleaning procedure was used for the hydrogen gas. The flow rate of hydrogen gas was kept at 0.2 L/min to flush away reaction products (H2O). Silver powder and magnesium turnings (99.98 pct, Alfa Aesar, size: 1 cm and smaller) were weighed into appropriate ratios, not exceeding a total weight of 15 g. Thereafter, the silver powder and magnesium turnings were mixed and pressed into pellets. In each experiment, approximately 15 g iron was used. The Ag-Mg pellet together with the iron powder was put in an MgO-crucible (supplied by Aremco Products Inc.).
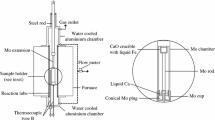
A high-temperature furnace with graphite heating elements was used in the experiments. A schematic of the experimental setup is shown in Figure 2. An alumina tube was used for the reaction chamber. To quench the sample rapidly, the alumina reaction tube was interconnected to a water-cooled brass tube. This arrangement allowed the sample to be quenched without withdrawal from the furnace. A lifting device was placed on top of the furnace, consisting of a lifting unit holding a stainless steel tube that was inserted through an O-ring sealed hole in the top of the brass chamber. A Mo-tube was connected to the lower part of the stainless steel tube.
After placing the MgO crucible containing the sample into the Mo-vessel in an argon atmosphere, the Mo conical lid was put on by hand or with a small hammer if necessary. Attention was given to use as little force as possible, just enough to ensure that the connection could bare the weight of the vessel when the Mo-vessel was hung in the furnace. The sample assembly was then attached to the lifting device using a small Mo-rod. Note that the sample assembly was hung on the Mo-rod by the friction force between the conical lid and the Mo vessel. After lowering the sample assembly to the even temperature zone of the furnace using the lifting system, a B-type thermocouple was placed just below the Mo-vessel for temperature measurement. The reaction chamber was sealed by the use of O-rings. The system was then evacuated with a vacuum pump and thereafter filled with argon. This procedure was repeated at least three times. Before heating, the system was flushed with purified Ar-gas for 2 hours. In the gas cleaning system, first the gas passed through silica gel, ascarite, and magnesium perchlorate. The argon gas was then led through a silica tube with Cu turnings kept at 973 K (700 °C) and finally through an iron tube with magnesium chips kept at 773 K (500 °C). The purified Ar gas was passed through the furnace at 0.02 L/min throughout the whole heating and equilibration period.
The heating rate was kept low (2 K/min) to ensure homogenization of the Ag-Mg alloy and prevent magnesium boil-off. After reaching 1823 K or 1848 K (1550 °C or 1575 °C), the sample was kept in the furnace for 6 hours. Fujiwara et al.[12] used approximately the same setup for the solubility of calcium in liquid Fe + Ca + O alloys and determined that 3 hours were enough for reaching equilibrium. After equilibrating of 6 hours, the sample was lifted to the quench chamber. The lifting for the sample from the even hot zone to the quenching chamber took less than 1 second. The whole experiment took approximately 30 hours. After withdrawal from the furnace, the Ag-Mg alloy was separated from the iron part using a precision cutting machine. In all samples, it was observed that the iron phase was close to spherical and completely surrounded by the Ag-Mg alloy and nowhere in contact with either the crucible or the gas phase. The cutting was made with special attention to completely separate the two phases. The surfaces of the sample pieces were also polished to remove any possible MgO residues. The iron and Ag-Mg part were sent for ICP-AES analysis (Inductively coupled plasma atomic emission spectroscopy, relative error generally less than 10 pct of amount present).
The experimental results are listed in Table I. The content of magnesium in Fe is plotted as a function of Mg content in silver in Figure 3. The activities of Mg in Fe can be evaluated using the thermodynamic data for the Ag-Mg system.
Activity measurements in the liquid Ag-Mg binary alloys were made recently.[13] The excess Gibbs energy for magnesium in liquid Ag-Mg alloy is given in Eq. [2].
The magnesium activity in silver can then be calculated using the following relationship:
The calculated activities of magnesium along with the calculated activity coefficients of magnesium in iron \( \left( {\gamma_{{{\text{Mg}}\left( {\text{Fe}} \right)}} } \right) \) are presented in Table II.
A plot of \( ln\gamma_{{{\text{Mg}}\left( {\text{Fe}} \right)}} \) vs X Mg for 1823 K (1550 °C) is presented in Figure 4.
Despite the limited number of experiments at this temperature, a clear decreasing trend of \( \gamma_{{{\text{Mg}}\left( {\text{Fe}} \right)}} \) with X Mg can be observed. A linear regression yields
Presented as the ε or interaction parameter definition
where \( \gamma_{{{\text{Mg}}\left( {\text{Fe}} \right)}}^{0} \) is the activity coefficient of magnesium in liquid iron at infinite dilution and \( \varepsilon_{\text{Mg}}^{\text{Mg}} \) is the self-interaction parameter of magnesium in liquid iron, one can conclude
The magnitude of \( \varepsilon_{\text{Mg}}^{\text{Mg}} \) is unusually high for self-interaction but is in accordance with the value calculated by Tarby[11] based on the data from Zhang et al.[9]
As the number of experimental points is fewer at higher temperatures than 1823 K (1550 °C), no attempt is made to do any regressions for these points. However, as shown in Figure 3, the data for 1848 K (1575 °C) follow the same trend as the data for 1823 K (1550 °C).
From an industrial perspective, the most common way to express activities of dissolved elements in liquid iron is to use the “wt pct” standard state. The free energy change for the change of standard state from “pure element” to the wt pct standard state is calculated according to
where M Fe and M Mg are the atomic weights of iron and magnesium, respectively. At 1823 K (1550 °C), \( \Updelta G^{0} \) equals 4125 J/mole, using \( \gamma_{{{\text{Mg}}\left( {\text{Fe}} \right)}}^{0} \) = 57.
The formation of MgO by the reaction between dissolved Mg and O can be expressed as
The standard Gibbs energy \( \Updelta G_{7}^{0} \) for reaction [7] can be calculated using data from References 14 to 16 as follows:
Hence, \( \Updelta {\text{G}}_{7}^{0} = \Updelta {\text{G}}_{8}^{0} + \Updelta {\text{G}}_{9}^{0} + \Updelta {\text{G}}_{10}^{0} + \Updelta {\text{G}}_{11}^{0} = - 281522{\text{ J/mol}} \) at 1823 K (1550 °C). This value leads to \( {\text{logK}}_{ 6} = 8. 0 7 \) at 1823 K (1550 °C). This value is compared with the literature data in Table III.
It is reported by Beskow et al.[17] that the total magnesium content is less than 2 ppm in the liquid metal after vacuum treatment of Al-deoxidized tool steel. In their work, the oxygen activity according to Celox measurements is approximately 10−4 (“wt pct” standard state). As the analyzed Mg content includes both dissolved Mg and the Mg in the oxide inclusions, the dissolved magnesium content is definitely much lower. A calculation using the current result based on reaction [7] reveals a dissolved magnesium content of 0.9 ppm (the MgO activity is assumed to be unity). This value is in accordance with the steel analysis. It is worthwhile to mention that it is unlikely that the steel-slag-refractory system has reached a final equilibrium state. The activity of MgO in the slag is always somewhat lower than 1. Although lower MgO activity in the slag would imply lower Mg content in the liquid metal, the current thermodynamic data is still in line with the steel analysis from industry. The evaluated Mg contents using the current thermodynamic data and the data from the literature are listed in Table 3. In all the calculations, the oxygen activity is assumed to be 10−4 (wt pct in Fe standard state) and the MgO activity equal to one. It is observed that the Mg content is calculated to be 59 ppm using the data from Han et al.[8] with log K equal to 6.23, and 83 ppm using the data by Nadif et al.[4] Such high Mg contents are unreasonable.
The findings are summarized as follows:
-
(a)
The activity coefficient of magnesium in liquid iron at infinite dilution, \( \gamma_{{{\text{Mg}}\left( {\text{Fe}} \right)}}^{0} \) was evaluated to be 57, and the self-interaction parameter of magnesium \( \varepsilon_{\text{Mg}}^{\text{Mg}} \) was evaluated to be –495.
-
(b)
The current data led to a value of 8.07 for the log K of the reaction Mg(wt pct Fe) + O(wt pct Fe) = MgO(s) at 1823 K (1550 °C). This value was found to be reasonable in explaining the industrial observation in comparison with the literature data.
References
V. Brabie: Scand. J. Metall., 1996, vol. 25, pp. 148-60.
B. Hallberg: Clean Steel 5: 5th Int. Conf. on Clean Steel, Balatonfured, Hungary, 1997, pp. 165-74.
S. Jansson, V. Brabie, and P. Jönsson: Ironmaking Steelmaking, 2006, vol. 33, pp. 389-97.
M. Nadif and C. Gatellier: Rev. Métall. Cah. Inf. Tech., 1986, vol. 83, pp. 377-94.
H. Itoh, M. Hino, and S. Ban-ya: Tetsu-to-Hagané, 1997, vol. 83, pp. 623-28.
I.S. Kulikov: Izv Akad. Nauk SSSR, Met., 1985, pp. 9–15.
A.P. Gorobetz: Metall. Koksokhim, 1980, vol. 69. pp. 34-37.
Q. Han, D. Zhou, and C. Xiang: Steel Res., 1997, vol. 68, pp. 9-14.
X. Zhang, Q. Han, and D. Chen: Metall. Trans. B, 1991, vol. 22B, pp. 918-21.
M. Hillert: Metall. Trans. B, 1992, vol. 23B, p. 665.
S.K. Tarby: Metall. Trans. B, 1993, vol. 24B, pp. 909-10.
H. Fujiwara, M. Tano, K. Yamamoto, and E. Ichise: ISIJ Int., 1995, vol. 35, pp. 1063-71.
J. Gran: Ph.D. Dissertation, Royal Institute of Technology, Stockholm, Sweden, 2011.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, pp. 5-26.
G.K. Sigworth and J.F. Elliott: Met. Sci., 1974, vol. 8, pp. 298-310.
P.J. Guichelaar, P.K. Trojan, T.McCluhan, and R.A. Flinn: Metall. Trans., 1971, vol. 2, pp. 3305-13.
K. Beskow, Du Sichen, and N. Sano: Iron Steel Tech., 2006, vol. 3, pp. 103–17.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 8, 2011.
Rights and permissions
About this article
Cite this article
Gran, J., Sichen, D. Experimental Determination of Mg Activities in Fe-Mg Solutions. Metall Mater Trans B 42, 921–924 (2011). https://doi.org/10.1007/s11663-011-9557-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-011-9557-7